Cow's milk allergy diagnosis many times requires double-blind placebo-controlled food challenge (DBPCFC), which presents high accuracy but involves risks, specifically in infants and anaphylactic patients. The identification of the cut-off values for specific IgE to milk or its components would contribute to cow's milk allergy (CMA) diagnosis. The aim of this study was to compare discriminating concentration of a cow's milk specific IgE and its fractions (¿-lactoalbumin, ¿-lactoglobulin, casein) in children for the CMA diagnosis.
Methodsthis study included 123 patients (M:F=1.3:1) median age at diagnosis=1.91 years, (3.5m to 13.21y) with CMA diagnosis via DBPCFC (n=26), proven anaphylaxis due to cow's milk (n=46) or a suggestive clinical history associated with a positive skin prick test (n=51) and open oral food challenge. The control group included 61 patients (1 male:1.1 female) ages ranging from 0.66 to 16.7 years (median=6.83 years). Receiver operator characteristics (ROC) curves were constructed to determine the best cut-offs that guarantees high specificity (>95%) for cow's milk and its components.
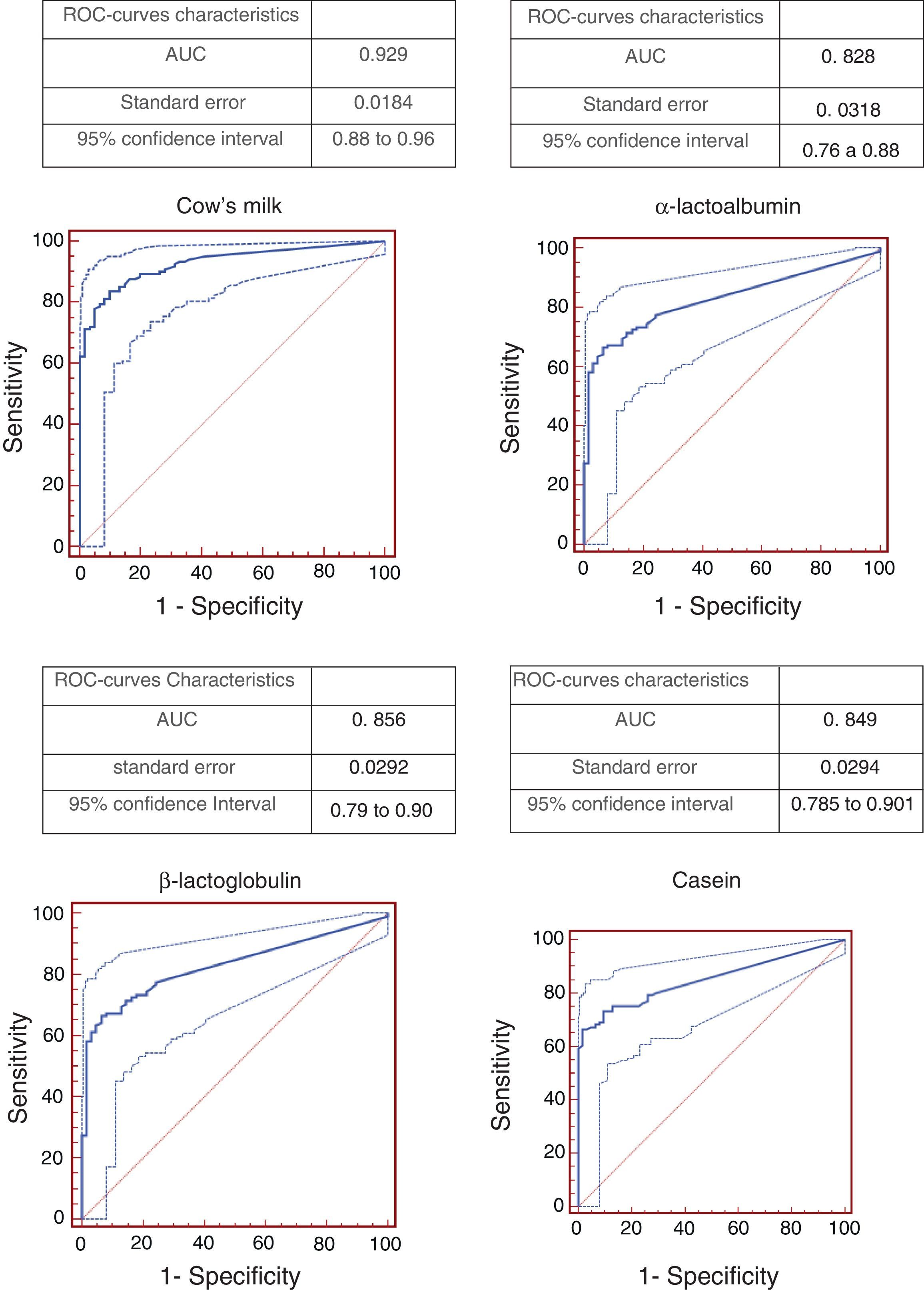
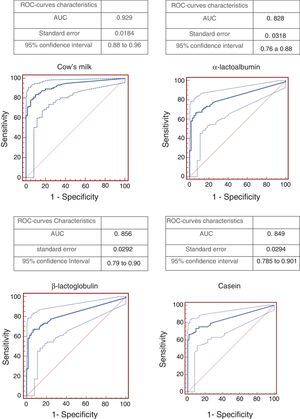
Resultsconsidering 98% specificity, cut-off points were: 3.06kU/L for cow's milk, 2.06kU/L for ¿-lactalbumin, 1.85kU/L for ¿-lactoglobulin and 1.47kU/L for casein. The best ROC curve (area under the curve=0.929) was obtained evaluating cow's milk.
Conclusionthis study showed that the cut-off point detected for whole cow's milk revealed a better discriminatory capacity for CMA diagnosis without the necessity of the milk components testing.
The prevalence of food allergy (FA) varies from 6% to 8% in children, and it is currently increasing in many countries.1 Among all food allergens, cow's milk is one of the most common and often the first food introduced in the infant diet, even during breastfeeding. Cow's milk allergy (CMA) affects approximately 2.5% of children and may occur early in life, even during the neonatal period.2 Clinical findings of CMA include a large spectrum of clinical manifestations including anaphylaxis, which can be life-threatening.3
The diagnosis of an Immunoglobulin E (IgE)-mediated FA depends on the clinical history and specific IgE detection through skin prick test or laboratory evaluation, including the investigation for the specific IgE (sIgE) of the triggering food and the double-blinded, placebo-controlled food challenge (DBPCFC). Although the DBPCFC is still considered the gold standard for FA diagnosis, it involves risks, demands adequate local conditions and is expensive.4,5 Consequently, it is necessary to develop other laboratory tools with adequate accuracy and feasibility.
Several studies have been developed with the aim of determining the serum level cut-off of specific IgE for CMA diagnosis.4–8 It was observed that this determinant concentration depends on the characteristics of the evaluated populations. Most of the studies included patients with atopic dermatitis, who usually present with high IgE serum levels that may interfere with the cut-off values.4–8 Moreover, there are few studies that evaluated milk components as a tool in cow's milk allergy diagnosis.6 The aim of this study was to establish a cut-off for the serum levels of specific IgE to milk and its components for CMA IgE-mediated diagnosis from a Brazilian food allergy reference centre. The hypothesis is that the local cut-offs in our population may have different characteristics than those previously evaluated in other populations.
MethodsStudy populationThis was a retrospective study evaluating 184 consecutive children and adolescents referred to a food allergy centre in Brazil for evaluation of suspected IgE-mediated food hypersensitivity, in a four-year period from March 2004 to March 2009. Inclusion criteria for CMA diagnosis were: (1) children who had a suggestive history of allergy to cow's milk and a positive DBPCFC for cow's milk; (2) children with at least one episode of anaphylaxis triggered by milk in the previous year and positive skin prick test (SPT); and (3) children with a suggestive CMA history associated with a positive SPT and positive open food challenge. Positive open challenge was defined as a known and confirmed ingestion of milk followed by immediate IgE-mediated symptoms.3 The criteria adopted for anaphylaxis were defined by the “Second symposium on the definition and management of anaphylaxis”.9 Exclusion criteria were: children with previous CMA history but already tolerant, without symptoms after milk ingestion, or a dosage of serum-specific IgE to cow's milk one year after the last report of symptoms. All children were submitted to sIgE detection to milk and individual components.
From 184 children (1.2M:1F), 123 had a confirmed diagnosis of cow's milk allergy: 51 through a positive open food challenge, 46 with confirmed anaphylaxis to cow's milk, and 26 had positive DBPCFC. Sixty-one had negative challenges to milk (open challenge) and were considered the control group.
DiagnosisSPTs were performed with glycerinated food extract to whole milk, ¿-lactalbumin, ¿-lactoglobulin and casein, a positive control (histamine 1%), a diluent as the negative control (IPI ASAC Brasil, ASAC Pharma – Alicante, Spain) and with a drop with fresh cow's milk (prick to prick). A wheal 3mm larger than the negative control was considered positive.10 The DBPCFCs were performed according to Williams and Bock11 and modified for the use at Allergy and Immunology Paediatric Unit.12 Patients were referred to the outpatient clinic for testing after six hours of fasting, and they received fixed volumes of 60ml at each intake at intervals of 15–30min to a total of 360ml. During the period of cow's milk administration, increasing doses of milk were administered (5, 10, 15, 20, 25 and 25ml) after being added to a vehicle (soy formula) to complete the total volume of 60ml. Low lactose cow's milk was used to exclude clinical manifestations due to lactose intolerance. The test was considered positive when IgE-mediated reactions occurred up to two hours after the ending of the test.12 After the test, the family was instructed to record any symptoms that the child may have during the following week. Serum samples from all patients and the control group individuals were collected during regular visits and analysed for specific IgE for cow's milk, ¿-lactalbumin, ¿-lactoglobulin and casein (ImmunoCAP – by Thermo Fisher Scientific Inc., USA) according to the manufacturer's instructions.13 The age of the patients at blood collection varied from 0.3 to 13.21 years (median=1.9 years), and the ages of the control group patients ranged from 0.6 to 16.7 years (median=6.83 years).
The study received approval from the Department of Paediatrics and Clinical Hospital from University of Sao Paulo Institutional Review Board. Informed consent forms are routinely obtained to challenge test.
Data analysisThe cut-off levels of specific IgE for cow's milk and its components were determined by an analysis with the ROC curve. The analysis was performed using Med Calc 10 1.2.0 (MedCalc Software, Mariakerke, Belgium). The area under the curve (AUC) was calculated to quantify the test accuracy and was considered adequate when greater than 0.8.14 The cut-off was considered adequate if the specific IgE cut-off levels presented specificity≥95% and a positive likelihood ratio≥10 (LR).15 GraphPad Instat 3 (GraphPad Software Inc., CA 92037, USA) was used to calculate medians.
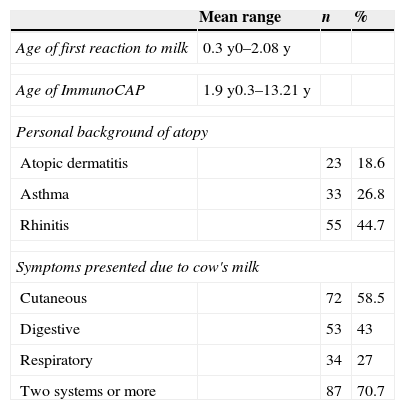
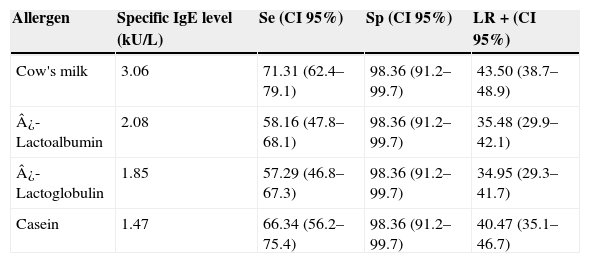
ResultsThe study included 123 children with confirmed CMA. Of these, 41.4% had a suggestive history and a positive oral food challenge, 37.4% had anaphylaxis and 21.2% had a positive DBPCFC. Only 21.1% of the patients were submitted to DBPCFC and the main reasons for this were: previous anaphylaxis, the young age and refusal to perform DBPCFC because of a recent accidental exposure. The majority of the patients (87.8%) had the symptoms onset before six months of age. Table 1 shows the clinical and epidemiological characteristics of these patients. Patients aged between 0.3 and 13.21 years old (median=1.9 years) when the ImmunoCAP was performed. In the control group of 61 patients, the ages varied from 0.6 to 16.7 years old (median=6.83 years). Atopic dermatitis was diagnosed in 23 (18.6%) patients. The relationship between sensitivity and specificity from cow's milk and its components was obtained by calculating ROC curves as shown in Fig. 1. The AUC was considered excellent (0.92) for cow's milk and good for its components (0.828 for ¿-lactalbumin, 0.856 for ¿-lactoglobulin and 0.849 for casein). According to the proposed criteria for the establishment of an optimal cut-off point (specificity≥95% and a positive likelihood ratio≥10), the best specific IgE concentrations found were: 3.06kUI/l for whole milk, 2.08kUI/l for ¿-lactalbumin, 1.85kUI/l for ¿-lactoglobulin and 1.47kUI/l for casein. Table 2 shows the sensitivity, specificity, and LR for cow's milk and its components regarding the obtained cut-offs.
Clinical and epidemiological characteristics of the patients with CMA.
| Mean range | n | % | |
|---|---|---|---|
| Age of first reaction to milk | 0.3y0–2.08y | ||
| Age of ImmunoCAP | 1.9y0.3–13.21y | ||
| Personal background of atopy | |||
| Atopic dermatitis | 23 | 18.6 | |
| Asthma | 33 | 26.8 | |
| Rhinitis | 55 | 44.7 | |
| Symptoms presented due to cow's milk | |||
| Cutaneous | 72 | 58.5 | |
| Digestive | 53 | 43 | |
| Respiratory | 34 | 27 | |
| Two systems or more | 87 | 70.7 | |
Sensitivity (Se), specificity (Sp) and positive likelihood ratio (LR+) of specific IgE levels to cow's milk, ¿-Lactoalbumin, ¿-lactoglobulin and casein obtained from ROC curves.
| Allergen | Specific IgE level (kU/L) | Se (CI 95%) | Sp (CI 95%) | LR+(CI 95%) |
|---|---|---|---|---|
| Cow's milk | 3.06 | 71.31 (62.4–79.1) | 98.36 (91.2–99.7) | 43.50 (38.7–48.9) |
| ¿-Lactoalbumin | 2.08 | 58.16 (47.8–68.1) | 98.36 (91.2–99.7) | 35.48 (29.9–42.1) |
| ¿-Lactoglobulin | 1.85 | 57.29 (46.8–67.3) | 98.36 (91.2–99.7) | 34.95 (29.3–41.7) |
| Casein | 1.47 | 66.34 (56.2–75.4) | 98.36 (91.2–99.7) | 40.47 (35.1–46.7) |
Cow's milk is frequently the first food introduced to the infant diet, even during breastfeeding.2,16 Cow's milk has a great nutritional importance, but it is also one of the major allergens in childhood, especially in early ages, affecting 1.5–5% of children in the first year of life.3,16 Cow's milk allergy patients may present clinical symptoms very early, and it was observed in patients included in this study; this can make the diagnosis and the DBPCFC test very difficult. Consequently, new diagnostic cut-off approaches for specific IgE for trigger allergens are strongly suggested.17 Some studies revealed a close relationship between the specific serum IgE levels and symptomatic food intake.4–8 After reviewing previous studies, it was observed that there is a significant difference between the proposed cut-offs.4–8 This is probably due to the age and clinical characteristics of the population and the chosen control groups. Except for Garcia-Ara et al.,6 all the studies included a large sample of patients with atopic dermatitis, who have elevated IgE levels and high rates of sensitisation.18 It was our interest to evaluate if it is possible to identify a cut-off point in a population with different symptoms, including patients with anaphylaxis, and this casuistic has a substantial number of anaphylactic patients. Although our series of cases did not include all patients through DBPCFC, the inclusion criteria were quite strict in order to assume that all patients presented CMA. Considering patients with anaphylaxis, there are some studies in the literature ruling out the performing of DBPCFC, especially for diagnostic evaluation. For some authors, challenge testing in patients with anaphylaxis is “unnecessary, undesirable and unsafe”.19,20 In 2007, Niggemann and Beyer reviewed the diagnostic methods for food allergy and suggested clinical indications for the realisation of DBPCFC. In their review, the authors suggested that patients with a history of anaphylaxis associated with a specific IgE detection (radioallergosorbent test, RAST, or SPT), as occurred with the patients included in this study, would not require any kind of challenge. The challenge would be performed in doubtful cases only.21 When it is necessary to evaluate the discriminatory power of a test, like serum-specific IgE, a case control study is a natural study design, but the choice of controls is crucial.22 In this study, the control group represented a real situation in clinical practice where patients with suspected food allergy presented a misdiagnosis or some kind of aversion to cow's milk, but not CMA. When Sampson and Ho4 established their first cut-off values, the control group consisted of patients with atopic dermatitis; this was the correct choice for that population, but it may lead to a higher cut-off.
The establishment of a specific IgE concentration that can replace a test that is considered the gold standard requires a high positive predictive value and a high specificity. In the present study, ROC curves showed that the cut-off points that best fulfil these requirements were values where the specificity was 98% and the confidence interval was very narrow. Obviously, every time the specificity rises, sensitivity is lost. Taking the proposed value to whole cow's milk, it was observed that the sensitivity was 73.31%, which indicates that almost 30% of patients with CMA do not reach the levels proposed to dispense them from DBPCFC; therefore, in these latter patients it would be interesting to perform challenge tests.
It is difficult to establish reliable positive and negative predictive values because they are dependent on the disease prevalence. Thus, in a situation where the prevalence of food allergy is high, such as in patients with moderate or severe atopic dermatitis, the positive predictive value (PPV) remains high. However, in populations with a lower prevalence there would be a reduction of PPV for the same cut-off values.23 In this study there was no possibility of applying this formula because there are no population-based studies evaluating the prevalence of food allergy in Brazil. An alternative is the adoption of the likelihood ratio (LR), defined as sensitivity/1−sensitivity. If it is higher than 10, it confers strong evidence of a correlation between the presence of food allergy and a positive test.16 In this study, the chosen cut-off points have a high likelihood ratio, valorising the obtained results. The obtained cut-off point was lower than in many previous studies, but very similar to the concentration proposed by Saarinen et al. (3.5kU/L).24 These differences can be explained by the characteristics of the sample, especially related to the number of patients with atopic dermatitis. Patients with this disease present a high rate of sensitisation, therefore the cut-off should be higher to detect food allergy. In a recent study including 2096 children with atopic dermatitis, it was detected that 54.5% of them were sensitised, with 41.9% sensitised to egg and 27.4% to milk.20 Therefore, it was important to evaluate a population with a lower rate of atopic dermatitis. Another aspect is related to patients with anaphylaxis after milk ingestion; this is a very characteristic symptom, which does not demand high specific IgE levels to confirm diagnosis. In this study, seven patients with confirmed and recurrent anaphylactic episodes present specific IgE levels for cow's milk lower than 0.7kU/L, contributing to a lower cut-off. It is important to point out that the cut-off points are useful but related to the specific evaluated population or others with similar characteristics; therefore, local studies are necessary to reproduce the cut-off points obtained here. There are few studies of discriminator specific IgE values for cow's milk components in CMA diagnosis, despite the fact that these allergens are important for sensitisation and the triggering of symptoms. However, our group had a previous experience regarding CMA patients where specific IgE for ¿-lactalbumin, ¿-lactoglobulin and casein components through SPT contributed to an improved diagnosis in patients who had negative SPT to whole milk.25 This fact motivated the evaluations of serum IgE for these components, in order to determine the cut-off values for a CMA diagnosis. This study showed that this assessment did not contribute to the diagnosis, because the AUC of the ROC curves for the components had less accuracy than for whole milk. Moreover, the sensitivity values were lower than those obtained for whole cow's milk. One reason to justify this result may be the adequate quality of the solid phase of whole milk allergen in ImmunoCAP®, dispensing the components.26 Thus, the assessment of the association of both cow's milk and the components is unnecessary and could represent a higher cost for the patient or the health service. Studies involving the casein fraction are more associated with aspects related to the severity and persistence of CMA, therefore detection of specific IgE levels of casein can be useful to evaluate prognosis of CMA, but these outcomes were not addressed in this study.27
ConclusionIn conclusion, it was possible to establish a concentration of specific IgE to cow's milk that dispenses the need to perform the oral double-blind placebo-controlled food challenge for CMA diagnosis, and the lower value in comparison with previous studies is probably due to the characteristics of this population. The cow's milk fraction did not provide improvement in CMA diagnosis, and was considered dispensable. Local studies must be encouraged in order to recognise what is the optimum cut-off point for each population.
Ethical disclosuresPatients’ data protectionConfidentiality of Data. The authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human subjects and animals in researchProtection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this investigation.
FundingFinancial support from governamental agency CNPq-PQ2-308105/2012-5 to Cristina MA Jacob.
Conflicts of interestThere are no potential conflicts.
We would like to thank Prof Sonia Regina Testa Ramos, Professor of Paediatrics, Department of Paediatrics University of São Paulo, for her careful review.
Part of these results were a poster presentation at 29 European Academy of Allergy and Clinical Immunology, http://www.postersessiononline.com/173580348_eu/congresos/29EAACI/aula/-P_1084_29EAACI.pdf.