The present study explores the professional opinion of a wide range of experts from the Iberian Peninsula (Spain and Portugal) and their degree of consensus about CMPA's prevention, diagnosis, treatment and progression.
Material and methodsA 57-item survey divided in four blocks: Prevention (14 items), Diagnosis (10 items), Treatment (19 items) and Progression (14 items) was completed by 160 panellists, experts in CPMA management (116 Spain, 44 Portugal). Each one answered the questionnaire, formulated in Portuguese and Spanish, by individually accessing an online platform in two consecutive rounds. Five possible answers were possible: “completely agree”, “agree”, “neither agree nor disagree”, “disagree” and “completely disagree”. A modified Delphi method was used.
ResultsConsensus (more than 66% agree) was reached in 39 items (68.4%) and Discrepancy (less than 50% agree) in nine items (15.7%). Block separated analysis offers valuable differences regarding consensus. The Prevention block only reached 50%; the Diagnosis block 90%; the Treatment block 73.68%, showing a high degree of agreement on dietary treatment (15/16 items), and discrepancy or less agreement on immunotherapy treatments. The Progression block reached 71.4% consensus with discrepancy with regard to the time to perform oral food challenge and negatives prognosis consequences of accidental milk ingestion.
ConclusionsThis study displays the current opinions of a wide group of experts on CMPA from the Iberian Peninsula and evidence discussion lines in CMPA management. The questions on which there were situations of discrepancy, provide us with very useful information for promoting new, rigorous research enabling us to draw conclusions on these controversial aspects.
IgE-mediated cow's milk protein allergy (CMPA) is the most common allergy in infants and small children. Prevalence in the first year of life was estimated in our environment, using strict criteria, with results below 1%.1,2
In recent years, updated guidelines have been published for the diagnosis and management of CMPA in infants.3–8 Indications for diagnosis and substitution treatment are considered well defined, but there is greater contention about prevention and progression. Additionally, clinical practice does not always follow the recommendations of the guidelines.9 Therefore, new prevention and treatment strategies focusing on promoting tolerance, which are not clearly reflected in these documents, are currently being drawn up.
This study (CIBAL Consenso Iberico sobre Alergia a Leche) aims to explore the opinions of a wide range of experts from the Iberian Peninsula (Spain and Portugal), two countries which share a racial and cultural identity, to assess the degree of consensus on the prevention, diagnosis, treatment and progression CMPA.
Material and methodsThe study was undertaken as a joint initiative of the Spanish Society of Pediatric Allergy, Asthma and Clinical Immunology (SEICAP) and the Portuguese Society of Pediatric Allergy (SPAP). There were 160 panellists with expertise in CMPA management: 116 from Spain and 44 from Portugal. Danone Nutricia and OH Strategy & Digital Communication provided technical support in the study.
After a literature review by the four authors of the study, a series of statements were prepared, some of these are accepted in CMPA guidelines and some issues are more controversial or have insufficient scientific evidence. The result was re-examined and reduced by the authors. The final version of the questionnaire included 57 items categorised under four blocks: Prevention (14 items), Diagnosis (10 items), Treatment (19 items) and Progression (14 items). Experts’ opinions were gathered using a modified Delphi method10 with two rounds of consultation. Results were expressed using one of five possible answers: “completely agree”, “agree”, “neither agree nor disagree”, “disagree” and “completely disagree”.
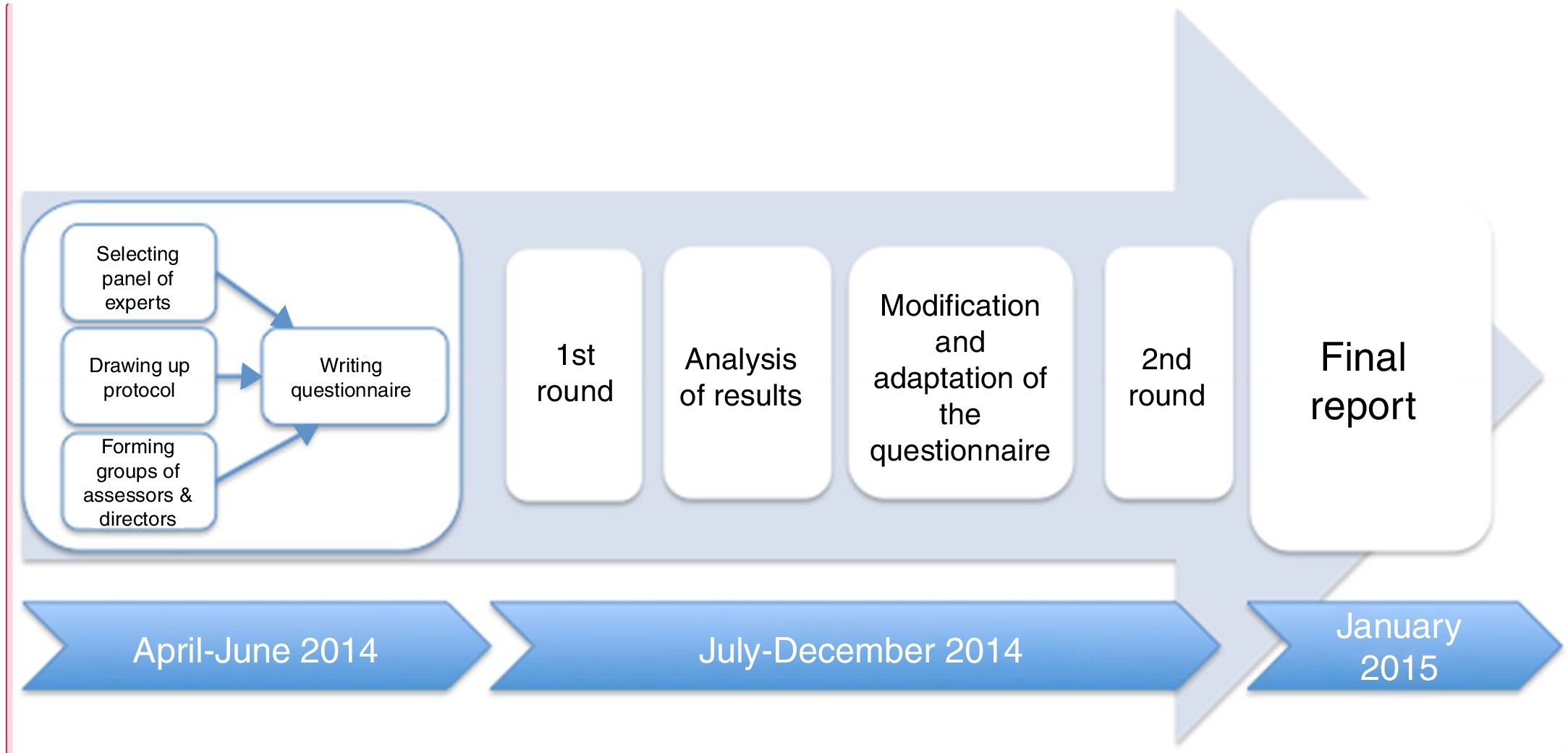
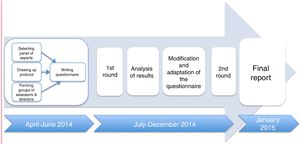
Each expert answered the questionnaire, formulated in Portuguese and Spanish, by individually accessing an online platform in two consecutive rounds. The results of the first round were analysed and sent back to the specialists to reconsider. Answers were collected between April 2014 and January 2015 (see Fig. 1).
There was considered to be consensus when at least 66% of panellists gave the same answer to a question. When this consensus was not initially reached, trends were evaluated by comparing the number of “agree” and “completely agree” responses to the number of “disagree” and “completely disagree” responses. Responses were grouped according to the following criteria defined a priori:
- •
Unanimity: the panel of experts gave the same answer (86–100%).
- •
Qualified majority: a large majority gave the same answer (66–85%).
- •
Simple majority: a majority gave the same answer (50–65%).
- •
Discrepancy: same answer was not given by at least 50% of panellists.
Tables 1, 2, 3 and 4 express jointly and as percentages the results from Portugal and Spain, divided into four blocks: Prevention, Diagnosis, Treatment and Progression. Differences greater than 20% between the two countries are identified separately in the discussion text.
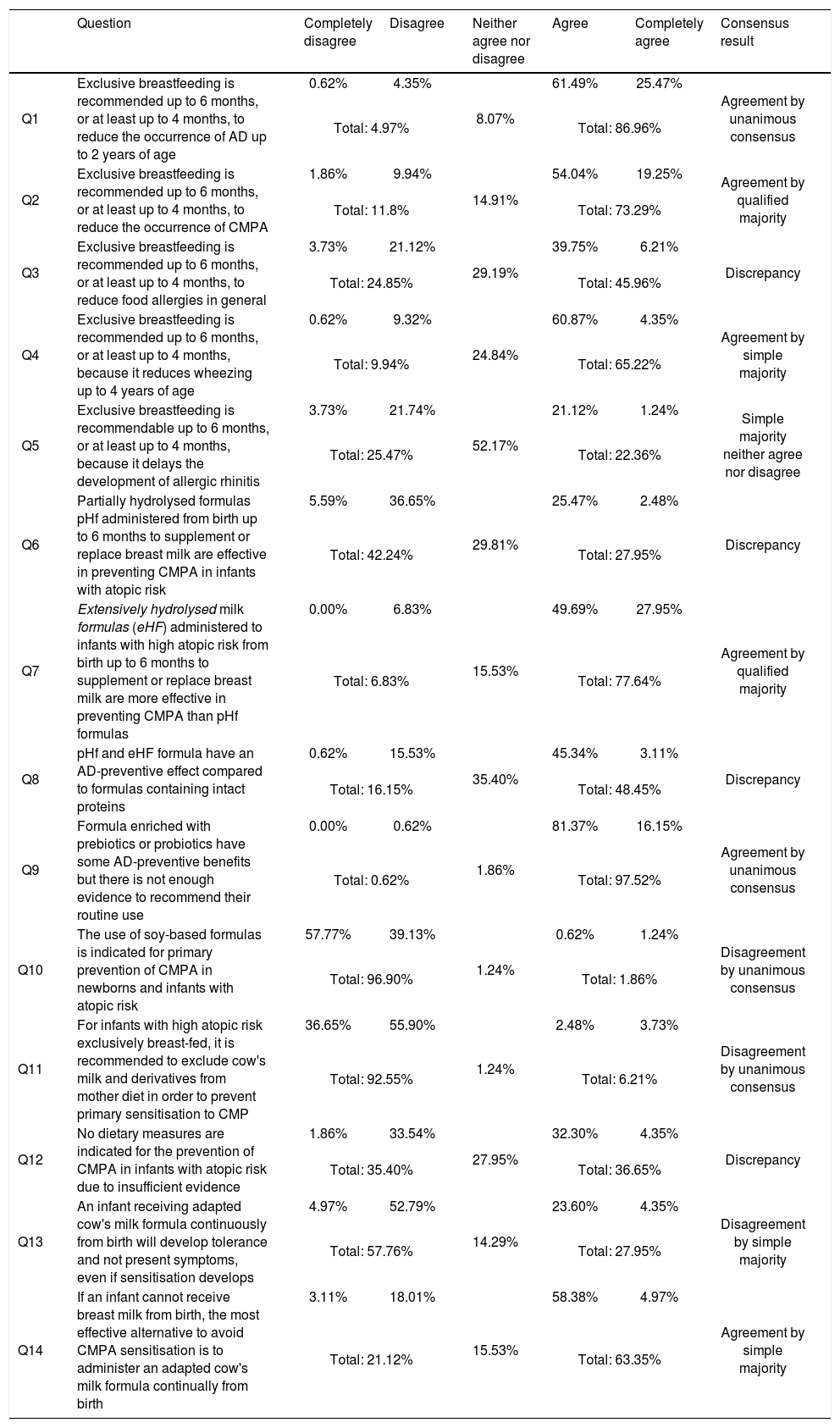
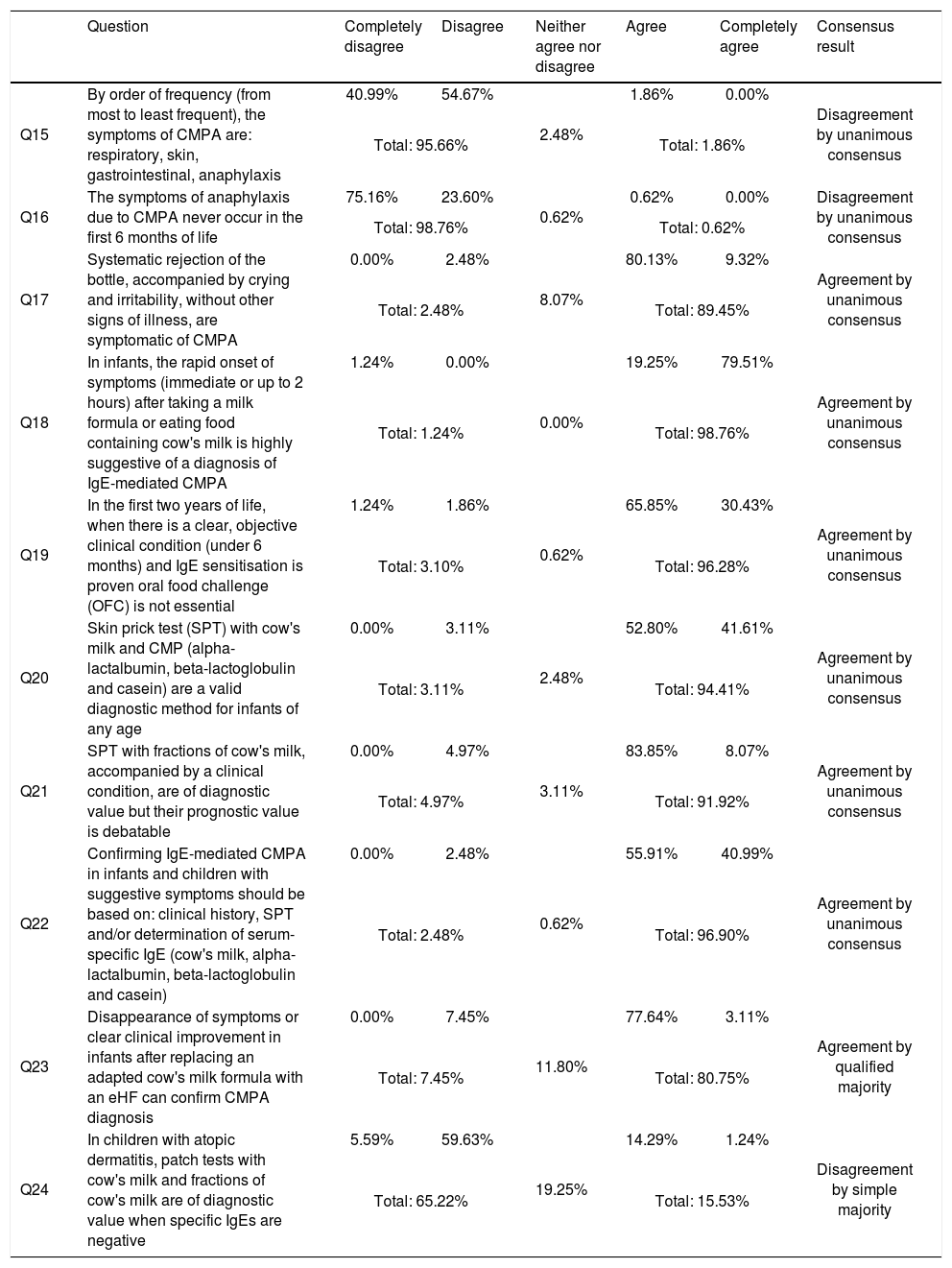
Results for block I prevention, and degree of agreement (in the second round).
| Question | Completely disagree | Disagree | Neither agree nor disagree | Agree | Completely agree | Consensus result | |
|---|---|---|---|---|---|---|---|
| Q1 | Exclusive breastfeeding is recommended up to 6 months, or at least up to 4 months, to reduce the occurrence of AD up to 2 years of age | 0.62% | 4.35% | 8.07% | 61.49% | 25.47% | Agreement by unanimous consensus |
| Total: 4.97% | Total: 86.96% | ||||||
| Q2 | Exclusive breastfeeding is recommended up to 6 months, or at least up to 4 months, to reduce the occurrence of CMPA | 1.86% | 9.94% | 14.91% | 54.04% | 19.25% | Agreement by qualified majority |
| Total: 11.8% | Total: 73.29% | ||||||
| Q3 | Exclusive breastfeeding is recommended up to 6 months, or at least up to 4 months, to reduce food allergies in general | 3.73% | 21.12% | 29.19% | 39.75% | 6.21% | Discrepancy |
| Total: 24.85% | Total: 45.96% | ||||||
| Q4 | Exclusive breastfeeding is recommended up to 6 months, or at least up to 4 months, because it reduces wheezing up to 4 years of age | 0.62% | 9.32% | 24.84% | 60.87% | 4.35% | Agreement by simple majority |
| Total: 9.94% | Total: 65.22% | ||||||
| Q5 | Exclusive breastfeeding is recommendable up to 6 months, or at least up to 4 months, because it delays the development of allergic rhinitis | 3.73% | 21.74% | 52.17% | 21.12% | 1.24% | Simple majority neither agree nor disagree |
| Total: 25.47% | Total: 22.36% | ||||||
| Q6 | Partially hydrolysed formulas pHf administered from birth up to 6 months to supplement or replace breast milk are effective in preventing CMPA in infants with atopic risk | 5.59% | 36.65% | 29.81% | 25.47% | 2.48% | Discrepancy |
| Total: 42.24% | Total: 27.95% | ||||||
| Q7 | Extensively hydrolysed milk formulas (eHF) administered to infants with high atopic risk from birth up to 6 months to supplement or replace breast milk are more effective in preventing CMPA than pHf formulas | 0.00% | 6.83% | 15.53% | 49.69% | 27.95% | Agreement by qualified majority |
| Total: 6.83% | Total: 77.64% | ||||||
| Q8 | pHf and eHF formula have an AD-preventive effect compared to formulas containing intact proteins | 0.62% | 15.53% | 35.40% | 45.34% | 3.11% | Discrepancy |
| Total: 16.15% | Total: 48.45% | ||||||
| Q9 | Formula enriched with prebiotics or probiotics have some AD-preventive benefits but there is not enough evidence to recommend their routine use | 0.00% | 0.62% | 1.86% | 81.37% | 16.15% | Agreement by unanimous consensus |
| Total: 0.62% | Total: 97.52% | ||||||
| Q10 | The use of soy-based formulas is indicated for primary prevention of CMPA in newborns and infants with atopic risk | 57.77% | 39.13% | 1.24% | 0.62% | 1.24% | Disagreement by unanimous consensus |
| Total: 96.90% | Total: 1.86% | ||||||
| Q11 | For infants with high atopic risk exclusively breast-fed, it is recommended to exclude cow's milk and derivatives from mother diet in order to prevent primary sensitisation to CMP | 36.65% | 55.90% | 1.24% | 2.48% | 3.73% | Disagreement by unanimous consensus |
| Total: 92.55% | Total: 6.21% | ||||||
| Q12 | No dietary measures are indicated for the prevention of CMPA in infants with atopic risk due to insufficient evidence | 1.86% | 33.54% | 27.95% | 32.30% | 4.35% | Discrepancy |
| Total: 35.40% | Total: 36.65% | ||||||
| Q13 | An infant receiving adapted cow's milk formula continuously from birth will develop tolerance and not present symptoms, even if sensitisation develops | 4.97% | 52.79% | 14.29% | 23.60% | 4.35% | Disagreement by simple majority |
| Total: 57.76% | Total: 27.95% | ||||||
| Q14 | If an infant cannot receive breast milk from birth, the most effective alternative to avoid CMPA sensitisation is to administer an adapted cow's milk formula continually from birth | 3.11% | 18.01% | 15.53% | 58.38% | 4.97% | Agreement by simple majority |
| Total: 21.12% | Total: 63.35% | ||||||
Results for block II diagnosis, and degree of agreement (in the second round).
| Question | Completely disagree | Disagree | Neither agree nor disagree | Agree | Completely agree | Consensus result | |
|---|---|---|---|---|---|---|---|
| Q15 | By order of frequency (from most to least frequent), the symptoms of CMPA are: respiratory, skin, gastrointestinal, anaphylaxis | 40.99% | 54.67% | 2.48% | 1.86% | 0.00% | Disagreement by unanimous consensus |
| Total: 95.66% | Total: 1.86% | ||||||
| Q16 | The symptoms of anaphylaxis due to CMPA never occur in the first 6 months of life | 75.16% | 23.60% | 0.62% | 0.62% | 0.00% | Disagreement by unanimous consensus |
| Total: 98.76% | Total: 0.62% | ||||||
| Q17 | Systematic rejection of the bottle, accompanied by crying and irritability, without other signs of illness, are symptomatic of CMPA | 0.00% | 2.48% | 8.07% | 80.13% | 9.32% | Agreement by unanimous consensus |
| Total: 2.48% | Total: 89.45% | ||||||
| Q18 | In infants, the rapid onset of symptoms (immediate or up to 2 hours) after taking a milk formula or eating food containing cow's milk is highly suggestive of a diagnosis of IgE-mediated CMPA | 1.24% | 0.00% | 0.00% | 19.25% | 79.51% | Agreement by unanimous consensus |
| Total: 1.24% | Total: 98.76% | ||||||
| Q19 | In the first two years of life, when there is a clear, objective clinical condition (under 6 months) and IgE sensitisation is proven oral food challenge (OFC) is not essential | 1.24% | 1.86% | 0.62% | 65.85% | 30.43% | Agreement by unanimous consensus |
| Total: 3.10% | Total: 96.28% | ||||||
| Q20 | Skin prick test (SPT) with cow's milk and CMP (alpha-lactalbumin, beta-lactoglobulin and casein) are a valid diagnostic method for infants of any age | 0.00% | 3.11% | 2.48% | 52.80% | 41.61% | Agreement by unanimous consensus |
| Total: 3.11% | Total: 94.41% | ||||||
| Q21 | SPT with fractions of cow's milk, accompanied by a clinical condition, are of diagnostic value but their prognostic value is debatable | 0.00% | 4.97% | 3.11% | 83.85% | 8.07% | Agreement by unanimous consensus |
| Total: 4.97% | Total: 91.92% | ||||||
| Q22 | Confirming IgE-mediated CMPA in infants and children with suggestive symptoms should be based on: clinical history, SPT and/or determination of serum-specific IgE (cow's milk, alpha-lactalbumin, beta-lactoglobulin and casein) | 0.00% | 2.48% | 0.62% | 55.91% | 40.99% | Agreement by unanimous consensus |
| Total: 2.48% | Total: 96.90% | ||||||
| Q23 | Disappearance of symptoms or clear clinical improvement in infants after replacing an adapted cow's milk formula with an eHF can confirm CMPA diagnosis | 0.00% | 7.45% | 11.80% | 77.64% | 3.11% | Agreement by qualified majority |
| Total: 7.45% | Total: 80.75% | ||||||
| Q24 | In children with atopic dermatitis, patch tests with cow's milk and fractions of cow's milk are of diagnostic value when specific IgEs are negative | 5.59% | 59.63% | 19.25% | 14.29% | 1.24% | Disagreement by simple majority |
| Total: 65.22% | Total: 15.53% | ||||||
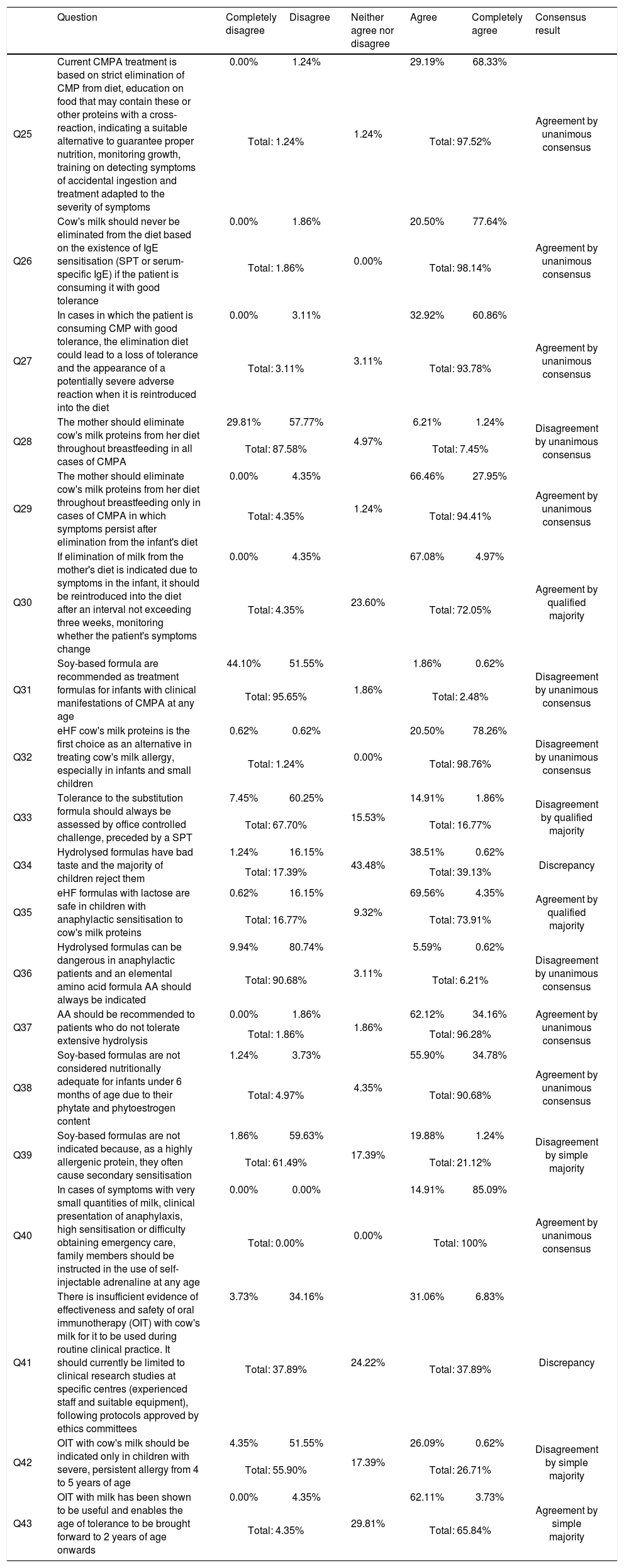
Results for block III treatment, and degree of agreement (in the second round).
| Question | Completely disagree | Disagree | Neither agree nor disagree | Agree | Completely agree | Consensus result | |
|---|---|---|---|---|---|---|---|
| Q25 | Current CMPA treatment is based on strict elimination of CMP from diet, education on food that may contain these or other proteins with a cross-reaction, indicating a suitable alternative to guarantee proper nutrition, monitoring growth, training on detecting symptoms of accidental ingestion and treatment adapted to the severity of symptoms | 0.00% | 1.24% | 1.24% | 29.19% | 68.33% | Agreement by unanimous consensus |
| Total: 1.24% | Total: 97.52% | ||||||
| Q26 | Cow's milk should never be eliminated from the diet based on the existence of IgE sensitisation (SPT or serum-specific IgE) if the patient is consuming it with good tolerance | 0.00% | 1.86% | 0.00% | 20.50% | 77.64% | Agreement by unanimous consensus |
| Total: 1.86% | Total: 98.14% | ||||||
| Q27 | In cases in which the patient is consuming CMP with good tolerance, the elimination diet could lead to a loss of tolerance and the appearance of a potentially severe adverse reaction when it is reintroduced into the diet | 0.00% | 3.11% | 3.11% | 32.92% | 60.86% | Agreement by unanimous consensus |
| Total: 3.11% | Total: 93.78% | ||||||
| Q28 | The mother should eliminate cow's milk proteins from her diet throughout breastfeeding in all cases of CMPA | 29.81% | 57.77% | 4.97% | 6.21% | 1.24% | Disagreement by unanimous consensus |
| Total: 87.58% | Total: 7.45% | ||||||
| Q29 | The mother should eliminate cow's milk proteins from her diet throughout breastfeeding only in cases of CMPA in which symptoms persist after elimination from the infant's diet | 0.00% | 4.35% | 1.24% | 66.46% | 27.95% | Agreement by unanimous consensus |
| Total: 4.35% | Total: 94.41% | ||||||
| Q30 | If elimination of milk from the mother's diet is indicated due to symptoms in the infant, it should be reintroduced into the diet after an interval not exceeding three weeks, monitoring whether the patient's symptoms change | 0.00% | 4.35% | 23.60% | 67.08% | 4.97% | Agreement by qualified majority |
| Total: 4.35% | Total: 72.05% | ||||||
| Q31 | Soy-based formula are recommended as treatment formulas for infants with clinical manifestations of CMPA at any age | 44.10% | 51.55% | 1.86% | 1.86% | 0.62% | Disagreement by unanimous consensus |
| Total: 95.65% | Total: 2.48% | ||||||
| Q32 | eHF cow's milk proteins is the first choice as an alternative in treating cow's milk allergy, especially in infants and small children | 0.62% | 0.62% | 0.00% | 20.50% | 78.26% | Disagreement by unanimous consensus |
| Total: 1.24% | Total: 98.76% | ||||||
| Q33 | Tolerance to the substitution formula should always be assessed by office controlled challenge, preceded by a SPT | 7.45% | 60.25% | 15.53% | 14.91% | 1.86% | Disagreement by qualified majority |
| Total: 67.70% | Total: 16.77% | ||||||
| Q34 | Hydrolysed formulas have bad taste and the majority of children reject them | 1.24% | 16.15% | 43.48% | 38.51% | 0.62% | Discrepancy |
| Total: 17.39% | Total: 39.13% | ||||||
| Q35 | eHF formulas with lactose are safe in children with anaphylactic sensitisation to cow's milk proteins | 0.62% | 16.15% | 9.32% | 69.56% | 4.35% | Agreement by qualified majority |
| Total: 16.77% | Total: 73.91% | ||||||
| Q36 | Hydrolysed formulas can be dangerous in anaphylactic patients and an elemental amino acid formula AA should always be indicated | 9.94% | 80.74% | 3.11% | 5.59% | 0.62% | Disagreement by unanimous consensus |
| Total: 90.68% | Total: 6.21% | ||||||
| Q37 | AA should be recommended to patients who do not tolerate extensive hydrolysis | 0.00% | 1.86% | 1.86% | 62.12% | 34.16% | Agreement by unanimous consensus |
| Total: 1.86% | Total: 96.28% | ||||||
| Q38 | Soy-based formulas are not considered nutritionally adequate for infants under 6 months of age due to their phytate and phytoestrogen content | 1.24% | 3.73% | 4.35% | 55.90% | 34.78% | Agreement by unanimous consensus |
| Total: 4.97% | Total: 90.68% | ||||||
| Q39 | Soy-based formulas are not indicated because, as a highly allergenic protein, they often cause secondary sensitisation | 1.86% | 59.63% | 17.39% | 19.88% | 1.24% | Disagreement by simple majority |
| Total: 61.49% | Total: 21.12% | ||||||
| Q40 | In cases of symptoms with very small quantities of milk, clinical presentation of anaphylaxis, high sensitisation or difficulty obtaining emergency care, family members should be instructed in the use of self-injectable adrenaline at any age | 0.00% | 0.00% | 0.00% | 14.91% | 85.09% | Agreement by unanimous consensus |
| Total: 0.00% | Total: 100% | ||||||
| Q41 | There is insufficient evidence of effectiveness and safety of oral immunotherapy (OIT) with cow's milk for it to be used during routine clinical practice. It should currently be limited to clinical research studies at specific centres (experienced staff and suitable equipment), following protocols approved by ethics committees | 3.73% | 34.16% | 24.22% | 31.06% | 6.83% | Discrepancy |
| Total: 37.89% | Total: 37.89% | ||||||
| Q42 | OIT with cow's milk should be indicated only in children with severe, persistent allergy from 4 to 5 years of age | 4.35% | 51.55% | 17.39% | 26.09% | 0.62% | Disagreement by simple majority |
| Total: 55.90% | Total: 26.71% | ||||||
| Q43 | OIT with milk has been shown to be useful and enables the age of tolerance to be brought forward to 2 years of age onwards | 0.00% | 4.35% | 29.81% | 62.11% | 3.73% | Agreement by simple majority |
| Total: 4.35% | Total: 65.84% | ||||||
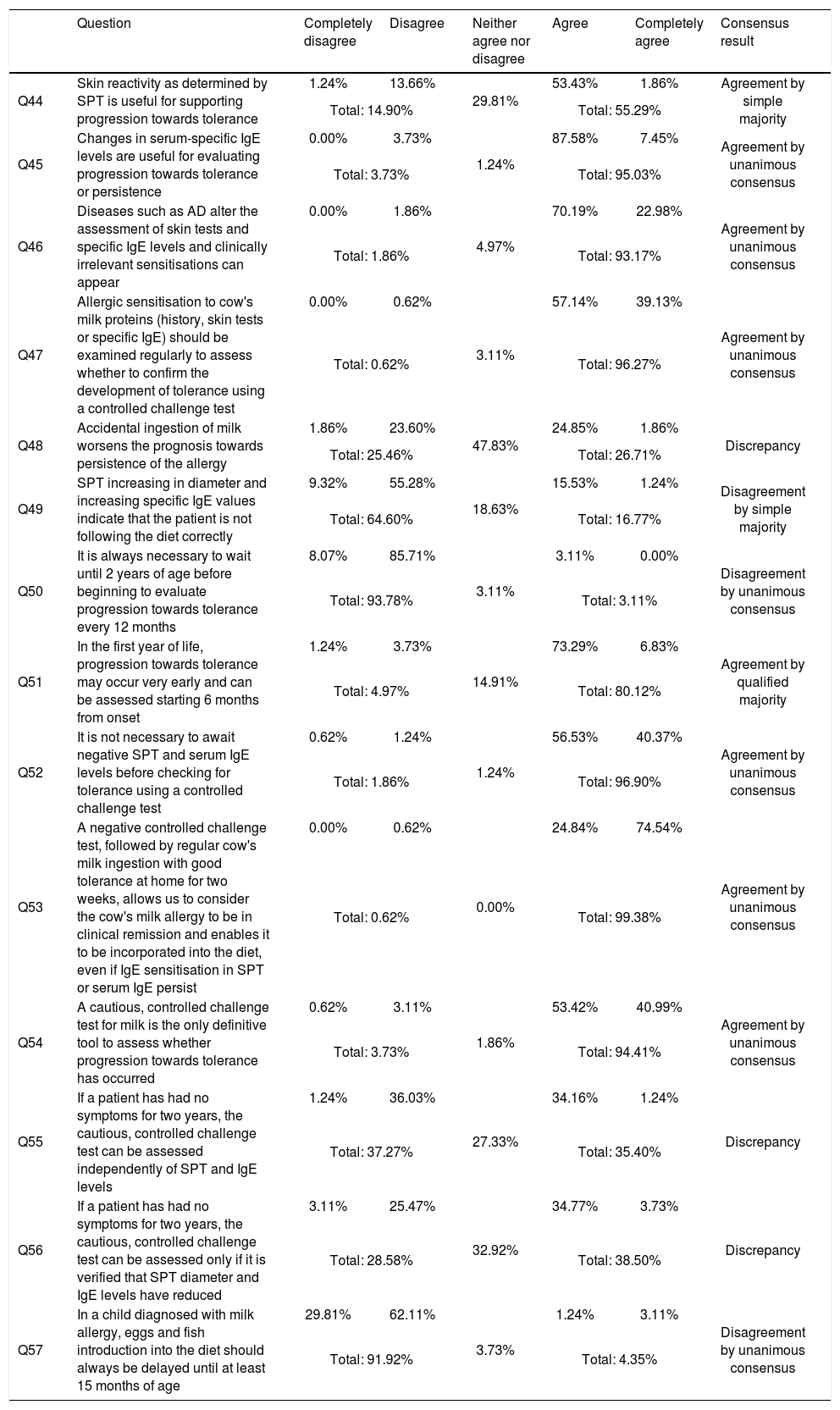
Results for block IV progression, evolution, prognosis and degree of agreement (in the second round).
| Question | Completely disagree | Disagree | Neither agree nor disagree | Agree | Completely agree | Consensus result | |
|---|---|---|---|---|---|---|---|
| Q44 | Skin reactivity as determined by SPT is useful for supporting progression towards tolerance | 1.24% | 13.66% | 29.81% | 53.43% | 1.86% | Agreement by simple majority |
| Total: 14.90% | Total: 55.29% | ||||||
| Q45 | Changes in serum-specific IgE levels are useful for evaluating progression towards tolerance or persistence | 0.00% | 3.73% | 1.24% | 87.58% | 7.45% | Agreement by unanimous consensus |
| Total: 3.73% | Total: 95.03% | ||||||
| Q46 | Diseases such as AD alter the assessment of skin tests and specific IgE levels and clinically irrelevant sensitisations can appear | 0.00% | 1.86% | 4.97% | 70.19% | 22.98% | Agreement by unanimous consensus |
| Total: 1.86% | Total: 93.17% | ||||||
| Q47 | Allergic sensitisation to cow's milk proteins (history, skin tests or specific IgE) should be examined regularly to assess whether to confirm the development of tolerance using a controlled challenge test | 0.00% | 0.62% | 3.11% | 57.14% | 39.13% | Agreement by unanimous consensus |
| Total: 0.62% | Total: 96.27% | ||||||
| Q48 | Accidental ingestion of milk worsens the prognosis towards persistence of the allergy | 1.86% | 23.60% | 47.83% | 24.85% | 1.86% | Discrepancy |
| Total: 25.46% | Total: 26.71% | ||||||
| Q49 | SPT increasing in diameter and increasing specific IgE values indicate that the patient is not following the diet correctly | 9.32% | 55.28% | 18.63% | 15.53% | 1.24% | Disagreement by simple majority |
| Total: 64.60% | Total: 16.77% | ||||||
| Q50 | It is always necessary to wait until 2 years of age before beginning to evaluate progression towards tolerance every 12 months | 8.07% | 85.71% | 3.11% | 3.11% | 0.00% | Disagreement by unanimous consensus |
| Total: 93.78% | Total: 3.11% | ||||||
| Q51 | In the first year of life, progression towards tolerance may occur very early and can be assessed starting 6 months from onset | 1.24% | 3.73% | 14.91% | 73.29% | 6.83% | Agreement by qualified majority |
| Total: 4.97% | Total: 80.12% | ||||||
| Q52 | It is not necessary to await negative SPT and serum IgE levels before checking for tolerance using a controlled challenge test | 0.62% | 1.24% | 1.24% | 56.53% | 40.37% | Agreement by unanimous consensus |
| Total: 1.86% | Total: 96.90% | ||||||
| Q53 | A negative controlled challenge test, followed by regular cow's milk ingestion with good tolerance at home for two weeks, allows us to consider the cow's milk allergy to be in clinical remission and enables it to be incorporated into the diet, even if IgE sensitisation in SPT or serum IgE persist | 0.00% | 0.62% | 0.00% | 24.84% | 74.54% | Agreement by unanimous consensus |
| Total: 0.62% | Total: 99.38% | ||||||
| Q54 | A cautious, controlled challenge test for milk is the only definitive tool to assess whether progression towards tolerance has occurred | 0.62% | 3.11% | 1.86% | 53.42% | 40.99% | Agreement by unanimous consensus |
| Total: 3.73% | Total: 94.41% | ||||||
| Q55 | If a patient has had no symptoms for two years, the cautious, controlled challenge test can be assessed independently of SPT and IgE levels | 1.24% | 36.03% | 27.33% | 34.16% | 1.24% | Discrepancy |
| Total: 37.27% | Total: 35.40% | ||||||
| Q56 | If a patient has had no symptoms for two years, the cautious, controlled challenge test can be assessed only if it is verified that SPT diameter and IgE levels have reduced | 3.11% | 25.47% | 32.92% | 34.77% | 3.73% | Discrepancy |
| Total: 28.58% | Total: 38.50% | ||||||
| Q57 | In a child diagnosed with milk allergy, eggs and fish introduction into the diet should always be delayed until at least 15 months of age | 29.81% | 62.11% | 3.73% | 1.24% | 3.11% | Disagreement by unanimous consensus |
| Total: 91.92% | Total: 4.35% | ||||||
In the Delphi method employed in the completion of the questionnaire, the identities of the panel of experts consulted are kept secret until the study is completed to avoid leadership bias. Responses therefore correspond solely to the opinions and personal practice of each participant.
Overall, consensus (over 66%) is reached in only 39 items (68.4%). This figure, together with the fact that nine items (15.7%) have discrepancies, evidence that there are points of debate in CMPA management standards. However, breaking down the data by block and analysing it by trend offers additional information, with marked differences depending on the issue under discussion. For instance, 90% consensus was reached for Diagnosis, but only 50% for Prevention.
Prevention blockThere has recently been controversy over effectiveness of preventive measures against allergy in general, and more specifically against food allergy, including CMPA. This survey reveals discrepancies with respect to the indication of dietary measures to prevent CMPA and other allergic diseases. Consensus was reached only on seven of the 14 questions posed, with unanimous consensus in four. There were discrepancies for four items, which make up only 28.5% of the questions. On the rest, agreement was reached by simple majority.
This study addresses CMPA prevention in daily practice, and only for patients with atopic risk in relation to intervention with special formulas. A child with atopic risk is defined as per the American Academy of Pediatrics, the European Society of Paediatric Allergy and Clinical Immunology, and the European Society for Paediatric Gastroenterology, Hepatology and Nutrition,5,11,12 which consider atopic risk to be present if there is at least one first degree relative with a documented allergic condition. These are very sensitive criteria but are not very specific, as they are met by a very wide sector of the population and include patients with a history of respiratory and food allergies, both light and severe, and do not account for the number of family members affected. This imprecise definition, criticised for its ambiguity,13 should be taken into account when evaluating the results of many prevention studies, as they favour the inclusion of heterogeneous populations. Additionally, they often provide preventive strategies including CMPA, but overlap with the prevention of allergy to other foods and with other allergic diseases like atopic dermatitis (AD) and asthma, which depend on numerous other factors. The panellists’ responses reflect this indeterminate situation.
Breastfeeding. The optimal food for children in early months is breast milk; the guidelines5 also mention its potential usefulness in reducing the occurrence of allergic diseases. However, although breastfeeding (BF) benefits both mother and infant, evidence of its preventive action against developing food allergy is limited.14 Essential procedures for drawing conclusions, such as randomised trials with or without BF, are not practicable from an ethical standpoint. Therefore, studies assessing BF as a protective factor are based exclusively on observational analysis. In these studies, carried out on children with atopic risk, it is difficult to avoid the risk of reverse causality bias. The families with the highest atopic load are those who use preventive measures the most, which can alter results as the hereditary component of atopy is more important than in other populations without this load.
Regarding this point on the preventive effect of BF, the panellists distinguish between CMPA and AD and agreed (86.94%) on the importance of exclusive breastfeeding until six months of age, or at least up to four months, in reducing AD. Its role in reducing CMPA occurrence was accepted at a lower percentage (qualified majority, 73.29%), with differences between Portugal, at 90.9%, and Spain, at 66.5%, i.e. just within the threshold for consensus.
This protection-affirming opinion contrasts with data from systematic reviews comparing BF with feeding using conventional formulas in the development of allergic diseases, which found no evidence of exclusive BF having any protective effect against developing AD for at least three months, in comparison with the formula.15 Nor was there evidence of an association between exclusive BF for at least four months and a lower cumulative incidence of CMPA at 18 months of age.16 Our questionnaire's approach presumed longer-term BF, of up to six months, which may have contributed to the affirmative response.
There are considerable differences regarding other allergic diseases. There was discrepancy in the matter of whether breastfeeding was useful in generally preventing food allergy, whereas a simple majority was achieved agreeing that exclusive BF would reduce occurrence of wheezing up to four years of age. There was discrepancy regarding allergic rhinitis (AR), the prevailing opinion being that BF has no effect (52.1%). These differences are easy to understand from a paediatric perspective. Small children's respiratory problems differ from asthma and AR: in the early years of life, wheezing and rhinitis are associated with viral infections, which decrease with age and immune maturation.17 By very different mechanisms, BF reduces the number of infections and the breastfed infant has reduced nursery school contact with other children, decreasing the risk of infection. A systematic review18 found that the protective effect of BF against asthma (including wheezing, bronchiolitis and bronchitis) was higher in the group of 0–2-year olds, regardless of duration or exclusivity of BF. This protective effect decreases with age, and studies that follow-up to adolescence have found that BF does not have an effect against asthma in older children caused by inhalants.19,20
In accordance with the literature,21 the panellists unanimously (92.45%) reject excluding cow's milk and derivatives from the mother's diet as a CMPA primary prevention method in newborns with atopic risk.
Special formulas. Recent guidelines5 recommend the use of hypoallergenic formulas during the first four months as prevention against CMPA in children with high atopic risk, where it is not possible to begin BF. In this study, the experts differed on the CMPA preventive role of partially hydrolysed formulas (pHF) administered from birth to six months. The same conclusion is reached by a recent Spanish consensus of specialists in paediatric digestive medicine.22 In our study, the panellists agreed (qualified majority, 75.33%), that if they were used, extensively hydrolysed formulas (eHF) would be more effective in preventing CMPA than pHFs. The time lapse established makes it difficult to compare opinions.
The question about the protective effects of eHFs and pHFs against AD showed discrepancy among the panellists. Responses were very different in Spain and Portugal, with only 41% in agreement in Spain as opposed to 68.1% in Portugal. Some studies23 have found that pHFs administered from birth until six months of age would prevent AD and CMPA in children with atopic risk. In the light of the discrepancies found and the methodological difficulty of jointly assessing two different diseases like CMPA and AD, we believe that further investigation is necessary to re-evaluate this data.
It was unanimously accepted (92.5%) that soy-based formulas are not indicated to prevent CMPA5 and that formulas including prebiotics or probiotics show some benefits in preventing AD (97.5%), although under the proviso that, in accordance with the literature,24 there is insufficient evidence to recommend their routine use. On this topic, there is therefore a clear need to conduct research before making systematic recommendations.
Other attitudes. There is disagreement by simple majority (57.67%) with the statement that, if an infant receives an adapted cow's milk formula continually from birth, he/she develops tolerance and shows no symptoms, with significant differences between Portugal and Spain (47.86%/84.09% respectively). These are controversial issues that are not endorsed in the guidelines but are the fruit of the panellists “own experience”.25 One issue arising from this is the suggestion that if BF cannot be established from birth, the most effective alternative to prevent development of CMPA is continual administration from the beginning of a standard cow's milk protein (CMP) formula. A simple majority among (63%) of those surveyed agreed with this statement, although there is a marked difference between Portugal, with 34% agreement, and Spain, with 74.3%. There is no discussion of administering isolated baby bottles or repeated, isolated bottle feeding; only long-term bottle feeding is considered. There has recently been much interest in this,26,27 although due to ethical problems there have been no randomised trials. The indisputable nutritional and emotional advantages of breastfeeding do not necessarily imply that it is effective in preventing allergic sensitisation, as had previously been held. In any case, further research is needed to provide scientific evidence on the role of the early introduction of milk for infants,27 as is being done with other foods.
Diagnosis blockIn this block, there was consensus in nine out of the 10 questions posed (90%). It can therefore be concluded that diagnostic procedures are well standardised and are accepted and taken up by clinics.
Clinical manifestations. The panellists unanimously rejected the order of frequency of CMPA symptoms proposed in the question (respiratory, skin, gastrointestinal, anaphylaxis) and that this does not correspond with the clinical presentation usually observed in their experience.
Although epidemiological studies carried out in various countries differ slightly in terms of percentages, the order of symptoms does not vary and skin symptoms always predominate.28 Other symptoms that are rarely described, for example that the systematic rejection of the baby bottle accompanied by crying or irritability without other signs of illness can be suggestive of CMPA, achieve unanimous agreement (89.44%).
They also agreed (98.7%) that CMPA caused anaphylaxis which can occur in infants under six months of age, an opinion not widely held by other professionals. The course of symptoms, which begin soon after ingesting milk or dairy, is considered suggestive (98.7%) of a diagnosis of IgE-mediated CMPA. Additionally, the statement that the disappearance or improvement of symptoms in infants, after substituting a cow's milk formula for an eHF, can be used to confirm a CMPA diagnosis, was supported by qualified majority (80.7%). In other words, the panellists consider these clinical factors to be very valid criteria to guide a diagnosis of presumption of CMPA.
In vivo and in vitro diagnostic tests. In this survey unanimity was reached in that the cutaneous tests, SPT, and specific IgE levels for cow's milk and its proteins are the first recourse for diagnosing CMPA in infants of any age (94.4%), but that their value in prognosis is debatable (91.9%). Sensitivity and specificity of skin tests and serum IgE values must be established for each age group and cannot be extrapolated to other groups. 99% sensitivity and 38% specificity have been described in infants under one year of age.29 In agreement with this, as many as 94.41% of the experts consulted agreed that skin prick tests with cow's milk and fractions, along with a suggestive clinical history, are a valid diagnostic method for children of any age. There is unanimity among the participants that clinical history, together with skin tests and determination of specific IgE for cow's milk and fractions, are the first diagnostic step. This result corresponds with the literature to date and all consensus guidelines.3,5,30
Although the controlled oral food challenge OFC is considered the gold standard for diagnosis, the panellists believe (96.27%) that in the first two years of life, in routine practice, when there is objective, clear clinical presentation under six months and current IgE sensitisation is demonstrated to confirm diagnosis is always required, it is not necessary to carry out OFC.
In terms of evaluating the utility of epicutaneous skin testing (patch testing) with CMP to diagnose CMPA in patients with AD, the panellists disagree by simple majority (65.2%). Again, there are differences between countries, with more disagreement from Spanish (71.7%) than Portuguese experts (47.7%). This situation corresponds with the discrepancies found in the bibliography on this topic.31,32
Treatment blockIn this block there was consensus for 14 out of the 19 questions posed (73.68%), and they show a high degree of agreement about dietary treatment, and less agreement or discrepancy on immunotherapy treatments.
General attitudes. The panellists unanimously agreed (97.5%) that current CMPA treatment should be based on strict elimination and providing nutritionally suitable alternatives for proper growth, as well as the education of family members on the prevention of accidental ingestion and providing skill for symptom control. This education extends (100%) to training carers to use self-injectable adrenaline in cases in which symptoms present with small quantities of milk, where there is a clinical history of anaphylaxis and high sensitisation, or where access to emergency care is difficult. The participants agree (100%) that this indication is valid regardless of age. The absence of an age limit for treatment with adrenaline is a clear, longstanding paediatric therapeutic attitude, based on panellists’ experience, which is not so widely shared by other professionals and which should be disseminated.
Regarding other therapeutic attitudes, such as elimination of CMP from the diets of nursing mothers, the panellists agreed unanimously (94.4%) that this is only indicated when the child continues presenting symptoms despite elimination of CMP from his/her own diet. In this case, it was decided by simple majority (72%) that it should be reintroduced to the mother's diet within a time limit not greater than three weeks, to monitor any change in symptoms. In other words, the proposal is to use the elimination diet, either following this with reintroduction as a clinical diagnostic element.
A noteworthy consideration for daily practice is the attitude to detecting IgE sensitisation for CMP (by skin tests or serum-specific IgE) in patients who have clinical tolerance. It was decided unanimously (98.14%) that without any accompanying clinical presentation, CMP should never be eliminated. Similarly, the panellists also agree (93.79%) that this withdrawal of milk from the diet in a sensitised but tolerant patient can be the cause of severe allergic symptoms when it is reintroduced. This belief, clearly accepted by paediatric allergists and referred to in the literature,33 is not widely shared by other professionals, who sometimes indicate diets based solely on sensitisation data, which causes the loss of existing tolerance. On this point, differences arise between Spanish and Portuguese experts. Although both groups are equally in agreement on the potential severe risks, 100% of the Spanish panellists stated that they completely agree, compared with only 77.2% of the Portuguese panellists. The wording of the question did not mention the time duration of elimination, which is an important factor in whether tolerance is lost. This point of departure may prove useful for studies into the allergic risks of restrictive diets based on IgE determination without accompanying clinical presentation.
Replacement formulas. There was unanimous consensus (98.7%) that eHF is the first choice of alternative formula for infants and small children, including anaphylactic patients (96.27%). This confidence in the safety of eHFs also leads the panellists to agree by qualified majority (67.7%) that these can be indicated without the need to perform any type of test. Given that by definition an eHF has to be tolerated by at least 90% of allergic children in order to be put on the market, up to 10% of patients could in theory present symptoms. Some experts always perform a controlled OFC. This practice, which takes time and resources, could be valued on its efficiency. Some 73.92% accepted that eHFs with lactose were safe, even with clinical presentation of anaphylaxis. It was unanimously agreed (96.27%) that elemental amino acid formulas should only be recommended for patients who do not tolerate eHF.
Accepting the current recommendations of all guidelines5–7 the participants agreed (95.65%) that soy-based formulas are not considered suitable for those under six months of age for nutritional reasons, due to their phytate and phytoestrogen content. This questionnaire does not tackle the use of soy in older children who consume a varied diet. With the statement that soy should not be indicated because it can cause further sensitisation the panellists disagreed by simple majority (61.49%, higher in Spain [68.4%] than in Portugal [43.1%]). This problem, which is of concern in English-speaking countries due to the frequency of soy allergy, is not confirmed in Spanish studies, where only 4% of those allergic to milk showed sensitisation to soy without accompanying clinical expression.25 This discrepancy in opinion and situations between different countries also points to future lines of research.
Palatability is an essential factor when choosing a formula. The opinions are based on the judgement of adults, who evaluate the bitter taste and peculiar smell of hydrolysed formulas negatively as compared to the sweet taste and smell of milk, and assume that this might cause infants to reject them. The panellists made an objective assessment on this point, which manifests as discrepancy. This approach brings a professional perspective to an argument often made by laypeople about the taste of special formulas, which for children simply have a different taste. These considerations, which are handled arbitrarily and subjectively, could be assessed by carrying out blind studies with children.
Oral immunotherapy with cow's milk. Food oral immunotherapy (OIT) is a treatment that has surfaced recently, with the aim of inducing total or partial food tolerance in allergy sufferers. Although implementation is not yet generalised, guidelines are being developed to regulate its indication, standards and requirements.
On the first question on OIT with cow's milk, which suggests its use only in research projects but not in routine practice, there was a discrepancy in the experts’ opinions. When mentioned as requirements for indication severe allergy and ages from 4 to 5 years, the panellists disagreed by simple majority (55.9%). Here, differences arise between the two countries, with disagreement predominating in Spain (64.1%) as against Portugal (34%). This attitude reflects the uncertainty around this therapeutic procedure among the scientific community and the need to provide guidelines for action.
Another disputed aspect of OIT – its use to bring forward the age of tolerance – narrowly missed the category of agreement by qualified majority, at 65.8%, but was accepted by 73% of Spanish panellists. This difference can be explained by a Spanish study addressing precisely this topic.34 Clinical trials are currently underway with new initiatives for early intervention using OIT with milk, which could cause additional change in these opinions.
Progression blockIn this block, focusing on clinical progression in children with CMPA, there was consensus on 10 of the 14 questions posed (71.4%).
Prognosis of progression. The good prognosis of IgE-mediated CMPA is well known, as tolerance is observed in approximately 60–80% of allergic children by the age of three.25 Therefore, regular OFC assessments are needed to check for the development of tolerance.
There is agreement by qualified majority (89.1%) that in the first year of life progression towards tolerance can occur as early as six months from onset. There are publications documenting this statement,25,29 although it is very common for assessment to be delayed. As it would enable some allergic children to begin a normal diet rapidly, this concept of early progression is noteworthy and should be disseminated because it provokes changes in the timescales of allergy assessment. Similarly, there is unanimous disagreement (93.7%) that it is necessary to wait until two years of age before assessing progression towards tolerance every twelve months. The experts therefore reveal themselves to be in favour of early evaluation of CMP tolerance.
Skin tests and specific IgE. In terms of progression criteria, the panellists value in vitro more than in vivo tests. Only a simple majority (55.6%) considers skin tests useful in assessing progression towards tolerance. However, they agree unanimously (95%) that changes in serum-specific IgE levels are useful for assessing progression towards tolerance or persistence. A negative SPT result is a good indicator of the development of tolerance, but it is not clear that variations in the size of the papule have any relation with the establishment of tolerance, and the validity of cut-off points are highly debated. There are published studies35 in the field on cut-off points for CMPA in the first year of life. Generally, a reduction in specific IgE levels is a likely predictor of progression towards tolerance, while an increase in these levels is an indicator of persistence of clinical reactivity36; however further research on the matter is still needed. It is noteworthy that the participants agree unanimously (93.1%) that diseases such as AD, frequently linked to CMPA, modify both the evaluation of skin tests and specific IgE values, which is a necessary consideration before clinical decisions are made.
From a practical point of view, the panellists agree (96.8%) that it is not necessary to await negative SPT/serum IgE levels to confirm tolerance using OFC, which is a change compared with attitudes held by clinicians with less experience. It is unanimously accepted (98.3%) that a negative milk OFC and subsequent tolerance at home is enough to conclude that CMPA is in remission, even if sensitisation persists, expressed through positive skin or serum IgE tests.
To explore empirical beliefs to a certain extent, a simple majority (55.2%) disagree that SPT increasing in diameter and increasing specific IgE values indicate that the patient is not correctly following the diet. There is discrepancy over the statement that accidental milk ingestion worsens prognosis towards persistence of the allergy. Although little research has been published on this topic,37,38 it is striking that on topics as routine as unintentional ingestion, which will at some point affect a large number of allergic people, there is such a large margin of disagreement over its impact on tolerance. It is necessary to carry out research on these points, which are important from the perspective of their psychological impact on the patient and his/her family.
An important aspect which is beginning to be addressed by the guidelines5 and with which the panellists agree unanimously (91.9%) is that introduction of presumptive allergenic foods such as eggs and fish into the diets of CMPA patients should not be delayed.
Controlled oral food challenge tests. The experts consulted unanimously agreed (96.2%) that sensitisation to cow's milk should be examined regularly to evaluate the establishment of tolerance and should be checked using OFC. Other clinical elements have been introduced to decide when to conduct OFC and SPT and IgE tests. The participants show discrepancy that the total absence of symptoms over the two previous years could be a valid basis on which to conduct OFC. Neither does introducing the factor of a decrease in IgE values resolve the uncertainty. This dilemma is shared by other researchers and in addition to cut-off points in objective tests, there are now attempts to design normograms including multiple parameters39 to predict when a OFC becomes a confirmation of tolerance.
ConclusionsThis study shows the current opinions of a wide group of experts on CMPA in the Iberian Peninsula (Portugal and Spain). It is noteworthy that a high degree of consensus was reached on diagnosis and dietary treatment, with differences as regards prevention. Both for diagnosis and for progression, data are handled based on specific IgE valuation, but the use of clinical criteria is also stressed. Panellists highlight the differences between sensitisation and clinical allergy, reject the use of diets based solely on milk IgE detection and express their agreement with early evaluation of CMP tolerance.
The questions on which participants were ambivalent or in disagreement, such as on the palatability of hydrolysed formulas, the impact of inadvertent ingestion on progression or the preventive use of pHF, provide us with very useful information with which to promote new, rigorous research that would allow us to draw conclusions on these controversial aspects.
Authors’ contributionsEAL, LB, AMA and LR designed the study. They selected the paediatric allergist who took part in the study and analysed and discussed the results. All authors read and approved the final manuscript.
Expert clinical participants- •
Spain,
- •
Portugal.
The authors have no conflicts of interest to declare. The study was supported by Danone Nutricia.