Intraoperative neurophysiological monitoring has experienced a spectacular development in the past 20 years, particularly in the fields of neurosurgery and spine surgery. It has become a useful, almost indispensable tool in preventing nerve damage during surgery. The aim of this article is to describe the intraoperative technique and analyze its results in the field of peripheral nerve surgery.
ObjectiveTo describe the usefulness of a technique in peripheral nerve surgery, the technique used and the experience in a center.
Patients and methodsA retrospective study was conducted on 30 cases of peripheral nerve surgery performed in this center from 2009 to 2013, using the intraoperative monitoring technique.
ResultsOf the total of 13 peripheral nerve tumors recorded, there were 11 excellent results and 2 good results, one temporary hypoesthesia and one with almost complete sensory, except for motor recovery. Traumatic injury was recorded in 17 cases, of which 6 required performing a graft, and the remaining 11 cases only neurolysis was performed, with complete motor and sensory recovery.
ConclusionsIntraoperative neurophysiological monitoring is a useful tool in the secondary surgery of peripheral nerve injury and the intraneural tumor pathology.
La monitorización neurofisiológica intraoperatoria ha experimentado un espectacular desarrollo en los últimos 20 años, particularmente en campos como la neurocirugía y la cirugía de raquis. Se ha constituido en una herramienta muy útil en la prevención de daño neurológico durante la cirugía, si bien su utilidad en la cirugía del nervio periférico en el área de traumatología y ortopedia no ha sido constatada.
ObjetivoDescribir exhaustivamente la técnica de monitorización neurofisiológica intraoperatoria y secundariamente comunicar la experiencia de nuestro centro.
Pacientes y métodoEstudio descriptivo retrospectivo de 30 casos de cirugía de nervio periférico realizadas en nuestro centro en el período 2009–2013. Descripción pormenorizada de la técnica de monitorización neurofisiológica intraoperatoria utilizada.
ResultadosRegistramos 13 tumores del nervio periférico, de estos, obtuvimos 11 resultados excelentes y 2 buenos, uno con hipoestesia temporal y otro con recuperación motora casi completa aunque no sensitiva. Registramos 17 casos de lesiones traumáticas, en 6 casos fue necesaria la realización de injerto, en los 11 restantes solo realizamos neurolisis, con recuperación sensitiva y motora completa.
ConclusionesLa monitorización neurofisiológica intraoperatoria supone una herramienta útil en la cirugía secundaria de las lesiones del nervio periférico y en la enfermedad tumoral intraneural de dicho nervio.
Microsurgery of the peripheral nerve in the area of Traumatology and Orthopedics is mainly carried out in the treatment of acute neurological lesions or complications thereof (neuromas), as well as peripheral nerve tumors.1 Traumatic lesions of the peripheral nerve are usually secondary to fractures (open or closed) or to penetrating injuries in the limbs.1 Their diagnosis is mainly clinical, can affect all age groups, and is potentially devastating for patients, as they affect professional and daily life activities. Peripheral nerve tumors are rare lesions developed at the expense of the elements comprising the nerve, with Schwann cells being the main constituent element.2,3 They usually appear as a soft tissue mass in the path of the nerve, which may be painful upon palpation and present positive Tinel sign.2,4 So-called schwannoma or neurilemoma is the most frequent neurogenic tumor in peripheral nerves, accounting for approximately 5% of benign soft tissue neoplasms.5–12
Both traumatic lesions, particularly secondary ones once the neuroma is constituted, and tumoral lesions, particularly intraneural ones, require advanced anatomical knowledge and extensive experience in microsurgery in order to achieve the desired objectives, which include recovery of the maximum functional capacity possible and elimination of pain.1 Direct nerve repair or through a graft in the first case or exeresis of the lesion respecting the nerve of origin in the second case are the treatments of choice.13,14
Therefore, we can say that an essential goal of this procedure is the preservation of a maximum of the undamaged nerve fascicles, that is, not sacrificing healthy nerves, and it is in this aspect that intraoperative neurophysiological monitoring (INM) studies are of great value, as they provide the surgical team with basic, reliable and real-time information on the functionality of the explored nerve.4,5
The main uses of INM of the peripheral nerves and brachial plexus are14,15:
- 1.
Identifying the peripheral nerves.
- 2.
Locating preexisting lesions throughout the pathway of the nerve.
- 3.
Determining continuity across a nerve lesion.
- 4.
Determining whether there is root avulsion.
- 5.
Identifying the targets for nerve biopsy.
- 6.
Monitoring and preventing damage to healthy nerves during the intervention.
- 7.
Obtaining an evolutionary prognosis of the neurological lesion.
The main objective of this study was to describe the technique of intraoperative neurophysiological monitoring conducted at our center and, secondarily, to report the experience of the surgical team in a series of 30 cases, as well as to debate whether these reasons make it a useful technique in peripheral nerve surgery.
Materials and methodsThis was a retrospective, descriptive study conducted in January 2014 on 30 patients intervened during the period between January 2009 and September 2013 due to peripheral nerve pathology (Table 1), at the same center (Hospital Universitario de Canarias, third level of healthcare).
| Gender | Age | Diagnosis | Prior ENG (SEDDON) | Drugs used in anesthesia | Type of INM | Previous surgery | Treatment | |
|---|---|---|---|---|---|---|---|---|
| 1 | Female | 37 | Schw. median | Normal | Sevoflurane | NAP | No | Exeresis |
| 2 | Male | 13 | Schw. median | Normal | Propofol+remifentanil | NAP | No | Exeresis |
| 3 | Female | 20 | Section median | Neurotmesis | Sevoflurane | NAP | Neurorrhaphy | Sural graft |
| 4 | Female | 42 | Schw. posterior tibial | Normal | Propofol+remifentanil | NAP | No | Exeresis |
| 5 | Female | 51 | Section ulnar | Neurotmesis | Sevoflurane | NAP | Neurorrhaphy | Neurolysis |
| 6 | Male | 5 | Paralysis ulnar | Neuropraxia | Sevoflurane | NAP | No | Neurolysis |
| 7 | Female | 72 | Schw. ulnar | Normal | Sevoflurane | NAP | No | Exeresis |
| 8 | Male | 41 | Paralysis radial | Neurotmesis | Sevoflurane | NAP | No | Neurolysis |
| 9 | Male | 23 | Schw. tibial posterior | Normal | Propofol+remifentanil | NAP | No | Exeresis |
| 10 | Male | 17 | Paralysis ulnar | Neuropraxia | Sevoflurane | NAP | No | Sural graft |
| 11 | Female | 36 | Section median | Neurotmesis | Sevoflurane | NAP | Neurorrhaphy | Neurolysis |
| 12 | Female | 44 | Section ulnar | Neurotmesis | Sevoflurane | NAP | Neurorrhaphy | Neurolysis |
| 13 | Male | 34 | Schw. median | Normal | Sevoflurane | NAP | No | Exeresis |
| 14 | Female | 25 | Schw. median | Normal | Propofol+remifentanil | NAP | No | Exeresis |
| 15 | Male | 43 | Section ulnar | Neurotmesis | Sevoflurane | NAP | No | Sural graft |
| 16 | Male | 71 | Section radial | Neurotmesis | Sevoflurane | NAP | Neurorrhaphy | Sural graft |
| 17 | Male | 30 | Section deep fibular | Neurotmesis | Sevoflurane | NAP | Neurorrhaphy | Sural graft |
| 18 | Male | 26 | Schw. radial | Normal | Sevoflurane | NAP | No | Exeresis |
| 19 | Male | 55 | Section radial sensory | Neuropraxia | Sevoflurane | NAP | No | Neurolysis |
| 20 | Male | 63 | Paralysis radial | Neurotmesis | Sevoflurane | NAP | No | Neurolysis |
| 21 | Male | 18 | Schw. median | Normal | Sevoflurane | NAP | No | Exeresis |
| 22 | Female | 41 | Section posterior tibial | Neurotmesis | Sevoflurane | NAP | No | Sural graft |
| 23 | Male | 71 | Paralysis sciatic | Neuropraxia | Sevoflurane | NAP | No | Neurolysis |
| 24 | Male | 45 | Schw. CFN | Normal | Sevoflurane | NAP | No | Exeresis |
| 25 | Male | 57 | Fibrolipoma median | Neuropraxia | Sevoflurane | NAP | No | Neurolysis |
| 26 | Female | 46 | Section median | Neurotmesis | Sevoflurane | NAP | No | Neurorrhaphy |
| 27 | Male | 6 | Paralysis ulnar | Neuropraxia | Sevoflurane | NAP | No | Neurolysis |
| 28 | Male | 4 | Paralysis ulnar | Neuropraxia | Propofol+remifentanil | NAP | No | Neurolysis |
| 29 | Female | 19 | Schw. radial | Normal | Sevoflurane | NAP | No | Exeresis |
| 30 | Male | 34 | Schw. median | Normal | Sevoflurane | NAP | No | Exeresis |
CFN: common fibular nerve; ENG: electroneurogram; INM: intraoperative neurophysiological monitoring; NAP: nerve action potential; Schw.: schwannoma.
A clinical history was obtained from all patients, and all underwent physical exploration, especially focusing on motor and sensory function, before and after the surgery, conducted by the same head surgeon (FMM), given his knowledge and experience in microsurgery. We followed the criteria described by the British Medical Research Council (BMC) or modified versions classifying sensory recovery between S0 and S5 and motor recovery between M0 and M5.1
All patients underwent a prior neurophysiological study by the same neurophysiologist in charge of the intraoperative technique (PPL). The Seddon classification, considered to be the most widely used worldwide, was applied as it provides an understanding of the physiopathology of the lesion, establishes a likely prognosis and, above all, offers an adequate therapeutic approach.1 This classification distinguishes between: neuropraxia, a block in nerve conduction at a local level, without axonal involvement and therefore without distal wallerian degeneration, in which function is recovered rapidly, after a couple weeks at the most, and restitutio ad integrum is the norm; axonotmesis, an axonal lesion associated to distal wallerian degeneration in which the endoneurium and perineurium are intact, thus guaranteeing correct guidance of the axon in regeneration up to the distal end of the lesion site, with fiber displacement speed classically described as between 1 and 1.5mm daily, and where functional recovery is also the norm, although with a greater delay than neuropraxia, with up to 6 months; and lastly, neurotmesis, a more severe lesion, with complete section of the nerve, absolute loss of function and absence of any kind of spontaneous recovery.1,14
All patients with tumoral pathology also underwent a magnetic resonance imaging (MRI) study prior to the surgery, which was interpreted by the same radiologist (LDF).
Data collection, analysis and interpretation were carried out by the remaining authors of this work (MHP, JPB).
Al the patients were adequately informed about the necessary procedures. There was no need to obtain approval from the Ethics Committee of our center, as this diagnostic test was accepted for use in daily practice. Moreover, this study did not provide any personal information from patients, thus maintaining their confidentiality.
The inclusion criteria to participate in the study were the following:
- -
Open traumatic neurological lesions without clinical or neurological recovery after 6 months of evolution since the first intervention.
- -
Closed neurological lesions treated conservatively with worsening of the symptoms and neurophysiology during follow-up.
- -
Neural or extraneural tumoral lesions of the peripheral nerve.
- -
Existence of a neurophysiological study prior to the surgery.
The exclusion criteria were the following:
- -
Diabetic patients.
- -
Patients with prior sensory-motor alterations.
- -
Patients who did not sign the informed consent forms.
- -
Patients who did not complete the required follow-up.
Before describing our exploration technique, we should highlight a series of preliminary aspects to be taken into account:
- •
A decrease in temperature during the surgical process is difficult to prevent and, therefore, we should bear in mind that this temperature decrease will reduce nerve conduction speeds.
- •
A decrease in arterial tension during surgery reduces the amplitudes of somatosensory evoked potentials (SSEP) and motor evoked potentials (MEP).
- •
Peripheral ischemia, caused by the ischemia cuff, affects nerve response by altering the vasa nervorum, and may block nerve response.14,16,17 If a tourniquet is applied for over 60min, it should be removed at least 20–30min before starting intraoperative monitoring studies.13
- •
Anesthesia affects INM studies as it alters cortical excitability. In addition, we should also take into consideration the effect of neuromuscular relaxing agents.
In our series we did not use peripheral ischemia in any case, the anesthesia used was general with particular precaution regarding the use of neuromuscular relaxing agents which altered intraoperative measurements, and the temperature within operating rooms was maintained at 15°C in all cases.
Methodology: technical description of neurophysiological monitoringThe technique starts once the nerve to be explored has been identified and isolated. Since the main purpose of nerve conduction studies is to assess the integrity of the nerve, its functional continuity or to determine the location of a peripheral nerve lesion, the stimulation and reception electrodes should be placed on either side of the lesion. This is known as bipolar stimulation, and its main advantage is causing less current dispersion. The best alternative when bipolar stimulation is unfeasible, is to conduct monopolar stimulation with the cathode on the nerve and the anode on an inert surface at a certain distance. The bipolar stimulation electrode (preferable hooked electrodes) held by the surgeon is placed on the surface of the nerve, with the active electrode aimed toward the collecting electrodes.15 The distance between the poles of this bipolar electrode is generally of 3–4mm, although this mainly depends on the size of the nerve, as large nerves may require distances between poles close to 7mm.13
First, the neural response is evoked in a healthy part of the nerve, placing both the stimulation and reception electrodes proximally or distally to the lesion. Subsequently, the stimulation electrodes are placed proximally and the reception electrodes are placed distally to the lesion to evoke a NAP across it.
During the stimulation it is advisable to elevate the nerve with respect to the surgical field, in order to avoid contact with any fluids (blood, serum…) which would reduce the intensity of the stimulus received by the nerve. We must take into account that an excessive stimulation increases the intensity of the stimulus, which may become a considerable problem if the distances between the stimulation and collection electrodes are small.
Generally the duration of the stimulus is around 0.05–0.1ms with square pulse,7 and the intensity does not need to be above 1–5mA to produce a depolarization of the nerve and evoke a supramaximal response. Damaged nerves require stimulation at higher intensities in order to evoke a response (around 20–25mA or 25–50V).16 This causes an increase in the stimulation artifact that could interfere with response collection.
The nerve action potential (NAP) is an electrical potential that is generated and travels through nerve fibers once these have been stimulated above their threshold, physiologically or electrically.16 It can be registered both in the entire nerve and in its various fascicles.14 The presence of NAP is considered to require at least 4000 fibers of over 5μ in diameter. When assessing the continuity of the nerve, we must take into account that the presence of a few large-sized myelinated nerve fibers (out of the 4000) can evoke a NAP of normal characteristics. The process of studying a peripheral nerve starts with stimulation and reception of the NAP in a proximal location to the damaged region of the nerve, which allows the technique to be assessed (normal responses should be evoked).13 The electrodes are subsequently placed on both sides of the lesion to assess nerve continuity.
The distance between the stimulation and collection electrodes should be over 4cm.13,16 The collection electrode should also be bipolar, with a distance of 3–5mm between the electrodes (with the active electrode placed closer to the cathode of the stimulator), with a greater separation between them if there is a considerable distance between the stimulator and the receptor.
The ground electrode can be placed on a surface of the skin of the patient, away from the grounding of the electric scalpel.13
The filters required to obtain a NAP response are between 5 and 10Hz for the low frequency and between 2 and 3kHz for the high frequency.16
The amplitude of NAP is generally low, about 100μV, so the gain is around 20–50μV per division. Response averaging is necessary in order to evaluate it (less than 10 stimuli are generally necessary).
Naturally, the latency of the response depends on the distance between the stimulation and collection electrodes. Accepting a conduction speed of 50m/s, we can apply 1ms for every 5cm of nerve distance, so the sweep should be around 0.5–1ms per division and increase to 2ms if the distance between both electrodes is considerable.
Practical implications for intraoperative decision makingThe large majority of peripheral nerve lesions do not section the nerves, instead leaving continuity lesions that may not be observed during a visual inspection.17 The presence of structural continuity does not imply nerve functionality; therefore, a simple visual inspection by itself should not be taken into consideration in the surgical process, since electrophysiological studies will be the ones to determine the course of the intervention. Thus, the surgical decision in peripheral nerve lesions is based on the determination of continuity of nerve function through a lesion in the said nerve (ascertaining the presence of continuity).17,18
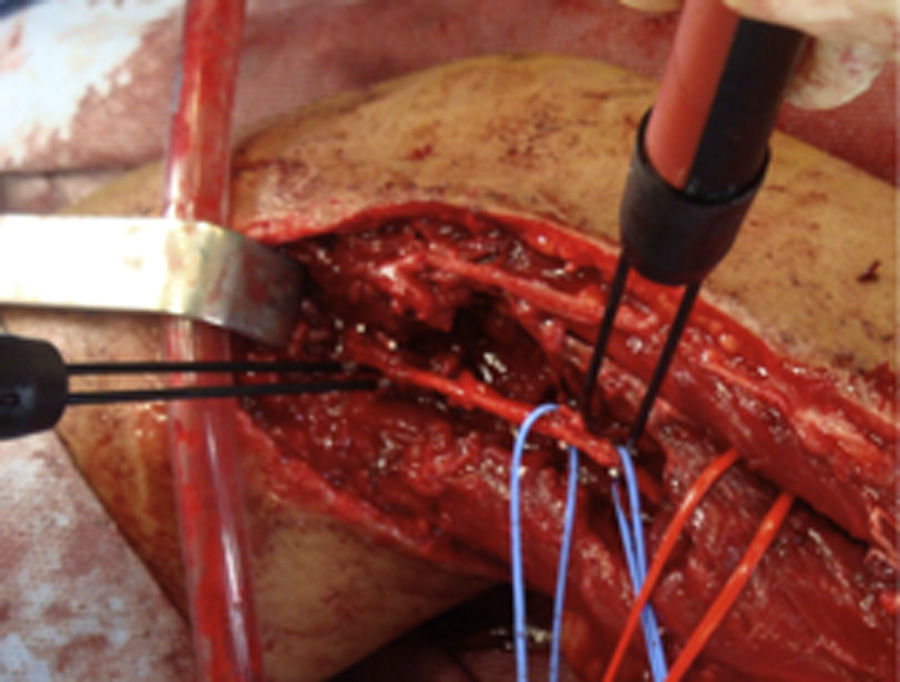
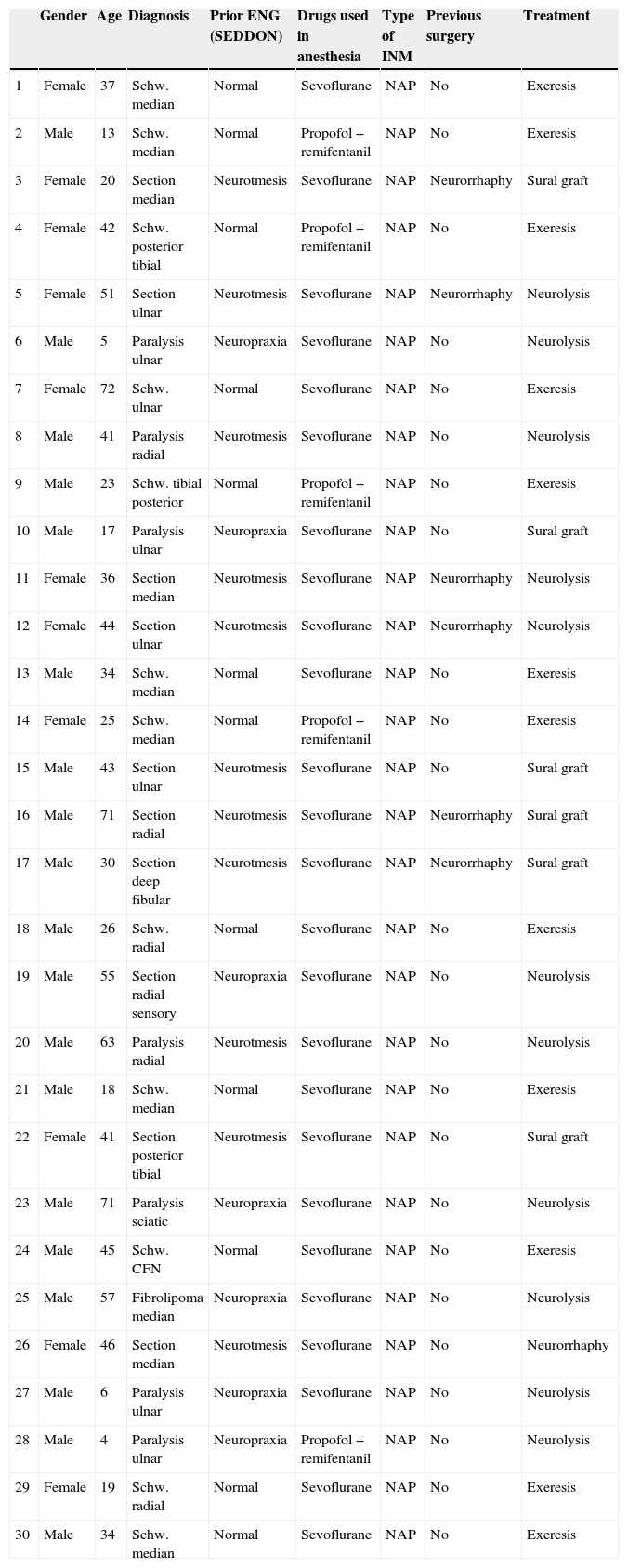
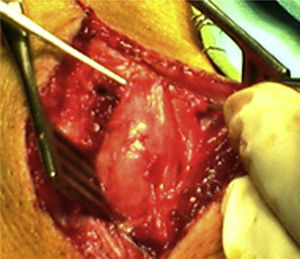
How do we know if an explored nerve is functional and therefore viable?The presence of NAP through a lesion is the gold standard technique and indicates that there is nerve continuity or collateral reinnervation (even though preoperative neurophysiological studies and symptoms may indicate otherwise).13,17 Therefore, surgical procedures should be limited to neurolysis (release of nerve adherences) of the lesion in continuity14 (Fig. 1), with this being the crucial point to bear in mind during the decision-making process in peripheral nerve surgery.1
What to do in case of lack of response to nerve action potential?In case of a lack of response to NAP, we should carry out the following steps sequentially14,16:
- 1.
Verify the presence of hypotension and hypothermia.
- 2.
Proximal to the lesion or in healthy segments, check the position of the electrodes and ensure that they are not too close.
- 3.
Verify whether the stimulating electrode is producing a stimulus.
- 4.
Make sure that there should be no fluids in the surgical field and the electrodes should not touch other tissues.
- 5.
There should not be excessive tension in the nerve tissue.
- 6.
If the surgical field has cooled, it should be warmed with warm serum.
- 7.
If the tourniquet is still in place, it should be removed and a period of 20min should be awaited before continuing with INM.
- 8.
No local anesthesia should be used.
- 9.
If a large artifact is observed, about 60Hz (network), the network filter of the device should be switched off, before checking that there are no other machines, such as X-ray devices in the field.
- 10.
Ensure that the signal obtained does not come from the inadvertent stimulation of other nerves.
In general, it is accepted that lesions in which NAP is not transmitted after an evolution of 3–4 months do not have a chance of spontaneous recovery and should be intervened through a graft or nerve transfer.13,16 On the other hand, the presence of NAP points to functional recovery within weeks or months.
Lastly, we must take into account the possibilities for false positive potentials14,16,17:
- 1.
Prenodal lesions (plexopathies) may respect the sensory fibers alongside severe damage of the motor fibers and generally the conductions are very high (around 65–70m/s of the sensory fibers instead of the 50m/s of the motor or mixed).
- 2.
We may be stimulating and collecting information in a different area from that of the nerve lesion.
- 3.
We may be exploring a different nerve or part of the plexus.
- 4.
Exploring the nerve more than 1 year after the nerve lesion.
- 5.
An excessive averaging may register fine fibers.
- 6.
It is important to be careful with near motor responses.
In the case of peripheral nerve tumors,14 the use of NAP can detect the peripheral nerve in those locations where the architecture or anatomy is confusing and even identify the functional fascicles (and protect as far as possible).13
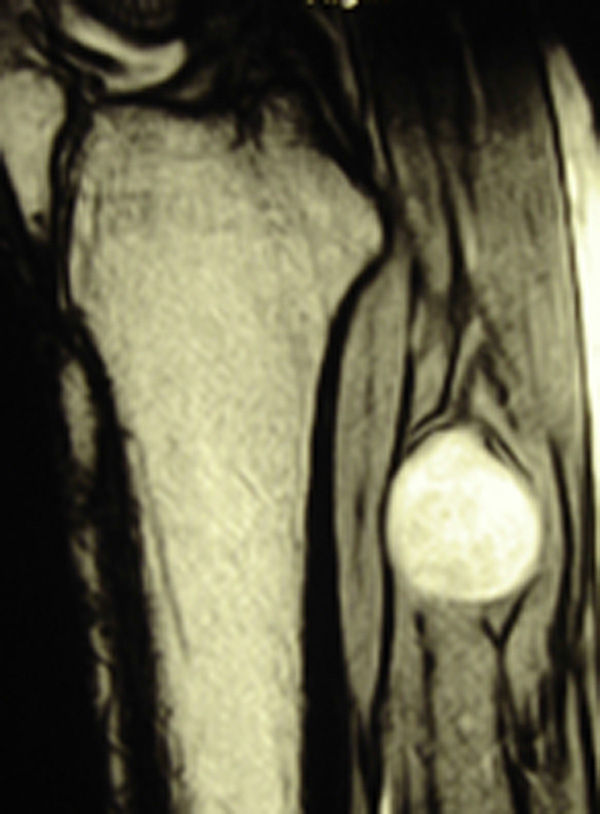
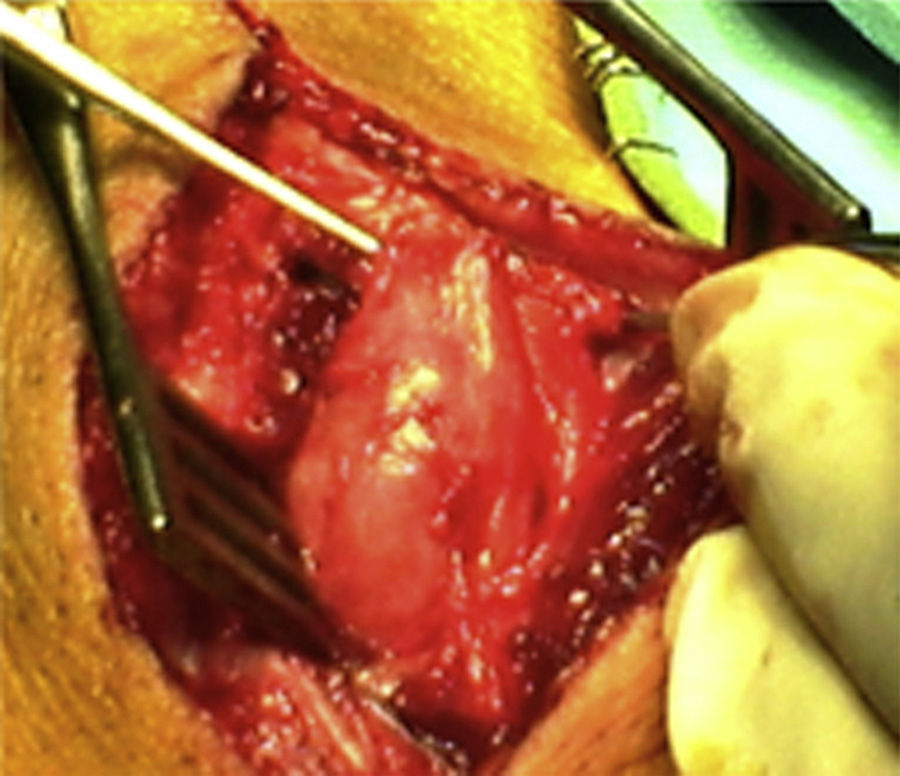
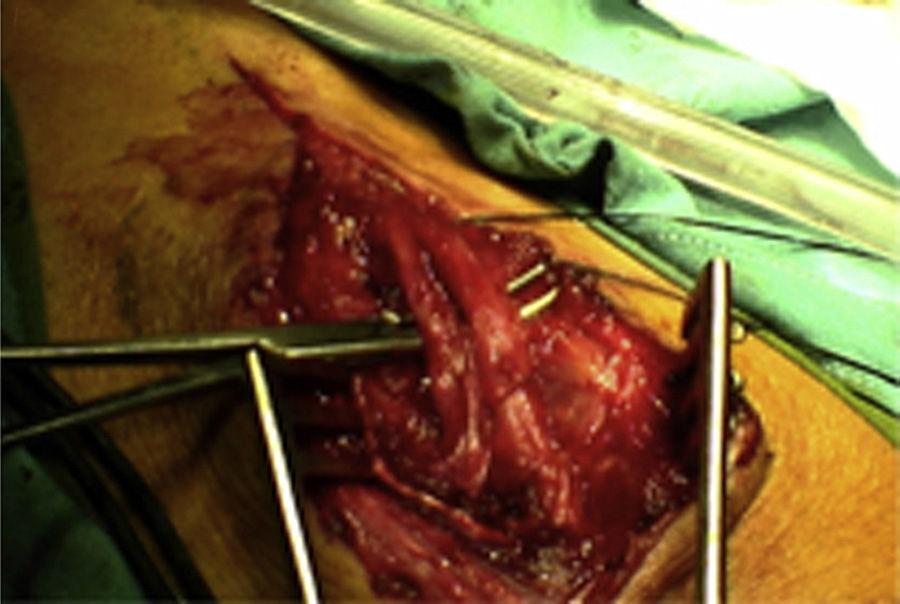
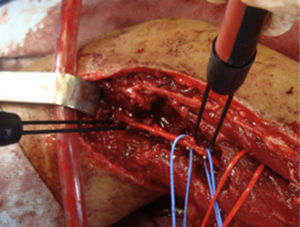
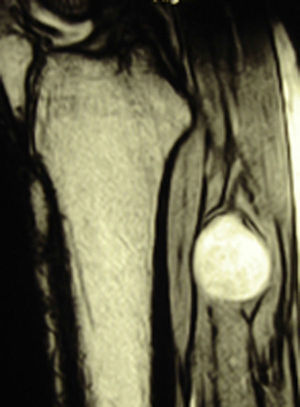
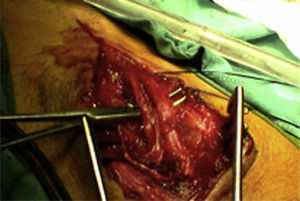
ResultsWe registered 12 peripheral nerve schwannomas, 9 in the upper limbs and 3 in the lower limbs (Table 1). In all cases, the surgery was carried out with no incidents and the tumor was resected from the main trunk of the nerve, except for 1 case where we had to sacrifice a only-sensory branch of the posterior tibial nerve after the neurophysiological monitoring showed that it had no motor component. In this case, a residual hypoesthesia was registered in the internal calf region (S2 according to the BMC classification), which lasted for 3 months (Figs. 2 and 3). As a permanent sequela we report 1 case of schwannoma in the external popliteal nerve which began with sensory and motor involvement (M1 and S1) (Figs. 4 and 5), with a persistent hypoesthesia in the dorsum of the foot (S1 according to the BMC classification), although without motor involvement. We registered 1 case of fibrolipoma of the median nerve, also known as neural fibrolipomatous hamartoma, perineural lipoma or neural fat infiltration, in a 57-year-old male which started as carpal tunnel syndrome and increased in size in the volar side of the wrist. We were able to treat it through section of the flexor retinaculum of the wrist and endoneurolysis of the median nerve. Therefore, we registered 11 cases with excellent results and 2 with good results, 1 with temporary hypoesthesia and another with almost full motor recovery, although not sensory.
We monitored 17 cases of traumatic lesions (Table 1). Ten of these cases were secondary to penetrating wounds, and in 6 of them we conducted direct neurorrhaphy of the damaged nerve. During the revision surgery we verified through INM that in 6 cases the neuroma did not allow distal conduction, so it was necessary to perform grafting with tissue from the sural nerve following the usual technique. In the 4 remaining cases we registered distal conduction so we only carried out neurolysis.
As for the remaining cases, 7 patients had suffered accidental closed trauma or else iatrogenic damage (ulnar nerve damage in surgery for supracondylar fracture). In this last group, intraoperative neurophysiological monitoring was very useful in 3 children suffering ulnar nerve lesion following incruental reduction and percutaneous fixation of a displaced supracondylar fracture of the humerus, due to a possible lesion whilst introducing the needle from the ulnar side. After being unable to observe functional recovery in the physical exploration or the serial neurophysiological studies after 9, 10 and 11 months, respectively, we decided to perform exploration and direct monitoring (Fig. 1). In the 3 cases we were unable to observe evident macroscopic lesions and registered that the nerve did conduct at a distal level, so we simply performed neurolysis. Function was recovered 3, 4 and 4.5 months after the surgery, respectively.
DiscussionPeripheral nerve surgery is a very demanding type of surgery, requiring advanced technical and anatomical knowledge in order to obtain good functional results.1,14 The decision to repair a damaged nerve should take into account both the condition of the nerve and the capacity of the surgical team and the hospital.18 One of the most important factors to obtain these results is the moment when the surgical reconstruction is carried out, that is, surgical timing. Given that, very often, the lost nerve function returns spontaneously, performing surgery too early would be therapeutically rash, as the patient would undergo an unnecessary surgical intervention. On the other hand, acting too late would reduce the chances of recovery, because muscles which do not receive innervation inevitably tend to become atrophied, so the longer the period elapsed between the lesion and its repair, the greater the atrophy and the smaller the chances of success. In general practice, the second scenario (patients referred belatedly) is much more common than the first. Two main groups can be established: closed and open lesions. The first are those that generally require an expectant initial approach and deferred surgery, not before 3 months since the trauma. On the other hand, open lesions should be explored rapidly in order to carry out direct neurorrhaphy whenever feasible, or else cleaning and repair of the ends of the sectioned nerve, in order to conduct a repair with an interposed graft. Other nerve exploration indications include: nerve lesions with arterial damage, lesions caused by traction on the brachial plexus, decrease of nerve functionality after an initially expectant approach, lack of neurological improvement following a closed lesion, lack of improvement following a conduction block within 6 weeks after the lesion, persistent pain and suspected formation of a neuroma.1,18
Following a nerve lesion, a neurophysiological study helps to determine the severity of the nerve involvement, as well as detect signs of functional recovery before these are reflected by the symptoms. Currently, decision-making in peripheral nerve pathologies is still the result of a combination of neurophysiological studies and serial physical examinations. However, the use of INM has sparked a considerable and increasing interest, as it provides real-time information, by stimulating neural structures directly, about the functionality of the main affected nerve branches and the presence or absence of an action potential in the distal nerve to the lesion, particularly when the nerve is not completely sectioned and a neuroma has formed.13,14,16,17 This technique can even distinguish between an intact fascicle and a neuroma. Clinically, the size and hardness of the neuroma is a negative factor for recovery, but good amplitude signals distal to the nerve lesion indicate a better prognosis because they point to the existence of intact fascicles crossing the damaged area.15,18 We find this technique particularly interesting in cases of posttraumatic closed lesions or after surgery in specific locations, as in the case of the ulnar nerve at the level of the elbow in supracondylar humeral fractures in children, as in 3 cases in our series. With this technique we were able to determine whether the nerve was functional or required a nerve graft, as in one of the cases described. Lastly, and although not part of the objective of this work, INM has also been used experimentally in animals in the implementation of biological techniques for nerve repair that will serve as coadjutants in nerve regeneration in the near future.19–24
In the case of tumors settled on the peripheral nerve, the goal of treatment is complete exeresis with minimal damage to the nerve. Within these tumors we must distinguish resectable nerve tumors from unresectable cases. The former avoid fascicular groups without penetrating them, that is, they are extraneural, so they can be enucleated without having to break nerve continuity, such as schwannomas, for example, with an excellent functional prognosis. There are no studies proving that optimal results would not be achieved without using INM.
On the other hand, it is in unresectable tumors (solitary neurofibroma, hemangioma of the Schwann sheath, neural fibrolipoma) that INM is most useful. These tumors infiltrate all the elements that constitute the nerve and it is impossible to excise them completely without altering the nerve fibers, as in the case of fibrolipoma of the median nerve reported in our series. Therefore, they are intraneural and it would be important to know which fascicles are functionally viable in order to preserve them, even though in many cases this will not be possible and we will need to resort to nerve grafts or nerve conduits.18
In the literature review we found very few specific references to the use of this technique in peripheral nerve tumoral surgery related to the field of Orthopedics, not being able to support its generalized use in our field, although we found numerous studies reporting excellent results in cases suffering traumatic lesions of the peripheral nerve and brachial plexus, as well as in facial nerve surgery.23–25 The majority of articles dealing with this technique refer to the fields of Otolaryngology and Neurosurgery, particularly surgery of the skull base, and are usually reviews of case series or isolated case reports.25–28 It is worth highlighting the extensive experience existing in the case of schwannomas of the vestibulocochlear nerve (eighth cranial nerve),25–30 including the article by Oh et al.,28 who perfectly described the monitoring technique for neurinomas in this location, very similar to that used at our center. Kwok et al.31 reported excellent results in the case of benign tumors of the brachial plexus, and maintained that its generalized use has minimized intraoperative complications.
We found up to 3 reports of the application of this technique in the case of schwannomas of the posterior tibial nerve as a cause of tarsal tunnel syndrome, a pathology which is included in the differential diagnosis of atypical talalgias.32–35 Two of these publications included recommendations for the systematic use of this technique to avoid iatrogenic lesions deriving from the surgical intervention.32,33 In our series, we reported 3 schwannomas of the posterior tibial nerve, with complete recovery and intact functionality in all cases (Figs. 2 and 3).
Following the considerations described by Mohler and Hanel36 in his article, there is a discrepancy between the previous neurophysiological condition and the symptoms, in the case of closed lesions of peripheral nerves, and the final functional results, with considerable variability between the different peripheral nerves and between one patient and another. For this reason, we need more precise tools that can determine if it is possible to perform conservative surgery of the affected nerve during the intervention.
Lastly, the authors believe that, as is already occurring in neurosurgery and spinal surgery, the advent and development of these monitoring techniques mean we are not far from the need, not only from a therapeutic standpoint but also from the medical-legal, to make these diagnostic tools available to our patients in order to achieve a better functional result, which is the ultimate goal of our treatment. Our results are excellent from the functional standpoint, with only 2 patients with schwannoma suffering a partial sensory deficit, and with this deficit persisting in only 1 of the patients after 3 years of monitoring. The best results in traumatic cases were obtained with median nerve grafts.
Study limitationsThe main limitations of our study came from its methodology, as it was a retrospective descriptive study. We have not compared our results with those of interventions performed without monitoring, although it is worth mentioning that this kind of surgery is not carried out without INM at our center since 2009.
ConclusionsOur results indicate that the use of INM in peripheral nerve surgery is a useful technique, especially indicated in secondary surgery of traumatic lesions in which the conventional neurophysiology and symptoms point to an irreversible neurological lesion. It would be necessary to conduct comparative prospective studies to ensure that the technique is essential and indispensable in all peripheral nerve interventions.
Level of evidenceLevel of evidence IV.
Ethical disclosuresProtection of people and animalsThe authors declare that this investigation adhered to the ethical guidelines of the Committee on Responsible Human Experimentation, as well as the World Medical Association and the Declaration of Helsinki.
Confidentiality of dataThe authors declare that they have followed the protocols of their workplace on the publication of patient data.
Right to privacy and informed consentThe authors declare having obtained written informed consent from patients and/or subjects referred to in the work. This document is held by the corresponding author.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Herrera-Pérez M, Oller-Boix A, Pérez-Lorensu PJ, de Bergua-Domingo J, Gonzalez-Casamayor S, Márquez-Marfil F, et al. Monitorización neurofisiológica intraoperatoria en la cirugía del nervio periférico: descripción técnica y resultados en nuestro centro. Rev Esp Cir Ortop Traumatol. 2015;59:266–274.