Hypersensitivity reactions to pine nuts in children have been occasionally encountered recently, although reports on pine nut allergy cases are rare worldwide. The study aimed to feature clinical and laboratory findings pertaining to pine nut allergy in Korean children.
MethodsForty-two subjects were enrolled through a retrospective review of medical records, from September 2010 to December 2015, at the Department of Pediatrics in Ajou University Hospital. The demographic profiles, clinical characteristics, and laboratory findings were evaluated.
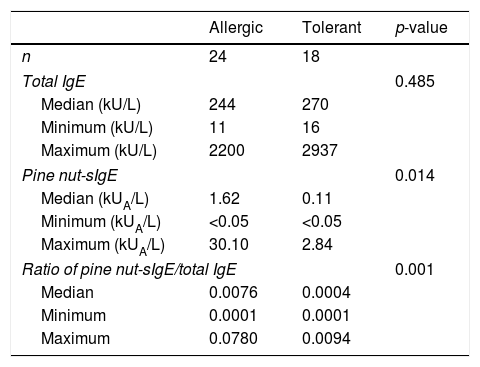
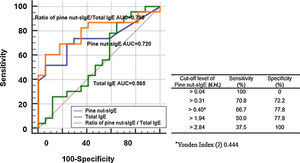
ResultsTwenty-four patients showed immediate-type reactions after exposure to pine nuts (the allergic group), while the remaining 18 were atopic controls, who exhibited no allergic symptoms (the tolerant group). The median age of the subjects in the allergic group was three years. More than half of the subjects in this group experienced allergic symptoms within 5min, and seven of them experienced anaphylaxis. The median level of pine nut-specific immunoglobulin E (sIgE) in the allergic group (1.62kUA/L) was significantly higher (p=0.014) than that in the tolerant group (0.11kUA/L), with an optimal cut-off level of 0.40kUA/L (sensitivity, 66.7% and specificity, 77.8%). The positive decision point of pine nut-sIgE (specificity, 100%) to distinguish the allergic and tolerant groups was 2.84kUA/L. However, there was no difference in pine nut-sIgE levels between the anaphylaxis and non-anaphylaxis cases.
ConclusionAbout 30% of children with pine nut allergy experienced anaphylaxis. The optimal cut-off level of pine nut-sIgE to distinguish the allergic and tolerant groups was 0.40kUA/L and the positive decision point was 2.84kUA/L.
A food allergy is a common disease especially in children, with increasing prevalence and a relatively high risk of anaphylactic reactions.1 Tree nuts, in particular, are a leading cause of severe allergic reactions and the prevalence of allergy to tree nuts is rapidly increasing.2,3 In addition, allergic reactions to tree nut are often severe, and frequently associated with fatal reactions.4
Pine trees (genus Pinus) belong to the gymnosperm group and are among the most widely distributed and prominent trees in the world.5 Pine nuts, the seeds of pine trees, are widely used for human consumption in Europe, America, and Asia. The most common pine nuts produced in Europe come from Pinus pinea, while in Korea, they are predominantly from Pinus koraiensis.6 They are used in the preparation of pastries, soup, pasta, salads, and sauces.7 In Korea, they are consumed either raw or are used as ingredients in a variety of traditional rice cakes, soup and cookies.8
Several case reports of hypersensitivity to pine nuts have been published. In most of these cases, severe anaphylactic reactions have been described.9–13 Even small amounts of pine nuts have been reported to cause dangerous allergic reactions in sensitised patients.14 The exact prevalence of allergic sensitisation to pine nuts is not known. In a recent study, pine nuts were found to be a cause of anaphylaxis, being responsible for 3% of child-related, food-induced anaphylaxis in Italy.15 In Korean children and adolescents, pine nuts accounted for 2.3% of food-induced anaphylaxis between 2009 and 2013, as per a large-scale, multi-centre case study,2 and were the third most common (7.1%) trigger among 126 anaphylactic cases caused by peanuts, tree nuts and seeds.16
Several major pine nut allergenic components were identified.12,17,18 Pine nut allergy seems to be characterised by a high monosensitisation rate 12,14,19 and low immunoglobulin (Ig) E cross-reactivity with other commonly consumed nuts, although cross-reactivity between pine nut and almonds has been described.10 A few studies have evaluated hypersensitivity to pine nut in patients with clinical symptoms and the level of pine nut-specific immunoglobulin E (pine nut-sIgE).17,18 However, the relationship between pine nut-sIgE levels and clinical symptoms has not been substantially studied.
The aim of this study is to feature the clinical characteristics and to evaluate the diagnostic values of sIgE in Korean children who have a pine nut allergy.
Materials and methodsForty-two subjects who underwent serum pine nut-sIgE assay, with a history of ingesting pine nuts, were enrolled through a retrospective review of medical records from September 2010 to December 2015, at the Department of Pediatrics in Ajou University Hospital, Suwon, South Korea. The allergic group (n=24) comprised children with a convincing history of immediate hypersensitivity following the ingestion of pine nuts, recognised by an experienced paediatric allergist using a systematic case report form, while the tolerant group (n=18) comprised children who were atopic controls and who exhibited no allergic reactions following the ingestion of pine nuts. Individual history was not confirmed by oral food challenges. The subjects in the allergic group were further classified into two sub-groups, based on their symptoms: pine nut allergy with anaphylaxis (anaphylaxis group) and pine nut allergy without anaphylaxis (non-anaphylaxis group). Anaphylaxis was diagnosed according to the clinical criteria proposed by the National Institute of Allergy and Infectious Disease and the Food Allergy and Anaphylaxis Network.20 Demographic profiles, clinical symptoms and laboratory findings were recorded. The study was approved by the Institutional Review Board of Ajou University Hospital.
Measurement of the total IgE and sIgE antibody levelsThe serum concentrations of total IgE and pine nut-sIgE for all the subjects were analysed by means of ImmunoCAP, following the manufacturer's instructions (Thermo Fisher Scientific, Uppsala, Sweden). The assay had a lower limit of pine nut-sIgE level of less than 0.05kUA/L and an upper limit of greater than 100kUA/L.
Statistical analysisThe Mann–Whitney U test was used for the analysis of continuous variables to compare characteristics and serologic parameters between study groups. A p-value of less than 0.05 was considered statistically significant. Receiver operating characteristic (ROC) curves were used to assess total IgE and pine nut-sIgE levels for diagnosing pine nut allergy. The area under the curve (AUC) was calculated to quantify the accuracy of the test.
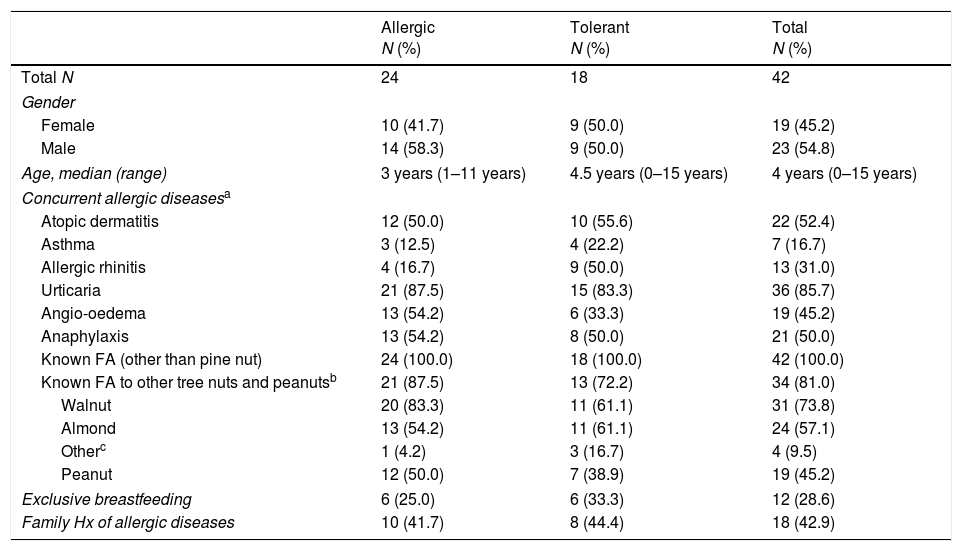
ResultsA total of 42 children, between the ages of zero and 15 years (mean age of four years), were enrolled in the study. The study population was highly atopic: 52% had atopic dermatitis and 85% had urticaria. Of the allergic group (n=24), 58.3% were males between the ages of one and 11 years (median age at the time of presenting of symptoms was three years). The characteristics of the all the subjects pointed to a history of food allergy: 81% had a food allergy to other tree nuts and peanuts. These food allergies were confirmed through the convincing history of clinical symptoms with sIgE sensitisation (>0.1kUA/L) to other tree nuts and peanuts. The most common tree nut allergies among them were walnut (74%) and almond (57%) allergies, while 45% experienced allergic reactions to peanuts (Table 1).
Clinical characteristic of study subjects.
| Allergic N (%) | Tolerant N (%) | Total N (%) | |
|---|---|---|---|
| Total N | 24 | 18 | 42 |
| Gender | |||
| Female | 10 (41.7) | 9 (50.0) | 19 (45.2) |
| Male | 14 (58.3) | 9 (50.0) | 23 (54.8) |
| Age, median (range) | 3 years (1–11 years) | 4.5 years (0–15 years) | 4 years (0–15 years) |
| Concurrent allergic diseasesa | |||
| Atopic dermatitis | 12 (50.0) | 10 (55.6) | 22 (52.4) |
| Asthma | 3 (12.5) | 4 (22.2) | 7 (16.7) |
| Allergic rhinitis | 4 (16.7) | 9 (50.0) | 13 (31.0) |
| Urticaria | 21 (87.5) | 15 (83.3) | 36 (85.7) |
| Angio-oedema | 13 (54.2) | 6 (33.3) | 19 (45.2) |
| Anaphylaxis | 13 (54.2) | 8 (50.0) | 21 (50.0) |
| Known FA (other than pine nut) | 24 (100.0) | 18 (100.0) | 42 (100.0) |
| Known FA to other tree nuts and peanutsb | 21 (87.5) | 13 (72.2) | 34 (81.0) |
| Walnut | 20 (83.3) | 11 (61.1) | 31 (73.8) |
| Almond | 13 (54.2) | 11 (61.1) | 24 (57.1) |
| Otherc | 1 (4.2) | 3 (16.7) | 4 (9.5) |
| Peanut | 12 (50.0) | 7 (38.9) | 19 (45.2) |
| Exclusive breastfeeding | 6 (25.0) | 6 (33.3) | 12 (28.6) |
| Family Hx of allergic diseases | 10 (41.7) | 8 (44.4) | 18 (42.9) |
As for the characteristics of the pine nut allergic reaction, 20 subjects (87%) in the allergic group experienced an allergic reaction on their very first exposure to pine nuts. Most subjects (83.3%) experienced symptoms only once because they strictly avoided pine nuts after experiencing symptoms. All the subjects had a reaction after oral ingestion; reactions have been reported after the consumption of raw pine nuts (75%) as well as in soups (17%), and sauces or cookies (12.5%). The amount of raw pine nuts ingested ranged from one to 15.
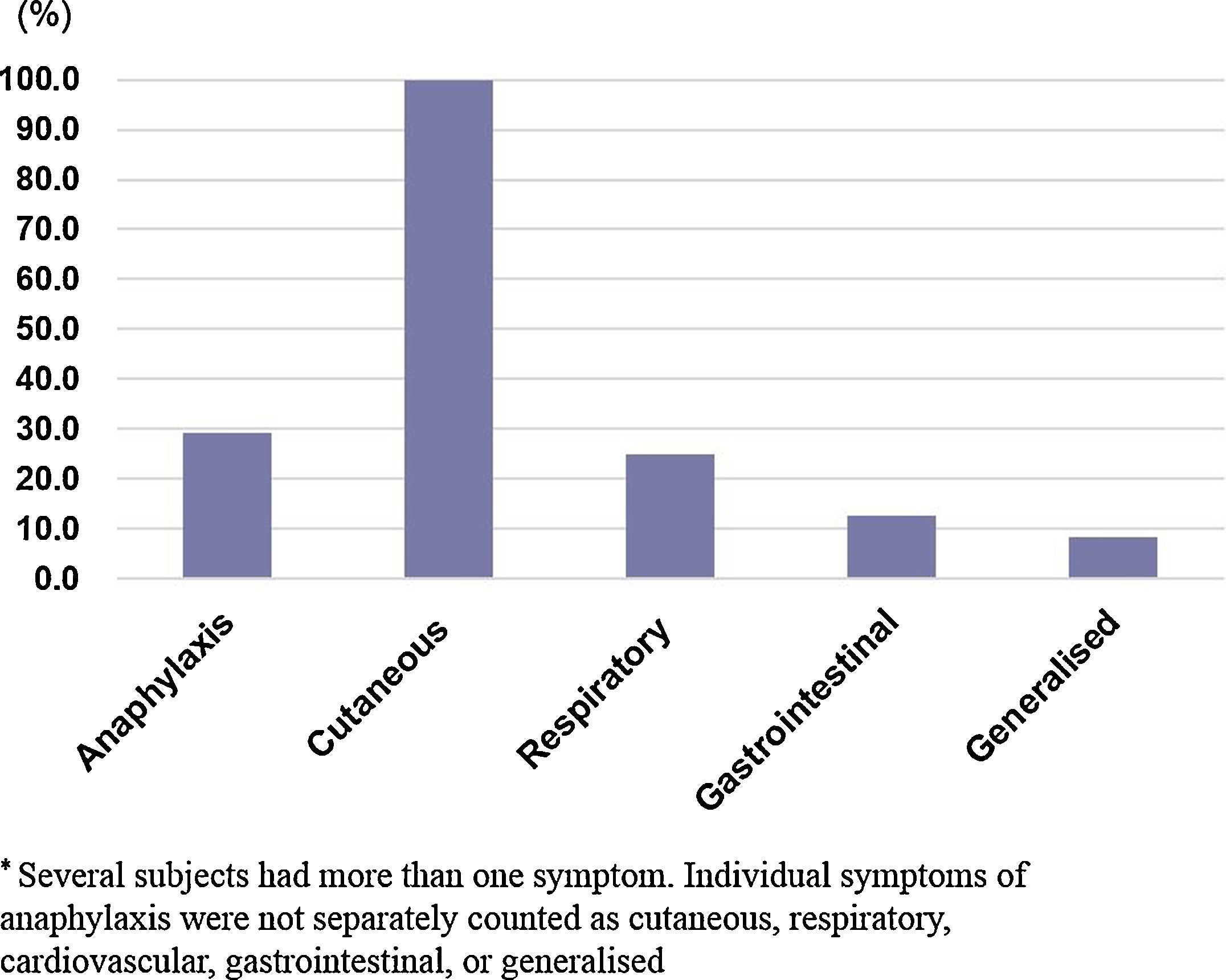
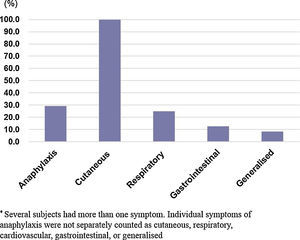
The clinical manifestations in the allergic group are shown in (Fig. 1). The most commonly-affected system was the cutaneous system (100.0%) followed by the respiratory system (25.2%). Seven children (29.2%) out of the 24 in the allergic group experienced anaphylaxis in reaction to pine nuts. Four of these subjects had a history of anaphylactic reactions to walnuts, cashew nuts, and nut mixtures.
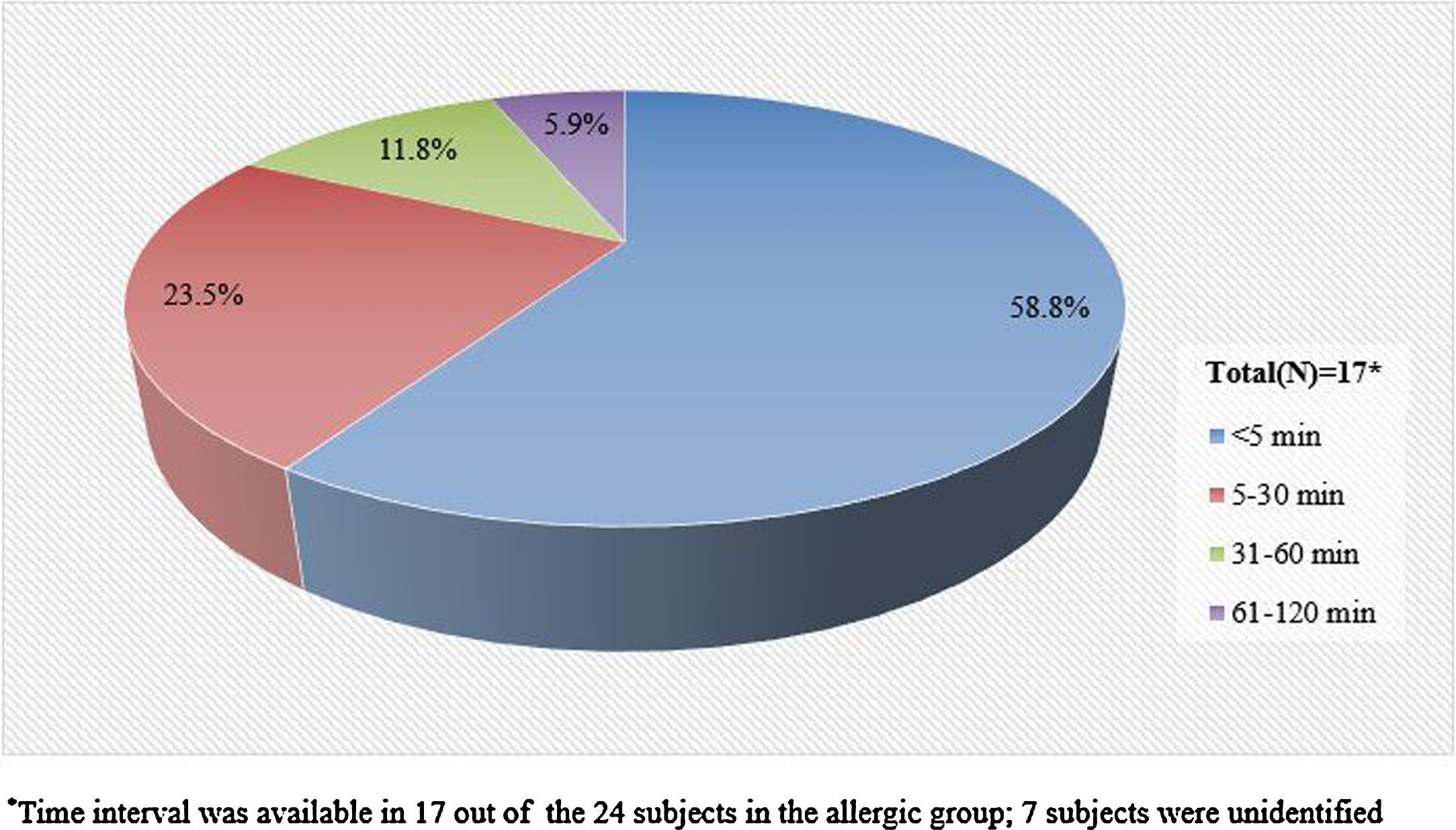
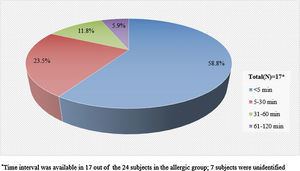
Information pertaining to the time interval between the exposure to pine nuts and the onset of symptoms was available in 17 (71%) of the 24 subjects in the allergic group. The mean time interval was 19min, with a range of zero to 120min. More than half of the 17 subjects experienced symptoms within 5min after exposure to pine nuts and 23% experienced symptoms within 30min (Fig. 2).
The median level of total IgE was 244kUA/L in the allergic group versus 270kUA/L in the tolerant group, with no significant difference between the two groups (p=0.485). The pine nut-sIgE level of the allergic and tolerant groups had different distributions. The median level of pine nut-sIgE was 1.62kUA/L (range, 0–30.10kUA/L) for the allergic group, which was significantly higher (p=0.014) than 0.11kUA/L (range, 0–2.84kUA/L) for the tolerant group. The ratio of pine nut-sIgE to total IgE showed significant differences between the allergic and tolerant groups (p=0.001) (Table 2).
Levels of total IgE and pine nut-sIgE in the allergic and tolerant groups.
| Allergic | Tolerant | p-value | |
|---|---|---|---|
| n | 24 | 18 | |
| Total IgE | 0.485 | ||
| Median (kU/L) | 244 | 270 | |
| Minimum (kU/L) | 11 | 16 | |
| Maximum (kU/L) | 2200 | 2937 | |
| Pine nut-sIgE | 0.014 | ||
| Median (kUA/L) | 1.62 | 0.11 | |
| Minimum (kUA/L) | <0.05 | <0.05 | |
| Maximum (kUA/L) | 30.10 | 2.84 | |
| Ratio of pine nut-sIgE/total IgE | 0.001 | ||
| Median | 0.0076 | 0.0004 | |
| Minimum | 0.0001 | 0.0001 | |
| Maximum | 0.0780 | 0.0094 | |
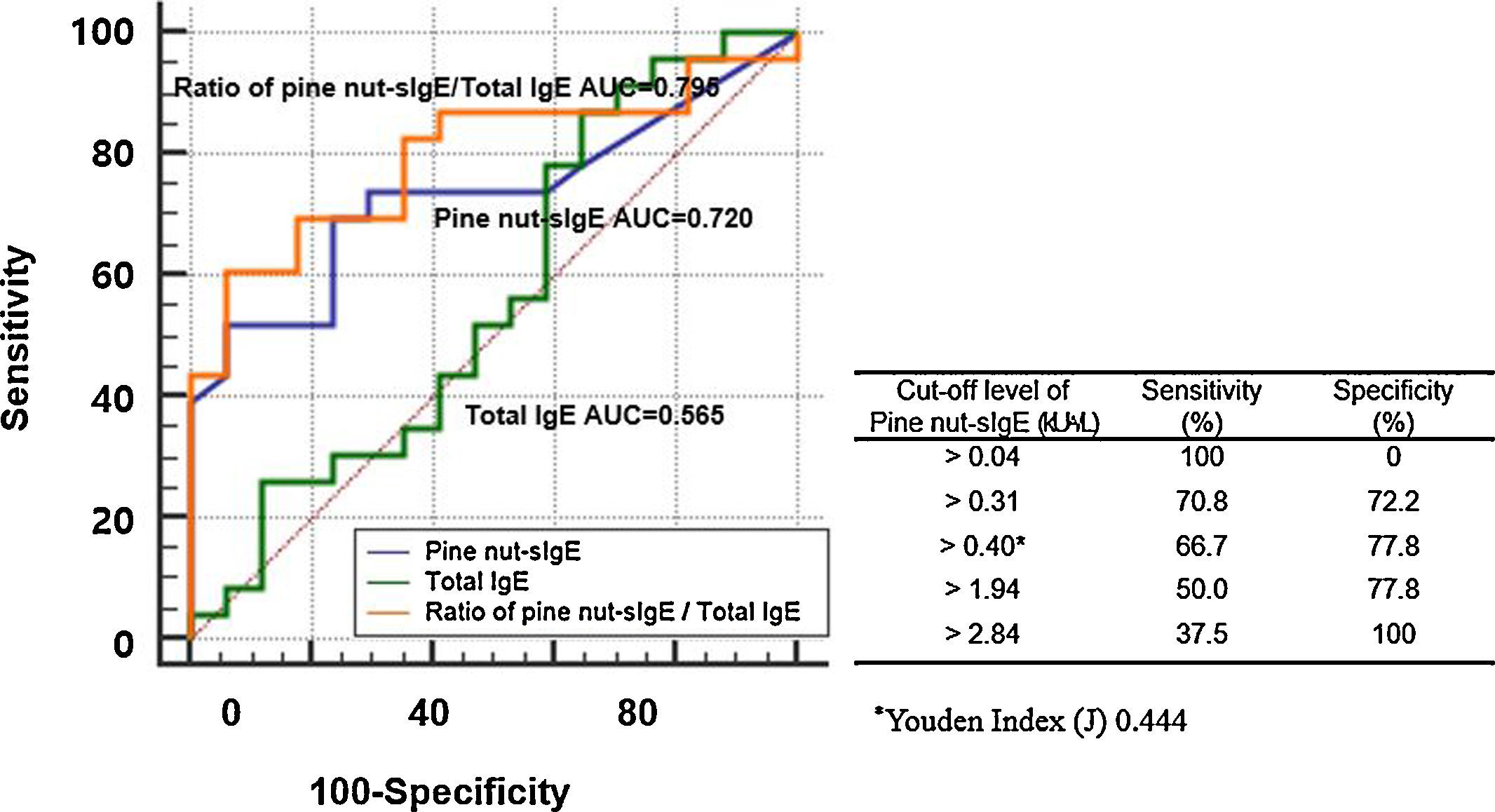
The ROC curves showed the diagnostic performance of total IgE, pine nut-sIgE, and ratio of pine nut-sIgE to total IgE to the allergic group and tolerant group (Fig. 3). The ratio of pine nut-sIgE to total IgE performed the best predictor with an AUC of 0.795, when compared to the total IgE (AUC 0.565) and pine nut-sIgE (AUC 0.734). The optimal cut-off value of pine nut-sIgE to predict the clinical reactivity to pine nuts was >0.40kUA/L with a sensitivity of 66.7% and a specificity of 77.8%, determined using the Youden Index. The positive decision point of pine nut-sIgE with a specificity of 100% to distinguish the allergic group from the tolerant group was 2.84kUA/L.
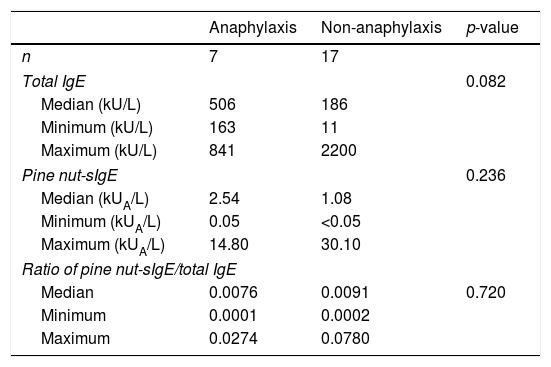
The median levels of total IgE and pine nut-sIgE in the anaphylaxis cases were higher than in the non-anaphylaxis groups. However, there were no significant differences between the anaphylaxis and non-anaphylaxis groups with regards to serum total IgE levels (p=0.082), pine nut-sIgE levels (p=0.236) and ratio of pine nut-sIgE to total IgE (p=0.720) (Table 3).
Levels of total IgE and pine nut-sIgE in anaphylaxis and non-anaphylaxis groups.
| Anaphylaxis | Non-anaphylaxis | p-value | |
|---|---|---|---|
| n | 7 | 17 | |
| Total IgE | 0.082 | ||
| Median (kU/L) | 506 | 186 | |
| Minimum (kU/L) | 163 | 11 | |
| Maximum (kU/L) | 841 | 2200 | |
| Pine nut-sIgE | 0.236 | ||
| Median (kUA/L) | 2.54 | 1.08 | |
| Minimum (kUA/L) | 0.05 | <0.05 | |
| Maximum (kUA/L) | 14.80 | 30.10 | |
| Ratio of pine nut-sIgE/total IgE | |||
| Median | 0.0076 | 0.0091 | 0.720 |
| Minimum | 0.0001 | 0.0002 | |
| Maximum | 0.0274 | 0.0780 | |
Pine nuts are nutrient-rich and are widely consumed raw or are added to soups, cookies, salads, sauces, and traditional rice cakes.7,8 Pine nut allergy is less common when compared to other tree nut allergies. However, it can trigger severe, life-threatening allergic reactions. In Western countries, several reports of pine nut allergy and anaphylaxis have been described,10,13,14,21–24 but studies on allergic reactions to pine nuts are not adequate. This study investigated the clinical features, onset time of allergic reactions and pine nut-sIgE levels in children with immediate hypersensitivity after the ingestion of pine nuts.
The study was performed in 42 children, who had a history of ingesting pine nuts. Twenty-four patients showed immediate hypersensitivity to the nuts, with a median age of three years (the age range of the subjects was one to 11 years), and 58.3% of them were male. The median age in this study was relatively younger than a previous study including five children (age range 5–13 years; female to male ratio 1:5) with severe anaphylactic reactions to pine nut.18 Although the difference did not attain statistical significance due to the small number of the subjects involved, the early onset of reactions to pine nuts probably reflects the early ages of exposure to pine nuts (especially in the form of soup) in Korea.
The cross-reactivity or co-sensitisation between pine nuts and other tree nuts remains unclear. A few previous studies have considered the cross-reactivity between pine nuts and other tree nuts such as almonds, brazil nuts, and hazelnuts.10,13,25 In this study, 87.5% of the subjects in the allergic group reported to have clinical allergies to other tree nuts and peanuts. In this group, walnut allergy was the most common (83.3%), followed by almond and peanut allergy. However, of the 45 reported cases, with a history of allergic reactions following the ingestion of pine nuts, most had severe anaphylactic reactions and were monosensitised to pine nuts.9 The 6kDa,17 17kDa,12,24 50kDa,10,17,18 30kDa22,26 and 44kDa21 proteins were recognised as the relevant allergic components in pine nut allergy patients. Further studies on cross-reaction proteins with other tree nuts have rarely been carried out. In our study, it is not possible to distinguish co-sensitisation or cross-reactivity for now, and therefore in the future, additional studies are necessary to clarify these issues.
Clinical allergic symptoms ranging from cutaneous effects to anaphylaxis have been described in patients with a pine nut allergy.13,21,27–30 In our study, all the patients in the allergic group experienced a cutaneous symptom, and 29.2% experienced anaphylaxis. In a previous study in 10 adolescents and adults who were diagnosed with a clinical allergy to pine nuts, anaphylaxis was reported in 70% of the allergic reactions.17 The percentage of anaphylaxis to pine nuts in this study is lower than reported in other studies. However, the difference was not of statistical significance due to the small number of patients and self-reported convincing history of reaction.
Our finding was that the time interval between the exposure to pine nuts and the onset of symptoms was within 0–120min and 58.8% of the subjects had symptoms within 5min. This result was consistent with an earlier study that stated that IgE-mediated food allergy reactions develop either within minutes or within an hour or two after ingesting the food.31 However, in our study, the percentage of reactions within 5min after ingestion of the pine nuts was higher than in previous studies on anaphylaxis to tree nuts.16
An earlier study reported that the levels of pine nut-sIgE of 10 consecutive patients diagnosed with a clinical allergy to pine nuts ranged from 0.02 to 48kUA/L, with a median level of 2.2kUA/L.17 In a more recent study, the median level of pine nut-sIgE was 5.3kUA/L in five children with a history of immediate allergic reaction following the ingestion of pine nuts.18 However, there have been no studies comparing the levels of pine nut-sIgE in subjects with clinical pine nut allergy and those without. In our study, the median level of pine nut-sIgE and the ratio of pine nut-sIgE/total IgE in the allergic group were significantly higher than those in the tolerant group, with an optimal cut-off level of pine nut-sIgE 0.40kUA/L. At this cut-off level, sensitivity and specificity were 66.7% and 77.8%, respectively. The positive decision point of pine nut-sIgE with a specificity of 100% to distinguish the allergic group from the tolerant group was 2.84kUA/L. These findings in our study may suggest that a diagnosis of clinical pine nut allergy could be established without oral food challenges, if the level of pine nut-sIgE exceeds the cut-off level of 0.40kUA/L with a convincing history of pine nut allergy, or if the level of pine nut-sIgE exceeds 2.84kUA/L even with previously-unknown exposures to pine nuts. The median level of pine nut-sIgE in the anaphylaxis group in this study was higher than that in the non-anaphylaxis group, but the difference was not statistically significant, probably due to the relatively small number of subjects. The median level of pine nut-sIgE in the anaphylaxis group in our study was lower than the levels presented in a recent report of pine nut anaphylaxis in Korean children16 (2.54kUA/L vs 4.61kUA/L).
The major limitation of our study was its retrospective design and that it was conducted at a single centre. Additionally, the diagnosis was based on self-reported history of allergic reactions, and the reaction features were evaluated in a relatively small number of children. However, the results of our study are rather noteworthy considering the fact that there is only one previous study including five patients in paediatric pine nut allergy so far. To our knowledge, this is the first study to identify clinical features and laboratory findings which may help the diagnosis of clinical pine nut allergy. Therefore, our study provides an opportunity to explore the clinical and laboratory parameters that most accurately predict pine nut allergy.
In conclusion, our results show that almost 30% of pine nut-allergic children experience anaphylaxis. The median pine nut-sIgE level was significantly higher in the allergic group compared to the tolerant group, with an optimal cut-off level of 0.40kUA/L. Further studies will be necessary to investigate the prevalence of pine nut allergy, cross-reactivity with other tree nuts, and the relationship between pine nut-sIgE and clinical symptoms, on a large scale.
Conflict of interestThe authors have no conflict of interest to declare.