Ingestion of small amounts of cow's milk (CM) can elicit adverse reactions in patients with IgE-mediated CM allergy. Knowing the dose eliciting allergic reactions and the factors affecting it can be of great help in avoiding these reactions.
ObjectiveTo analyse the eliciting doses of positive challenge test in patients with CM allergy and to determine its association with the level of CM specific IgE.
MethodsNinety-eight positive challenge tests in 56 children, median age of 11 months (3-80) with IgE-mediated CM allergy were retrospectively analysed. Open oral challenge tests were carried out by gradually increasing doses of milk (2-100ml). The relationship between challenge test doses and CM specific IgE levels were studied.
Results18 % of the challenge tests were positive with 2ml, 24 % with doses between 5 and 10ml, and the other 58 % with doses between 25 and 100ml. An inverse association between the doses of the positive challenge test and the level of CM specific- IgE was found, 13.9kU/L (0.54−>100kU/L) when the challenge test was positive with the smaller dose (2ml); and 1.73kU/L (<0.35− 76.4kU/L) with doses above 2ml (p=0.0001). The median age of the patients was 13 months (6-49) when the challenge test was positive with 2ml vs 9 months (3-80) with doses above 2ml (p=0.048).
ConclusionThe CM specific IgE level and patient's age should be considered in the assessment of the eliciting doses of positive challenge test in CM allergy.
Allergy to cow's milk proteins (CMP) mainly occurs in the first half year of life, coinciding with its introduction into the infant's diet. IgE mediated reactions are the best known and characterized of all food allergies with an incidence of 2 % in the first year of life. Clinical reactivity may remain for many years with the risk of secondary reactions to accidental exposure.
Ingestion of small amounts of an offending food can elicit adverse reactions in patients with IgE-mediated allergy. These reactions can be potentially life-threatening1,2. Standardised oral challenge tests are used to determine the reactive dose of food in IgE-mediated allergy. Over the last years, protocols for the determination of threshold doses for allergenic food have been developed3. Knowing the clinical reactivity dose could bring benefits to patients, as they would be aware of the risk of adverse reactions by ingestion of small amounts of food products.
CM specific IgE quantification has proven to be a useful method to predict the outcome of oral food challenge test4,5. As the IgE levels vary throughout the follow-up of patients allergic to CM6, the threshold dose might also be different in accordance with the specific IgE level, and it might also vary in the follow-up.
The aim of this study is to retrospectively analyse the results of positive oral challenge tests in patients with milk allergy and to determine the eliciting doses of clinical reactivity, and those factors that might be associated with it, such as CM specific IgE levels.
PATIENTS AND METHODSNinety-eight positive open oral challenge tests to milk in 56 children (29 males and 27 females, median age 11months) were retrospectively analysed. The challenge test was carried out to confirm CM allergy at the initial diagnosis or during the follow-up of the patients. Before the challenge test, skin prick test with whole CM extract and its proteins and the determination of specific IgE by CAP system (Phadia Uppsala, Sweden) to the same allergens were carried out in all patients.
Open oral controlled challenge tests to milk were performed in the allergy unit of the hospital under the close supervision of an allergist. Written informed consent to carry out the challenge test was previously obtained from the parents. The challenge test was performed within four days in our outpatient clinic5. On the first day of the challenge, increasing doses of CM infant formula (2, 5 and 10ml) were dispensed at 90-minute intervals; and 25, 50, and 100ml respectively were administered on the following days. When a clinical reaction occurred, the provocation test was discontinued. The challenge test was regarded as positive when any of the following symptoms developed: skin (urticaria, angio-oedema or erythematous rash), gastrointestinal (vomiting), or respiratory (upper respiratory and/or lower respiratory symptoms), or generalised (anaphylactic shock) manifestations within the first hour after the intake of milk. They were performed with an infant formula (1.7 gr of proteins for 100ml). A detailed follow-up of the observed symptoms during challenges with milk was accomplished.
StatisticsStatistical analyses were performed using SPSS 12. The median is the statistic used for the patients' age and specific IgE as the distributions are not normal. Chi square and Mann-Whitney U tests are used to compare proportions and medians respectively.
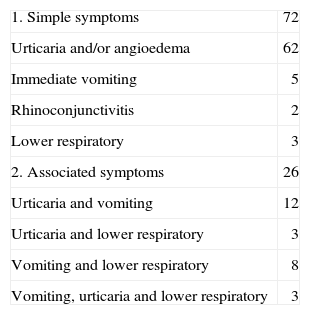
RESULTSThe median age at the 98 challenge tests was of 11months (range 3-80months). Fifty-six challenge tests were carried out in the initial diagnosis and 42 challenge tests in the follow-up. Symptoms of the positive challenge test appeared in all cases in the first 30 minutes. The clinical manifestations affected only one system in 72 reactions, whereas in the other 26 reactions two or more systems were involved. Skin reactions were most common. In 17 out of the 98 reactions, lower respiratory symptoms appeared, either as an isolated symptom, or associated with other symptoms (Table I). The CM specific IgE median was 2.5 kU/L (0.71 for the 25th percentile and 9.05 for the 75th percentile), range < 0.35 to > 100 kU/L.
Clinical symptoms to challenge test (n = 98)
| 1. Simple symptoms | 72 |
| Urticaria and/or angioedema | 62 |
| Immediate vomiting | 5 |
| Rhinoconjunctivitis | 2 |
| Lower respiratory | 3 |
| 2. Associated symptoms | 26 |
| Urticaria and vomiting | 12 |
| Urticaria and lower respiratory | 3 |
| Vomiting and lower respiratory | 8 |
| Vomiting, urticaria and lower respiratory | 3 |
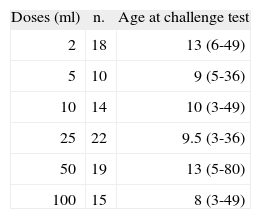
Table II shows the amount of milk that elicited a positive challenge test. It was positive with the smallest dose of 2ml in 18 % of the challenges, with doses ranging between 5 and 10ml in 24 %, and in the other 58 % with doses between 25 and 100ml. None of the patients required hospitalisation and all symptoms reversed easily. Oral antihistamines were administered in most patients and several drugs were only required in 5 % of the challenge tests.
We must bear in mind that this study was not carried out to calculate threshold doses since a starting dose of 2ml would be too high for that purpose. We have only considered that cut-off point to make some comparisons.
Table II also shows the age of patients who had positive challenge test with different doses of milk. The median age of the 18 patients who had a positive challenge test with the lowest dose (2ml) was 13months (6-49) vs 9months (3-80) in the patients who had positive challenge tests with doses above that amount (p = 0.048). In the initial diagnosis 12 % of the challenges were positive with a 2ml dose against 27 % that were positive in the follow-up, p = 0.03. No significant difference was found in the relationship between symptoms and doses of positive challenge test. There were symptoms in two or more systems in 22 % of the positive challenge tests with the smallest dose (2ml), and in 27 % of the challenge tests that were positive with doses above 2ml. There were no significant differences when lower respiratory symptoms were considered, 11 % with the smallest dose (2ml), and 18 % with doses above 2ml. The lower respiratory symptoms were more frequent (24 %) in children over 11months old than in children under that age (11.3 %), but no significant difference was found.
Dose of the positive challenge test and cow's milk specific IgE levelsThe CM specific IgE median was 2.5 kU/L (range < 0.35 kU/L to > 100 kU/L). An inverse association was found between the dose of the positive challenge test and the level of specific IgE to milk. The CM specific IgE median was 13.9 kU/L (0.54 to > 100) in patients with a positive challenge test with 2ml; and 1.73 kU/L (< 0.35-76.4) in those with doses above 2ml (p = 0.0001).
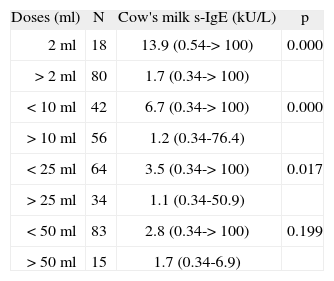
Table III displays different cut-off points in the positive challenge test dose and its association with the CM specific IgE levels. A significant association between CM specific IgE levels and the positive challenge test dose was found in all but the last cut-off point analysed.
Cow's milk specific IgE median (kU/L) and positive challenge test doses
| Doses (ml) | N | Cow's milk s-IgE (kU/L) | p |
| 2ml | 18 | 13.9 (0.54-> 100) | 0.000 |
| > 2ml | 80 | 1.7 (0.34-> 100) | |
| < 10ml | 42 | 6.7 (0.34-> 100) | 0.000 |
| > 10ml | 56 | 1.2 (0.34-76.4) | |
| < 25ml | 64 | 3.5 (0.34-> 100) | 0.017 |
| > 25ml | 34 | 1.1 (0.34-50.9) | |
| < 50ml | 83 | 2.8 (0.34-> 100) | 0.199 |
| > 50ml | 15 | 1.7 (0.34-6.9) |
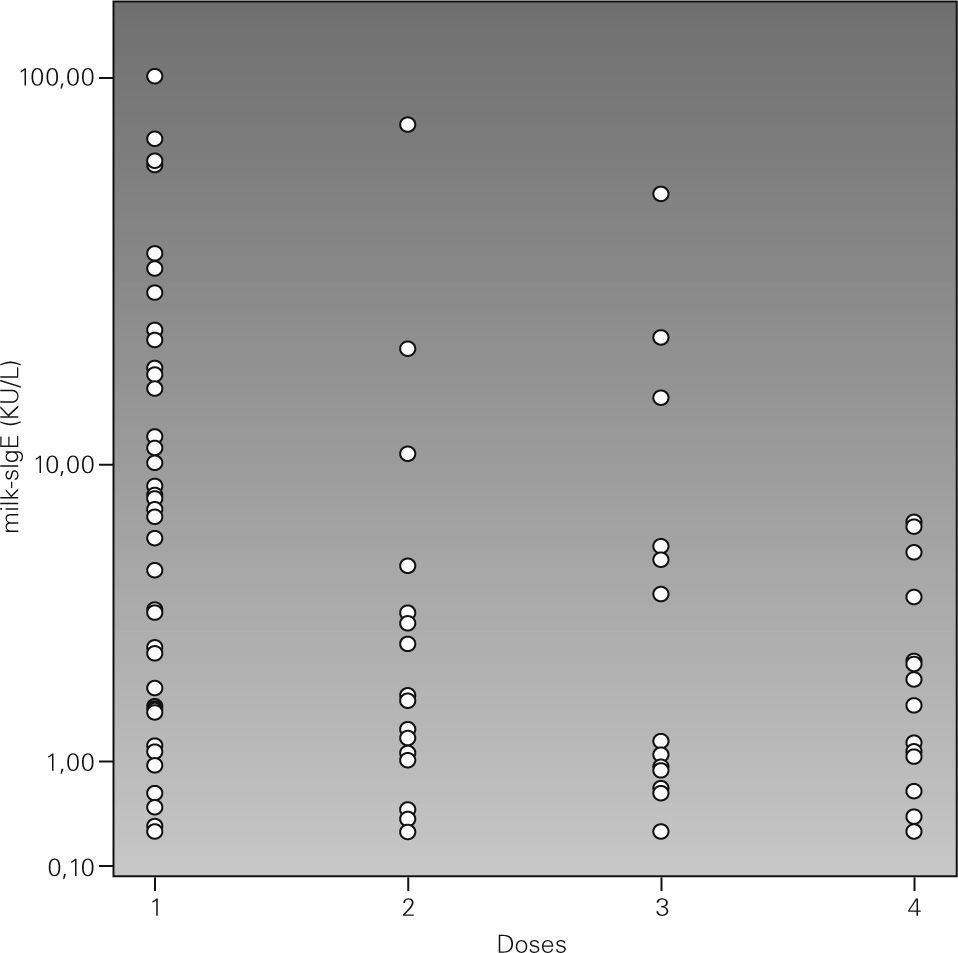
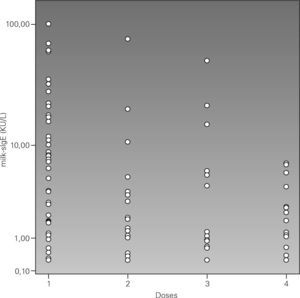
In figure 1 we arbitrarily gathered the challenge dose in four intervals and it was observed that 10 kU/L is the cut-off point of the CM specific IgE which shows that above that point positive challenges with low doses will prevail. Therefore all the positive challenge tests with a 100ml dose had a CM specific IgE level below 10 kU/L, although positive challenges were also found with low doses below that value. By analysing the challenge symptoms with regard to the CM specific IgE levels, it was observed that patients with lower respiratory symptoms had higher CM specific IgE levels (median 15.2 kU/L) than those who had urticaria and/or angio-oedema (median 1.9 kU/L) and other symptoms (median 2.9 kU/L). An association of CM specific IgE with age was found: those whose challenge age was above the median had higher CM specific IgE 6.2 kU/L (< 0.35-> 100 kU/L) vs 1.41 kU/L (< 0.35-61.7 kU/L) for those below the median age.
Therefore, a high CM specific IgE will be more likely to result in a positive challenge test with low doses and be more likely to have respiratory symptoms in the challenge test.
DISCUSSIONFood challenge tests allow us to confirm tolerance or clinical reactivity in a patient allergic to that food. Challenge protocols start with low-doses of allergenic food which are increased until the administered dose brings about an objective reaction or the oral tolerance is confirmed7,8. It is important to know the positive challenge dose in order to predict possible reactions. Research has recently been carried out3 to determine the threshold doses for allergenic foods at a time when the food industry is also making big efforts to protect the well-being of allergic consumers. Cow's milk proteins may sometimes be hidden allergenic food9,10 and may provoke undesirable reactions in children allergic to these proteins. The prevention of food anaphylaxis requires informative labelling of allergens added to foods. The threshold dose might be defined11 as the lowest amount of the offending food that would elicit mild, objective symptoms in the most sensitive individuals. This threshold dose may vary with different types of foods12. Different threshold doses11 have been reported in several studies on the diagnosis of allergy to CMP. In this study, the challenge test started with an initial dose of 2ml, which elicited 18 % of positive reactions, although it is likely that some of the 18 patients might have had positive reactions with doses lower than 2ml. These results are in line with other studies where 15 % of the patients allergic to milk react to values under 5ml doses13. Nevertheless, if we look for very small reactivity doses to CMP, we will find that few children will have problems with low doses. Thus, up to 82 % of patients tolerated 2 or more ml of milk.
In several studies14,15 the positive challenge test dose has been related to the symptoms of the positive challenge test. They show that patients with severe symptoms had lower threshold doses compared with those of patients with mild symptoms. Other authors have reported that the mean challenge dose is lower in anaphylaxis (life-threatening reaction) than in skin, gastrointestinal, respiratory, or multi-organ systems16. A relationship between reaction severity and the amount of food ingested in the challenge test was not found in this study, probably because of the patients' age (48 % under 11months). However, it was found that the challenge tests with more severe symptoms such as lower respiratory symptoms were very frequent in older patients. It is also possible that close observation and prompt intervention of positive challenges prevented the evolution to more serious reactions as they were discontinued at the first sign of the clinical symptoms. A significant relationship was found between the challenge dose and CM specific IgE. Children who had reactions with the smallest dose in the challenge test had higher CM specific-IgE levels than children who had positive reactions with doses above the smaller reactivity ones of our study. The fact that some authors17 do not report this association between dose and specific IgE could be explained on the basis of the selection of patients included in the study. In our study, nevertheless, the CM specific-IgE range was between < 0.35 and > 100 kU/L. A relationship between the positive challenge dose and the age of patients is also observed. Those children who had positive challenges with smaller doses were older than those who had reactions with higher doses. Older children had higher specific IgE levels and, consequently, higher risk of having a positive reaction to low doses in the challenge test and, probably, also higher risk of accidental reactions. Thus, it is essential to identify these patients mostly in order to prevent recurrent and potentially lethal accidents18.
In summary, this article shows that in CM allergy the eliciting dose of clinical reactivity in the challenge test is related to the specific IgE level. Patients with higher IgE levels will be more likely to react to smaller doses in the challenge test than patients with lower IgE levels. Age at the challenge test will be another factor to be taken into account since it seems that higher aged patients could have a positive challenge test with a smaller dose than lower aged patients. Thus, it is important to consider the patients' age and the level of the specific IgE levels at the moment of the challenge test.