In children with egg protein allergy (EA), the probability of overcoming the allergy decreases with age, and the possibility of suffering severe adverse reactions as a consequence of dietetic transgressions results in worsened quality of life. One treatment option in such cases is oral immunotherapy (OIT) with foods.
MethodsWe present a cohort of children with EA scheduled for OIT with pasteurized raw egg white, describing their clinical and allergic characteristics before the start of OIT.
ResultsThe median age was six years, and 93% of the patients also suffered other allergies (58% asthma and 38.6% allergy to more than two food groups). In the last year, 14.8% had suffered a severe reaction due to dietetic transgression with egg. The median IgE specific of egg white titer was 38.5kU/l. A double-blind placebo-controlled food challenge with cooked egg white was performed, and if the test proved positive, it was repeated with pasteurized raw egg white. The mean symptoms-provoking dose was 1.26g and 0.55g for cooked egg white and raw egg white, respectively. An IgE specific of ovomucoid titer of <2.045kU/l differentiated those patients that tolerated cooked egg white.
ConclusionsOIT with egg is regarded as an option in patients with persistent egg allergy. In the previous challenge test, an IgE specific of ovomucoid titer of <2.045kU/l differentiates those patients that tolerate cooked egg white.
Allergic diseases have increased in frequency in recent years. Food allergy is more common in childhood, with an estimated prevalence of 0.3–7.5% in the general population, and about 10% among atopic individuals.1 In Spain, egg is implicated in 78.9% of all cases of food allergy in children under five years of age,2 and most of these patients acquire tolerance during childhood – although in some cases the problem may persist into adult life. Some Spanish series have found 50% of the patients to tolerate egg by the age of three years – a figure that increases to 66% at the age of six years.3,4 However, other studies have reported lower tolerance rates of 4%, 12%, 37% and 68% at 4, 6, 10 and 16 years of age, respectively.5 Since heat processing reduces the allergenicity of different egg proteins,6 tolerance to cooked egg is reached more often and earlier than in the case of raw egg.7
Food allergy is moreover the most frequent cause of anaphylaxis in childhood,8,9 and in some cases such reactions can prove fatal. Most of them occur as a consequence of the consumption of processed foods.10 The only treatment option in the case of EA has been strict avoidance of egg in the diet. However, this measure is not always effective in avoiding unexpected adverse reactions, since foods such as egg are currently common ingredients in food products. In Spain, a study involving a series of children with EA found 21% of the patients to have suffered some adverse reaction as a result of dietetic transgression in the last year, and 66% of these cases were moreover of moderate to severe intensity.11 In a prospective cohort study of 514 infants under 15 months of age with allergy to cow's milk or egg and followed-up for three years, 71.7% of the patients suffered some reaction and over 50% experienced more than one reaction a year – an association was observed between the number of reactions and number of food allergies and the specific IgE titers corresponding to the causal foods. Most of the reactions were a consequence of accidental exposure (87.4%), and 11.4% were found to be serious.12 Furthermore, the risk of reactions increases in children with allergy to several food groups.
In view of the above, treatments are needed to reach tolerance or desensitization in those cases where natural tolerance is less likely to be reached, or when a very restrictive patient diet must be expanded in order to avoid the development of nutritional problems. In recent years, oral immunotherapy with foods (OIT) has been postulated as a treatment option in IgE-mediated food allergies, and the studies carried out to date with this therapeutic strategy in children with EA have yielded satisfactory results.13–22
The present study analyzes the clinical and immunological profile of a group of children with persistent EA scheduled for OIT with egg.
Material and methodsThe Spanish Society of Pediatric Allergy, Asthma and Clinical Immunology promoted a randomized, controlled multicenter trial of OIT in children with persistent EA (aged 6–9 years) to assess and compare the effectiveness and safety of various induction and maintenance OIT protocols with pasteurized egg white.
Patient selectionPatients with a diagnosis of EA were recruited from the allergy units of the Spanish public healthcare system. The parents of the patients were informed about and invited to participate in the study.
Inclusion criteria: (1) children with EA aged 6–9 years with at least one allergic reaction to egg over the last year; (2) signed parental consent to participate in the study; (3) egg white (EW, 10mg/ml) skin-prick test (SPT) mean diameter edema >3mm; (4) EW, ovalbumin (OVA) or ovomucoid (OVM) serum specific IgE levels >0.35kU/L; and (5) egg allergy confirmed by a pasteurized egg white (PEW) double-blind placebo-controlled food challenge (DBPCFC) at the time of inclusion.
Exclusion criteria: (1) severe or uncontrolled asthma; (2) severe atopic dermatitis according to an objective atopic dermatitis severity index; (3) autoimmune, cardiovascular or neuropsychiatric diseases; (4) omalizumab or betablocker treatment; (5) food OIT in the last year; and (6) immunotherapy with airborne allergens in the startup phase.
Information on personal and family allergic diseases and on the symptoms following egg consumption was collected.
Adverse reactions (ARs) – both documented in the case history secondary to transgressions in the last year and those occurring during OIT – were classified according to severity as follows:
Mild reactions: erythema, localized urticaria, vomiting, rhinitis, conjunctivitis and cough.
Moderate reactions: generalized urticaria, facial angioedema and mild bronchospasm responsive to salbutamol.
Severe reactions: severe bronchospasm with oxygen desaturation, breathing difficulty with inspiratory stridor, hypotension and anaphylactic shock.
Immunological markersSkin-prick testing was performed with EW extract (10, 5, 1 and 0.5mg/ml), with saline and histamine as negative and positive controls, respectively (Diater Laboratories S.A., Spain). The wheal size was calculated as the average of the largest and the perpendicular midpoint diameter, after subtracting the size of the saline wheal. Total and specific EW, OVA and OVM IgE and EW sIgG4 antibody levels in serum were measured using Immuno-CAP 100 (Thermo Fisher Scientific) at enrollment.
Oral food challengeEA was confirmed by a DBPCFC blinded with potato, carrot and olive oil mashed together. Challenges were performed in the hospital setting and supervised by a physician using one boiled egg white (at 100°C for 10min), starting with a dose of 2.5g and approximately doubling the dose every 30min, up to a cumulative dose of 45g (5g – 10g – 25g). If the patients passed this challenge, a second DBPCFC with PEW (1, 2, 4, 8, 15ml every 30min up to a cumulative dose of 30ml or 3.3g protein,23 corresponding to one medium egg) was performed. If allergic symptoms did not appear 2h after intake of the final dose, the patient was discharged. If the patient developed urticaria/edema, severe abdominal pain, vomiting, rhinitis, bronchospasm or hypotension, the challenge was stopped, the reaction treated, and the patient discharged 6h after treating the reaction.
Oral immunotherapy protocol (summary)The patients that met the inclusion criteria were randomized to either the active treatment group or the control group. The study design comprised six visits at intervals of 3–6 months over a period of two years of follow-up in the active treatment group (T0, T3, T6, T12, T18 and T24) and four visits in the control group (T0, T12, T18 and T24). Pasteurized egg white (Guillen, Valencia, Spain), whose allergenicity has been proven to be equivalent to that of raw EW,15 was used in an OIT scheme with three phases: (a) the initial dose escalation phase was performed in the hospital, starting with 1ml of a 1/1000 water solution of PEW. If the patient did not develop allergic symptoms, a double dose was administered every 30min until reaching undiluted PEW. If the patient did not develop ARs, discharge took place 2h later; (b) the buildup phase was begun the following day; on a weekly basis, all the OIT patients were given a 30% increased dose with respect to the last tolerated dose, in hospital. Patients included by La Paz University Hospital (Madrid) were assigned to pattern I (PI) and were given a daily 5% increased dose with respect to the last tolerated dose, at home. All other OIT patients were assigned to pattern II (PII) and were given the last tolerated dose throughout the week, on a daily basis, at home. Total desensitization was considered when 30ml of PEW was reached, at which point an open food challenge with one raw egg in the hospital confirmed total desensitization; (c) maintenance phase OIT with 30ml of PEW per day or every two days until completing one year of OIT.
The estimated recruitment period was 15 months (January 2011–March 2012). The patients were randomized to the different treatment groups in order of inclusion, using a centralized digital algorithm.
Ethical particularsThe study protocol and informed consent form were approved by the clinical research ethics committees of the participating centers. Informed consent was obtained from the parents or tutors, with approval from those patients over seven years of age.
The authors warrant adherence of the study to the established protocol, and the correctness of the data and their analysis.
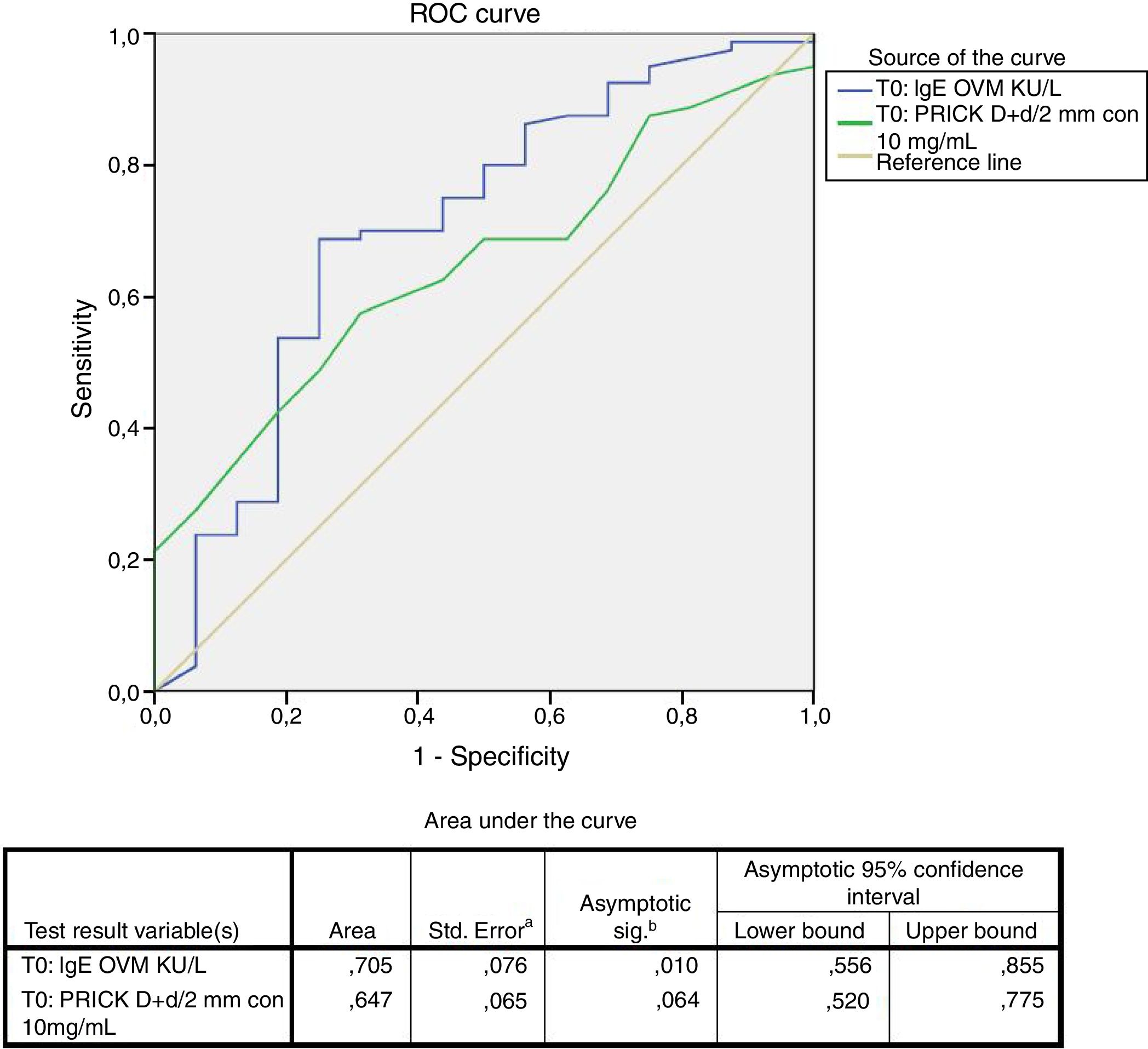
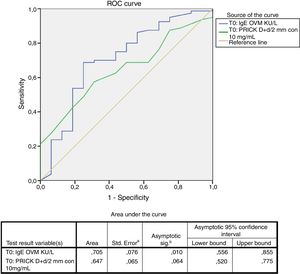
Statistical analysisThe statistical analysis was carried out using the SAS version 9.3 statistical package (SAS Institute, Cary, NC, USA). The clinical results were assessed on an intent-to-treat (ITT) basis. Quantitative variables were reported as the mean and standard deviation (median), and minimum and maximum, while qualitative variables were reported as frequencies and percentages. The discriminating capacity of specific IgE (sIgE) with respect to cooked egg tolerance was evaluated based on the receiver operating characteristic (ROC) curve. A threshold value was established based on Youden's (max sens+spec) index, with estimation of the sensitivity and specificity for that threshold value.
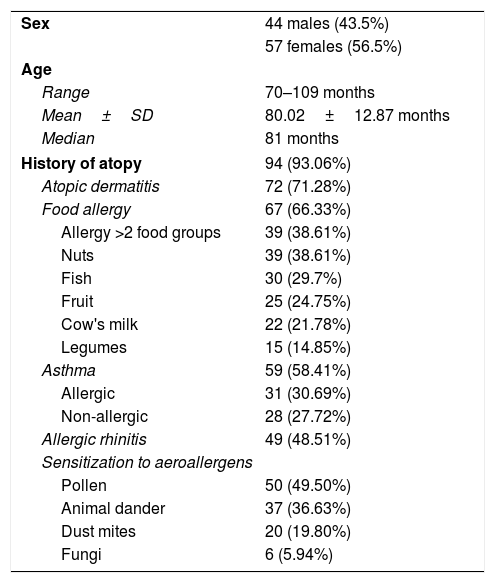
ResultsSample descriptionThe characteristics of the 101 patients included in the study are shown in Table 1.
Characteristics of the patients included in the study (n=101).
| Sex | 44 males (43.5%) |
| 57 females (56.5%) | |
| Age | |
| Range | 70–109 months |
| Mean±SD | 80.02±12.87 months |
| Median | 81 months |
| History of atopy | 94 (93.06%) |
| Atopic dermatitis | 72 (71.28%) |
| Food allergy | 67 (66.33%) |
| Allergy >2 food groups | 39 (38.61%) |
| Nuts | 39 (38.61%) |
| Fish | 30 (29.7%) |
| Fruit | 25 (24.75%) |
| Cow's milk | 22 (21.78%) |
| Legumes | 15 (14.85%) |
| Asthma | 59 (58.41%) |
| Allergic | 31 (30.69%) |
| Non-allergic | 28 (27.72%) |
| Allergic rhinitis | 49 (48.51%) |
| Sensitization to aeroallergens | |
| Pollen | 50 (49.50%) |
| Animal dander | 37 (36.63%) |
| Dust mites | 20 (19.80%) |
| Fungi | 6 (5.94%) |
The distribution of the severity of the reactions reported over the last year was as follows: mild (grade 1) in 44 (46.3%) children subsiding with oral antihistamines; moderate (grade 2) in 42 (41.6%) children affecting two organs or systems and requiring antihistamines, systemic corticosteroids or inhaled salbutamol; and severe (grade 3) in 15 children (14.8%) requiring the administration of inhaled salbutamol and/or intramuscular adrenalin.
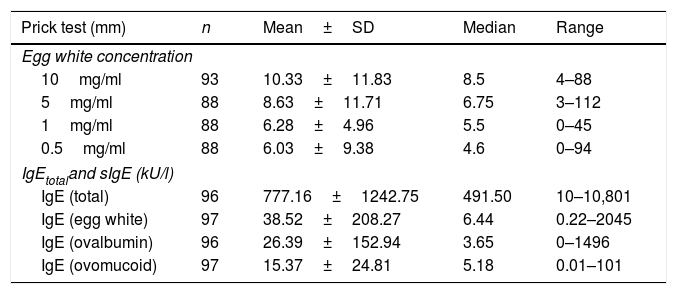
The results referred to the prick tests with egg white at different dilutions and the total IgE and specific IgE titers corresponding to egg white, ovalbumin and ovomucoid are reported in Table 2.
Results of the skin-prick tests and total and specific IgE (n=101).
| Prick test (mm) | n | Mean±SD | Median | Range |
|---|---|---|---|---|
| Egg white concentration | ||||
| 10mg/ml | 93 | 10.33±11.83 | 8.5 | 4–88 |
| 5mg/ml | 88 | 8.63±11.71 | 6.75 | 3–112 |
| 1mg/ml | 88 | 6.28±4.96 | 5.5 | 0–45 |
| 0.5mg/ml | 88 | 6.03±9.38 | 4.6 | 0–94 |
| IgEtotaland sIgE (kU/l) | ||||
| IgE (total) | 96 | 777.16±1242.75 | 491.50 | 10–10,801 |
| IgE (egg white) | 97 | 38.52±208.27 | 6.44 | 0.22–2045 |
| IgE (ovalbumin) | 96 | 26.39±152.94 | 3.65 | 0–1496 |
| IgE (ovomucoid) | 97 | 15.37±24.81 | 5.18 | 0.01–101 |
No significant correlation was found between the severity of the last documented reaction and the result of the prick test at different concentrations or the sIgE titers (data no shown).
Results of DBPCFC prior to the start of OITEighty-six patients (85.1%) developed immediate symptoms in DBPCFC with cooked egg white. The mean symptoms-triggering dose was 1.26±0.97g of egg white proteins (<1/16 of a cooked egg white). The 15 children (14.9%) that tolerated cooked egg white developed symptoms in the challenge with pasteurized raw egg white.
The symptoms-triggering dose of egg proteins and the sIgE values corresponding to the different egg proteins showed no statistically significant association to the results of the challenge tests.
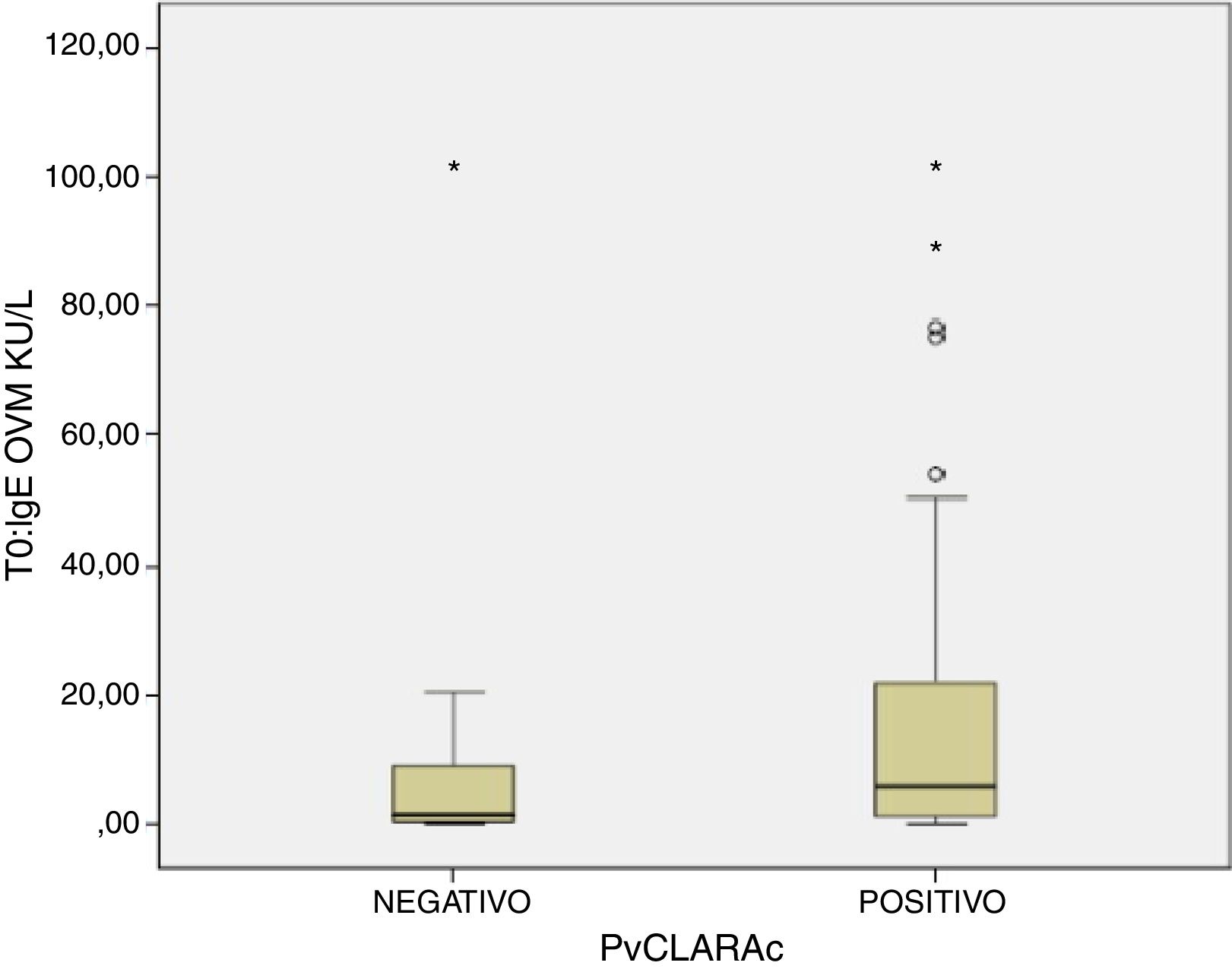
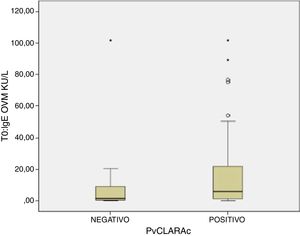
A positive cooked egg-white challenge test was only significantly correlated to the titers of sIgE against ovomucoid (p=.018). A cut-off point of sIgEOVM<2.045kU/l was found to be able to differentiate patients that tolerated cooked egg white with a sensitivity of 68.7%, a specificity of 75%, a positive predictive value of 41%, and a negative predictive value of 97%. The patients that tolerated cooked egg white showed significantly lower titers of sIgE against ovomucoid (Fig. 1) and a lesser response in the egg-white prick test at a concentration of 10mg/ml – although without reaching statistical significance (Fig. 2).
The present study describes a cohort of children diagnosed with persistent EA and enrolled in a randomized, controlled trial of OIT with pasteurized raw egg white. The mean patient age in our series is over six years, which is a period when the probability of acquiring natural tolerance to egg is seen to decrease. Fortunately, EA typically resolves in the first years of childhood, as evidenced by a number of studies,3,4 although the resolution rate may be decreasing, as suggested by a recent publication in which approximately 50% of the patients reached tolerance by 12 years of age.5 In Spain, most children with EA reach tolerance before six years of age.4 Consequently, in those patients that have not reached tolerance by that age, EA can be regarded as persistent, with little chance of acquiring tolerance naturally.
Egg protein allergy or sensitization has been associated to an increased risk of suffering other allergic disorders. In our series, over 93% of the patients also presented atopic dermatitis (71.2%), asthma (58.4%) and allergic rhinitis (48.5%). Likewise, 38.6% presented allergy to more than two food groups.
In children with persistent EA, the severity of the adverse reactions due to dietetic transgressions tends to increase. An effective egg exclusion diet is difficult to achieve, and symptomatic transgressions are frequent and can be serious.11 In our series, over 50% of the children experienced moderate and/or severe reactions as a result of accidental transgressions. This underscores the importance of starting treatment in the form of OIT, not only because the likeliness of achieving natural tolerance is low but also because the children are at risk due to the reactions that may be caused as a consequence of dietetic transgressions. We observed no relationship between the severity of the adverse reactions reported over the last year and the results of the prick test with different egg white concentrations or the titers of sIgE against egg proteins. This indicates that such data are more of use in predicting the existence of food allergy than in assessing the severity of such allergic disorders - although some studies suggest that high sIgE titers against egg may be correlated to the severity of the reactions and to the presence of persistent allergy.5,24,25 In our series, the mean sIgE titer for egg white was found to be high (38.52±208kU/l), and far higher than the cut-off points used to decide whether or not to perform a challenge test with egg, given the high probability that true EA exists.26,27
Food processing modifies the allergenic characteristics of many proteins, including egg proteins.7 In this regard, ovalbumin – the most abundant allergen in egg – is denaturalized by heat exposure, while in contrast ovomucoid is not modified by heat treatment. As a result, patients with EA can differ in their tolerance and reactivity to cooked and raw egg.8 Tolerance to cooked egg is usually reached first, particularly baked and matrix-bound egg (e.g., cereals), followed later on by raw or undercooked egg. The egg contained in commercial industrial food products is subjected to heat processing. It is therefore important for the quality of life of both the patient and the family to know whether the child tolerates cooked egg, since in this case the diet can be expanded and diversified, with the need to avoid only a few forms of egg preparation. Attempts have been made to define cut-off points for differentiating patients that can be expected to tolerate cooked egg.28 In our study we found that sIgEOVM<2.045kU/l significantly discriminates (p=.018) those patients that pass the challenge test with cooked egg white (Fig. 1), with a sensitivity of 68.7%, a specificity of 75%, and a positive and negative predictive value of 41% and 97%, respectively. These values are similar to those reported by other Spanish series.29 In contrast, we observed no differences with respect to sIgE against ovalbumin, or egg white, or the prick-test response to egg white.
The usual treatment of EA has been based on the elimination of the causal food from the diet and control of those adverse reactions that may arise as a consequence of symptomatic accidental transgressions. This management approach has a negative impact on the quality of life of the patients30; consequently, those families and children with persistent EA in which the chances of achieving natural tolerance are limited can benefit from OIT.
In summary, we have described the clinical and allergological characteristics of a cohort of highly sensitized children with persistent EA scheduled for OIT with egg, in which sIgEOVM<2.045kU/l was identified as a cut-off point capable of identifying those patients who are able to tolerate cooked egg white.
FundingThis work was supported by Spanish Society of Pediatric Allergy, Asthma and Clinical Immunology (SEICAP).
Conflict of interestThe authors have no conflict of interest to declare.