Early identification of septic patients at risk of mortality is important in their prognosis.
ObjectiveIdentification of septic patients at risk of mortality in Pediatric Intensive Care Units (PICUs) at Cairo University Hospitals, through measuring the levels of certain immunological parameters.
MethodsA hospital-based prospective cohort study was conducted in two PICUs at Cairo University Hospitals; all patients with diagnosis of severe sepsis or septic shock on admission were included. A total of 57 patients were prospectively followed at the selected PICUs and their demographic and clinical data were recorded. Microbiological and immunological workup (at days 1 and 7) was conducted for all patients to detect the causative organism of sepsis and to measure the levels of immunoglobulins (IgG, IgM and IgA), complement factors (C3 and C4), mature lymphocyte subpopulations (CD3+) and natural killer (NK) cells (CD3-CD16+CD56+), respectively.
ResultsMortality rate was 24.6%; the most frequent causes of death were multi-organ dysfunction and refractory shock. PELOD and PRISM III scores were significantly higher among non-survivors. At day 1, non-survivors had significantly higher levels of IgG, C4 and NK cells than survivors. However, from day 1 to day 7, survivors had a progressive increase in most of the immunological parameters (IgG, IgM, C4and CD3+ T lymphocytes). Survival curve analysis revealed the significant predictive ability of NK cells to detect early mortality.
ConclusionMonitoring the levels of cellular and humoral immunological parameters together with assessing PELOD and PRISM III scores can significantly affect prognosis and survival of septic children.
Egypt has made substantial progress in improving child health. According to the reported estimates, child mortality declined from 86 to 21 deaths per 1000 live births between 1990 and 2012, which represents a 75.4% drop. However, challenges remain to maintain the gains made through continued programmatic commitments.1 Therefore, continuous development of precise estimates of the number of deaths of under-five children by cause is crucial. Sepsis is one of the leading causes of death in infants and children worldwide accounting for about 10% of the under-five children mortality, together with pneumonia.2–4 In developing countries, sepsis is responsible for about 60–80% of children mortality, with more than 6 million neonates and children affected annually. Developing countries with large populations of children like Egypt bear the major burden of paediatric sepsis where a combination of environmental and socioeconomic factors like contaminated water, indoor air pollution, crowding and insufficient immunisation and nutrition, allow pathogens to invade and multiply relatively unchecked in the body.4–6 According to Cairo University Hospitals’ records in 2014, sepsis accounted for about 43% of cases admitted to the PICUs.
Despite extensive research about sepsis its pathophysiology remains poorly understood and the available data are controversial.7 Sepsis is a systemic inflammatory disorder that involves complex interactions between complement, coagulation and fibrinolysis systems, and also activated cell elements (macrophages/monocytes, neutrophils, endothelial cells, thrombocytes, lymphocytes) and potentially toxic mediators produced by them.8 In the most severe cases of infection, sepsis is associated with the release of huge amounts of pro-inflammatory cytokines and inflammatory mediators, which lead to devastating effects such as organ dysfunction and even death.9
Despite that, the important role of NK cells during sepsis was identified; however, they can play a beneficial or harmful function in the deleterious inflammatory process, depending on the circumstances and the type of bacterial infection.9 At least two subsets of circulating NK cells have been recognised, the CD3-CD56 dim, which induces enhanced cytotoxicity, and the CD3-CD56 bright subsets which produce greater amounts of cytokines9 including TNF, IFN-γ and GM- CSF, which are key mediators required to regulate the anti-infectious process.10
Early prediction of mortality in septic patients, through assessment of quantitative changes in key humoral and cellular parameters is crucial in prognosis and therapy.11,12 Evaluation of these parameters is easily available in the vast majority of hospitals with critical care units. However, to the researchers’ best knowledge, no data have been reported to fill the gap of knowledge in Egypt regarding this issue. The current study was conducted to identify septic patients at risk of mortality in the PICUs at Cairo University Hospitals, through measuring the levels of immunoglobulins (IgG, IgA and IgM), complement factors (C3 and C4) and lymphocyte subpopulations (T, B and NK cells) and finally setting recommendations based on the study results to improve the septic under-five children's prognosis and survival.
Subjects and methodsStudy design, period and settingThis is a hospital-based prospective cohort study conducted at two PICUs at Cairo University Hospitals for identification of patients at risk of mortality by measuring alterations of key humoral and cellular parameters. The included units were: the PICUs of the Japanese Children Hospital on the ground and 4th floors, which included 28 beds and 14 beds, respectively. These units receive about 1420 patients annually. The study took place over a 9-month period from March 2014 to December 2014.
Working definitions‘Sepsis’ was defined as suspected infection in the presence of two or more systemic inflammatory response syndrome criteria.13 ‘Severe sepsis’ was defined as sepsis plus sepsis-induced organ dysfunction or tissue hypo-perfusion.14 Sepsis-induced hypotension was defined as systolic blood pressure (SBP)<90mmHg, mean arterial pressure<70mmHg or SBP decrease >40mmHg or <2SD below normal for age in the absence of other causes of hypotension. ‘Septic shock’ was defined as hypotension (SBP<90mmHg) despite adequate fluid resuscitation (>1500mL) or the use of vasoactive agents.14
Study sampleAll patients admitted to the PICUs at Cairo University Hospital with severe sepsis or septic shock, were targeted for inclusion. A total of 67 patients were enrolled for the study and were prospectively followed at the selected PICUs from admission till hospital discharge or death. Ten patients were lost to follow up either due to death before the 7th day or due to insufficient samples to complete the laboratory workup, and that made a total of 57 patients enrolled. Severity of illness was assessed on the basis of two scores which are used for routine assessment following patients’ admission to the PICUs: the paediatric logistic organ dysfunction (PELOD) score and the paediatric risk of mortality (PRISM) III score for the first 24h following diagnosis. PELOD score is based on assessing the cardiovascular, neurological, respiratory, renal, haematological and hepatic organ dysfunctions. To calculate the PELOD score, each organ dysfunction received points; with the maximum number of points assigned for an organ being 20 and the maximum PELOD score being 71.15 PRISM III score is based on assessing the SBP, temperature, mental state, heart rate, arterial blood gases, kidney and haematological functions and blood glucose. To calculate the PRISM III score, each parameter received points, with the total score ranging from a minimum of 0 to a maximum of 74. In both scoring systems; higher scores indicate more severe disease.16
Exclusion criteria were: the presence of immunodeficiency or concomitant immunosuppressive therapy, cardiac arrest and patients with malignant tumours.
Study tools and data collectionData were prospectively collected by S.K. and M.R.; microbiological and immunological workup was done by E.M. and N.A.; and data quality was checked by Y.S. and A.S.
- (1)
A pre-tested data collection form was designed to collect and record the following data: the demographic characteristics, state of patients upon admission, associated co-morbidities, primary site of infection, and results of microbiological and immunological workup, PELOD and PRISM III scores and fate of the patient.
- (2)
Microbiological workup: standard cultures in biological samples guided by the presumptive source of the septic focus were performed to assess the presence of bacterial and fungal infections. Blood cultures were carried out by BACTEC blood culture system (Becton Dickinson Diagnostic Instrument Systems, Sparks, MD, USA); sputum and urine cultures were carried out with conventional culture methods (Blood, Chocolate and Mackonki agar systems).
- (3)
Immunological laboratory workup: a serum sample was collected from each patient at days 1 and 7 following admission to the PICUs. IgG, IgM and IgA, C3 and C4 levels in serum were measured by using Single Radial Immunodiffusion Plates (Diffu Plate, Biocientifica, S.A., Argentina). A blood sample was collected in parallel by using tubes containing ethylenediaminetetraacetic acid. Enumeration of mature human T (CD3+), and NK (CD3-CD16+CD56+) lymphocytes by using CYTOMICS FC 500 Flow Cytometer (Beckman coulter, FL, USA). Dyes used were CD16 (FITC), CD56 (PE) and CD3 (ECD). Consistent to previous similar studies, the choice of timing of samples collection at days 1 and 7 was based on the fact that NK cells act both in innate and adaptive immune responses. During the rapid innate immune response, they act during the first few days by killing through apoptosis or antibody-dependent cell mediated cytotoxicity. Later on, NK cells start to proliferate and release IFN-γ and other cytokines such as IL-1 and GM-CSF that lead to activation of cell-mediated immune response of the adaptive immune system and this reaches its maximum starting at one week of infection.17
Collected data were entered and analysed using the Statistical Package for Social Science Software (SPSS) program, version 21.0 IBM. Data were summarised using median and interquartile range for quantitative variables and frequency and percentage for qualitative variables. Comparison between groups was performed using Mann Whitney test for quantitative variables and Chi-square with Fisher's exact test for qualitative variables. Receiver operating characteristics (ROC) curve analysis was performed to explore the discriminate ability of NK cells in predicting mortality.
Kaplan Meier survival function was generated to explore the survival pattern among different groups. P values below 0.05 were considered statistically significant. Scatter Plot diagram was constructed to detect the correlation between PELOD and PRISM III scores and NK cells.
Ethical considerationsApproval of the study protocol was obtained from the Ethical Committee at the Faculty of Medicine, Cairo University. Informed consent was obtained directly from the legal guardian of each patient before data collection and after explanation of the study objectives. All procedures for data collection were treated with confidentiality according to the Helsinki declarations of biomedical ethics.18
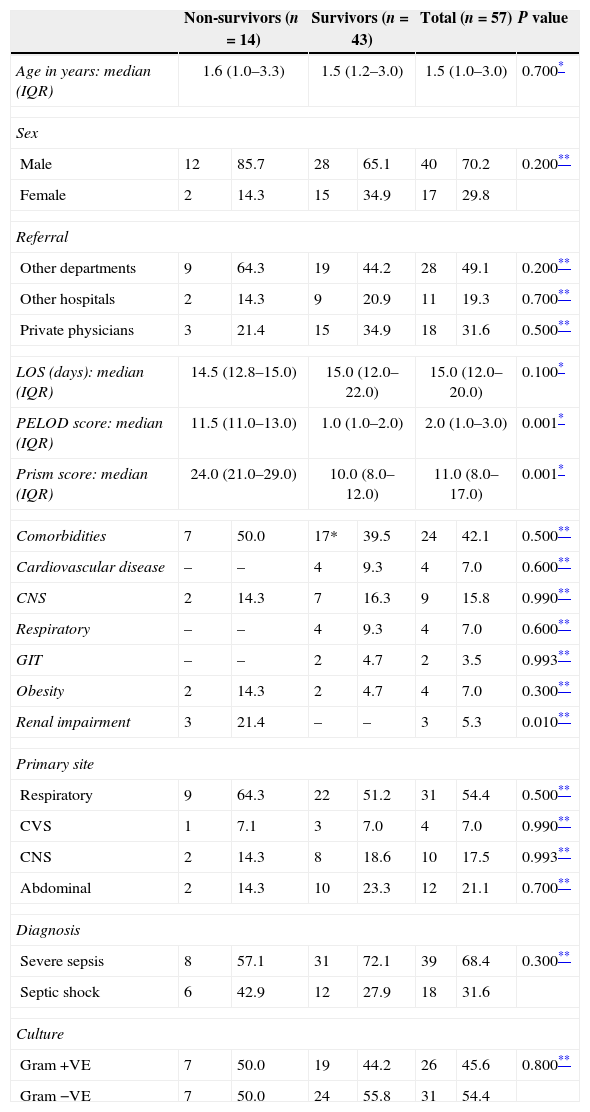
ResultsThe median age of the studied patients was 1.5 years (IQR: 1.0–3.0). The non-survivors were older than survivors. Nearly 64% of non-survivors were referred from other departments inside the Pediatric Hospital (Table 1).
Comparison between non-survivors and survivors groups regarding demographic and clinical characteristics.
| Non-survivors (n=14) | Survivors (n=43) | Total (n=57) | P value | ||||
|---|---|---|---|---|---|---|---|
| Age in years: median (IQR) | 1.6 (1.0–3.3) | 1.5 (1.2–3.0) | 1.5 (1.0–3.0) | 0.700* | |||
| Sex | |||||||
| Male | 12 | 85.7 | 28 | 65.1 | 40 | 70.2 | 0.200** |
| Female | 2 | 14.3 | 15 | 34.9 | 17 | 29.8 | |
| Referral | |||||||
| Other departments | 9 | 64.3 | 19 | 44.2 | 28 | 49.1 | 0.200** |
| Other hospitals | 2 | 14.3 | 9 | 20.9 | 11 | 19.3 | 0.700** |
| Private physicians | 3 | 21.4 | 15 | 34.9 | 18 | 31.6 | 0.500** |
| LOS (days): median (IQR) | 14.5 (12.8–15.0) | 15.0 (12.0–22.0) | 15.0 (12.0–20.0) | 0.100* | |||
| PELOD score: median (IQR) | 11.5 (11.0–13.0) | 1.0 (1.0–2.0) | 2.0 (1.0–3.0) | 0.001* | |||
| Prism score: median (IQR) | 24.0 (21.0–29.0) | 10.0 (8.0–12.0) | 11.0 (8.0–17.0) | 0.001* | |||
| Comorbidities | 7 | 50.0 | 17* | 39.5 | 24 | 42.1 | 0.500** |
| Cardiovascular disease | – | – | 4 | 9.3 | 4 | 7.0 | 0.600** |
| CNS | 2 | 14.3 | 7 | 16.3 | 9 | 15.8 | 0.990** |
| Respiratory | – | – | 4 | 9.3 | 4 | 7.0 | 0.600** |
| GIT | – | – | 2 | 4.7 | 2 | 3.5 | 0.993** |
| Obesity | 2 | 14.3 | 2 | 4.7 | 4 | 7.0 | 0.300** |
| Renal impairment | 3 | 21.4 | – | – | 3 | 5.3 | 0.010** |
| Primary site | |||||||
| Respiratory | 9 | 64.3 | 22 | 51.2 | 31 | 54.4 | 0.500** |
| CVS | 1 | 7.1 | 3 | 7.0 | 4 | 7.0 | 0.990** |
| CNS | 2 | 14.3 | 8 | 18.6 | 10 | 17.5 | 0.993** |
| Abdominal | 2 | 14.3 | 10 | 23.3 | 12 | 21.1 | 0.700** |
| Diagnosis | |||||||
| Severe sepsis | 8 | 57.1 | 31 | 72.1 | 39 | 68.4 | 0.300** |
| Septic shock | 6 | 42.9 | 12 | 27.9 | 18 | 31.6 | |
| Culture | |||||||
| Gram +VE | 7 | 50.0 | 19 | 44.2 | 26 | 45.6 | 0.800** |
| Gram −VE | 7 | 50.0 | 24 | 55.8 | 31 | 54.4 | |
Data expressed as number and % for qualitative variables and median with (interquartile range) for quantitative one. 17*=the total number of survivors with co-morbidity, it is less than the total number of co-morbidities (19) because some patients had >1 co-morbidity.
Severe sepsis and septic shock accounted for about 57% and 43% of diagnoses of non-survivors upon admission to the ICU. The principal suspected source of infection in this group was the lower respiratory tract in nine patients (64.3%).
The primary site of infection was recognised to be respiratory tract infection in 31 patients (54.4%), intra-abdominal in 12 patients (21.1%), CNS in the form of meningitis and encephalitis in 10 patients (17.5%), and CVS presenting as endocarditis and myocarditis in 4 patients (7%). In all patients, infection was confirmed by positive blood cultures, the Gram-negative bacteria was isolated from 31 patients (54.4%) where the most frequently encountered pathogen was Klebsiella in 22 patients (38.5%), followed by Pseudomonas aeruginosa in 7 patients (12.3%) and Escherichia coli in 2 patients (3.5%). Gram-positive bacteria was isolated from 26 patients (45.6%), where the most frequently encountered pathogen was Staphylococcus aureus in 20 patients (38%) followed by Acinetobacter in 6 patients (10.5%).
Severity of illness measured by PRISM scores was significantly higher among the non-survivor group (median (IQR): non-survivors=24.0 (21.0–29.0) and survivors=10.0 (8.0–12.0); P<0.001) and Organ Dysfunction Severity denoted by PELOD score was significantly higher among the non-survivor group (median (IQR): non-survivors=11.5 (11.0–13.0) and survivors=1.0 (1.0–2.0); P<0.001).
At day 1, levels of IgG and C4 were significantly higher among the non-survivor group compared to the survivor one (Table 2). Also, the relative concentrations of NK cells (CD3-CD56/16+ %) in blood (percentage of total lymphocytes) were significantly higher in the group of non-survivors (median (IQR): non-survivors=16.2 (12.1–19.5) compared to survivors=3.6 (1.4–8.6); P<0.001).
Comparison of immunological parameters between study groups based upon timing of investigation.
| Immunological parameters | Laboratory investigations | |||||
|---|---|---|---|---|---|---|
| At day 1 | At day 7 | |||||
| Non-survivors (n=14) | Survivors (n=43) | P value | Non-survivors (n=14) | Survivors (n=43) | P value | |
| IgG (mg/dl) | 1406.0 (1287–1739.25) | 604.0 (383–1116) | 0.001 | 317.5 (185–425) | 900.0 (604–1592) | 0.001 |
| IgA (mg/dl) | 71.5 (39–176.8) | 46.0 (21–83) | 0.100 | 68.0 (23–120.3) | 46.0 (23–125) | 0.900 |
| IgM (mg/dl) | 93.0 (60–136.5) | 95.0 (60–174) | 0.900 | 101.0 (17–112) | 142.0 (106–174) | 0.005 |
| C3 (mg/dl) | 103.0 (82.5–155) | 86.0 (70–135) | 0.100 | 84.0 (54–120.8) | 109.0 (64–148) | 0.200 |
| C4 (mg/dl) | 21.5 (11.5–34.3) | 12.0 (6–19) | 0.010 | 7.0 (5–22.8) | 19.0 (14–38) | 0.006 |
| CD3+ T (cells/mm3) | 58.5 (51.6–66.7) | 62.7 (41.1–68.9) | 0.900 | 41.2 (31.4–63.8) | 60.9 (51.4–72) | 0.040 |
| CD3-CD56/16+ % | 16.2 (12.1–19.5) | 3.6 (1.4–8.6) | 0.001 | 3.2 (2.3–7.2) | 3.1 (1.7–5) | 0.200 |
Data in the table are expressed in medians (interquartile ranges), Mann–Whitney test of significance was used for comparison.
At day 7, levels of IgG were significantly lower among non-survivor group (median (IQR): non-survivors=317.5 (185–425) and survivors=900.0 (604–1592); P<0.001). Also levels of IgM, CD3+ T lymphocytes and C4 were significantly lower among the non-survivor group compared to the survivor group (Table 2).
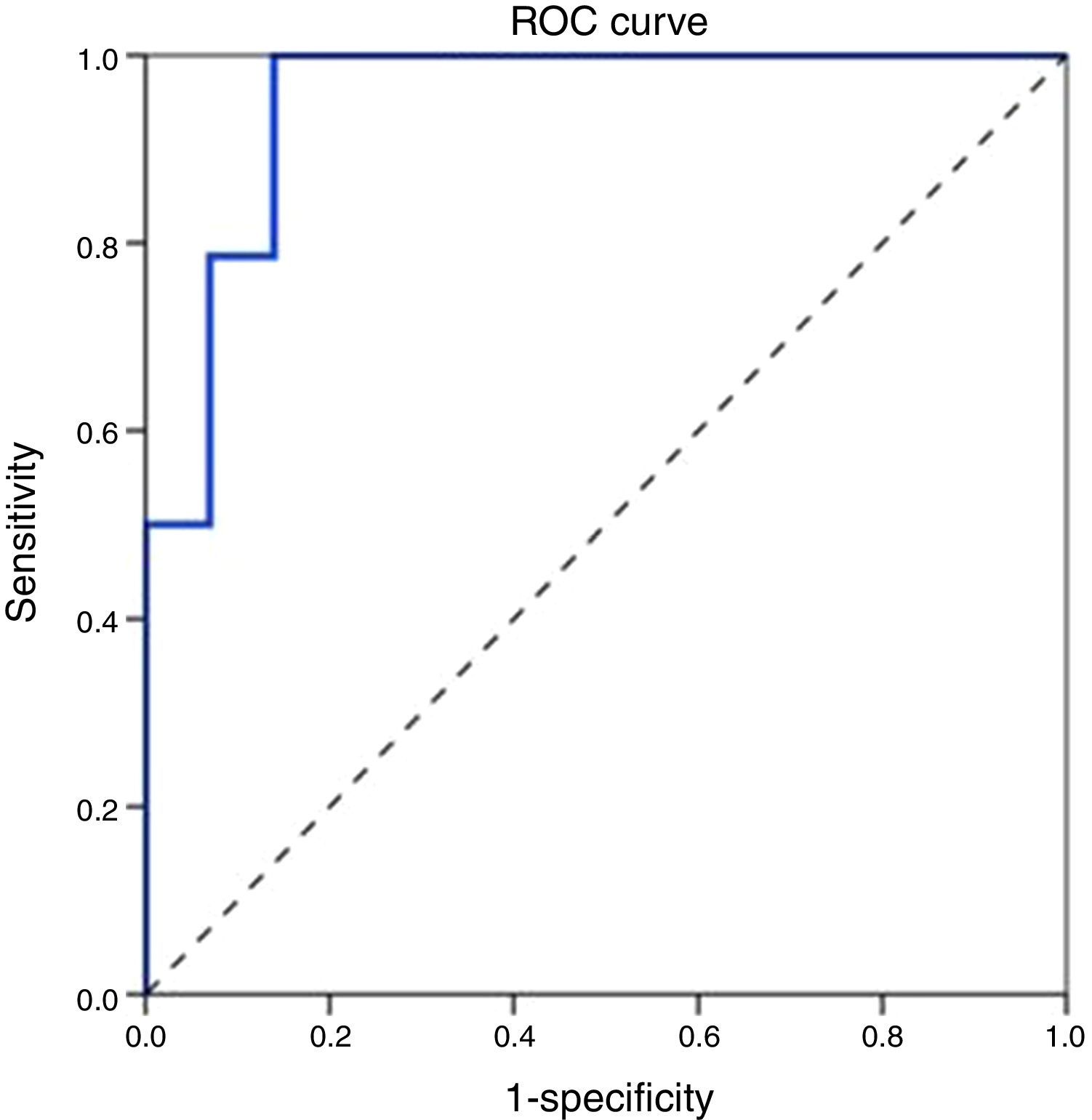
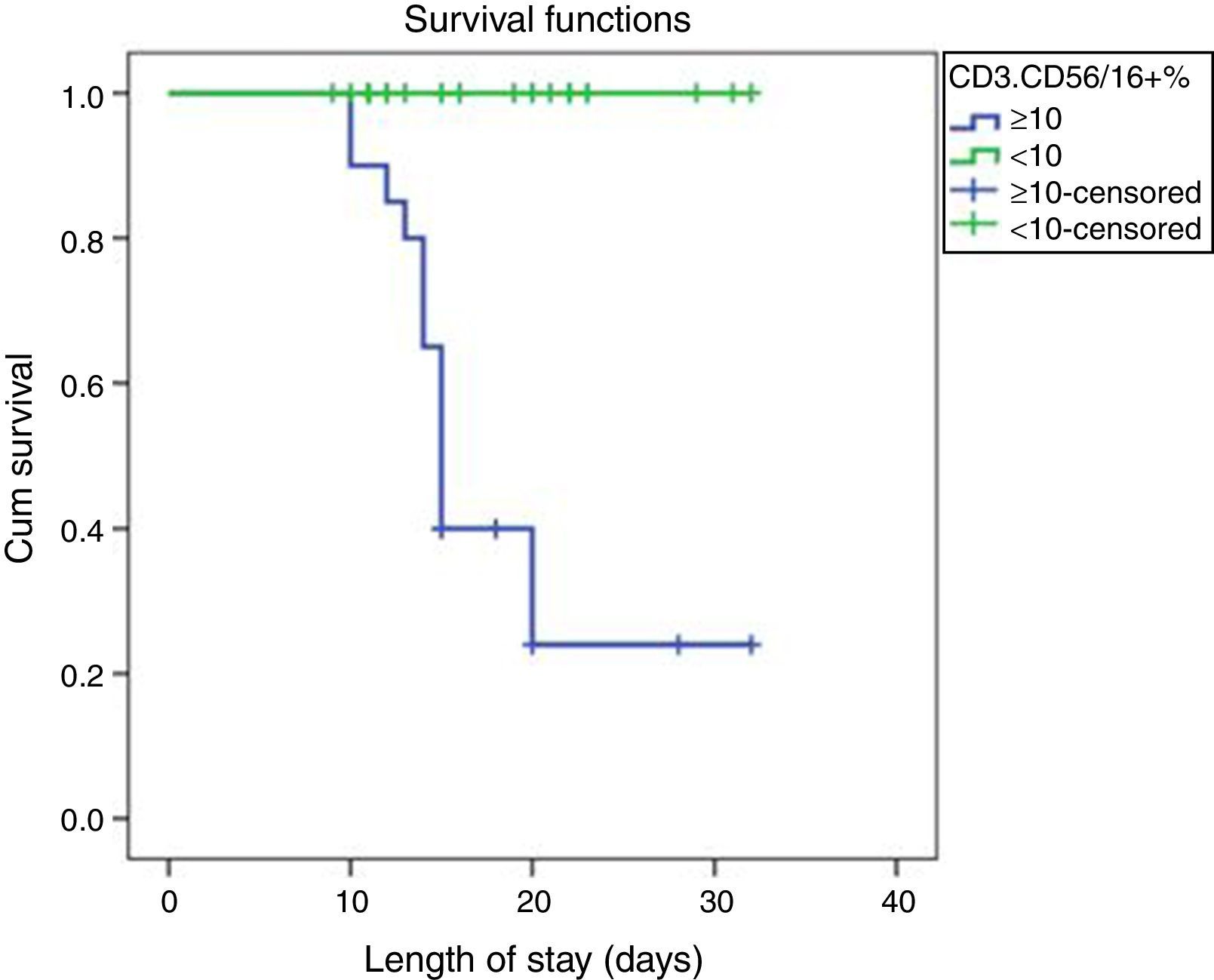
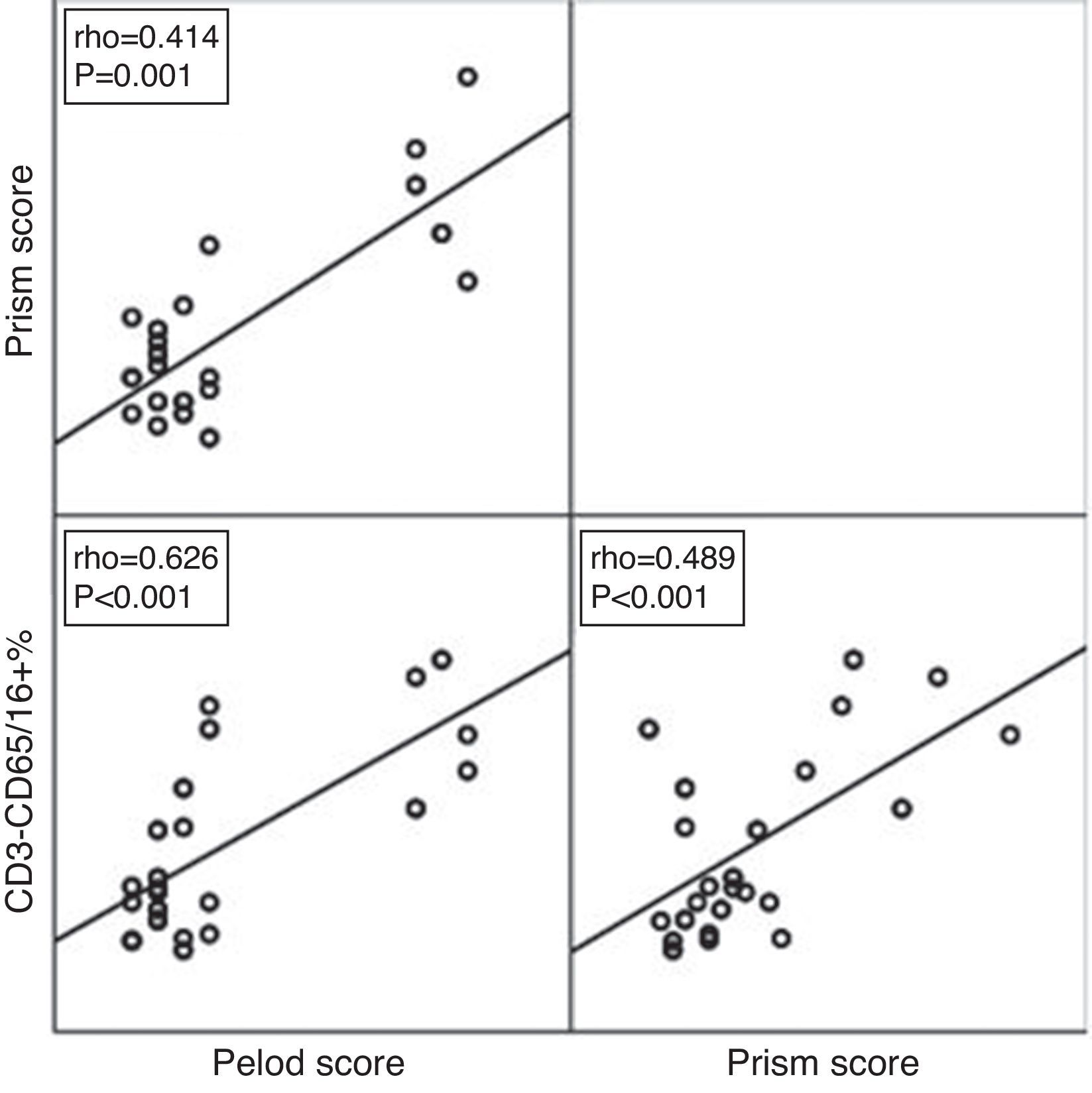
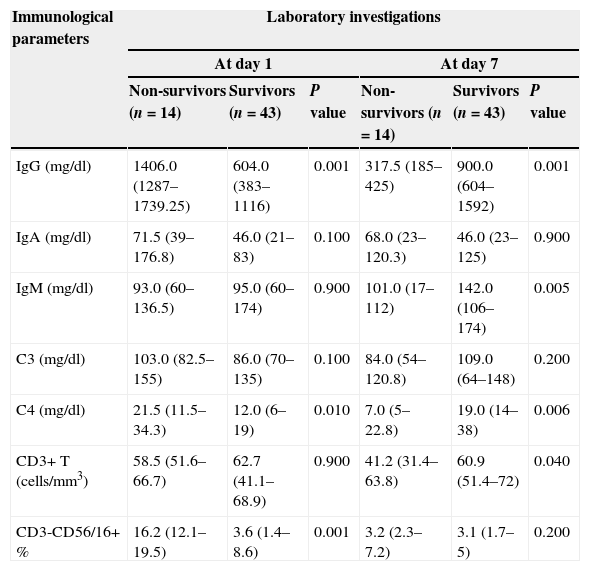
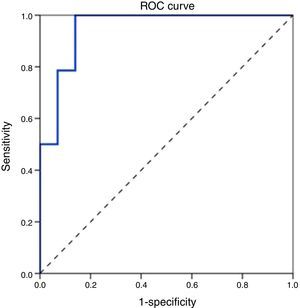
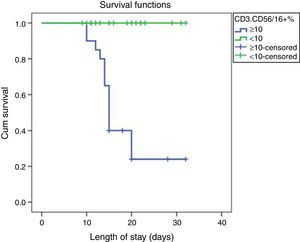
ROC curve analysis revealed the significant predictive ability (P<0.001) of the relative concentration of NK cell (CD3-CD56/16+ %) at day 1 to detect mortality with AUC (95% CI) 0.950 (0.889–1.0); the most suitable cut-off point was ≥10 with 100% sensitivity, 86.0% specificity, 70.0% PPV, 100.0% NPV and 89.5% accuracy (Fig. 1). Survival analysis illustrated that mortality pattern among patients having relative concentration of NK cells (CD3-CD56/16+ %) ≥10 at day 1 was significantly higher (Log rank P<0.001) compared to those having <10 level (Fig. 2). Scatter plot diagram showed a positive, moderate and significant linear relationship between NK cells, PELOD and PRISM scores, and between PELOD and PRISM scores (Fig. 3).
The current study demonstrated the prognostic role of immunoglobulins, complement factors, lymphocyte subpopulations, especially NK cells, in patients with severe sepsis and septic shock. Severe sepsis and septic shock are top priority causes of death in infants and children especially in developing countries4; therefore early prediction of poor prognosis among septic patients is decisive for the early initiation of therapy and consequently improving patients’ outcome.12 Several studies support the evidence that the final outcome of patients with severe sepsis and septic shock is influenced by quantitative changes in the systemic levels of a number of key host immunity elements.11,19
In the current study, there was no statistically significant difference between survivors and non-survivors regarding the cause of admission to the ICU; whether it was severe sepsis or septic shock (P=0.3). However, in a study conducted in Spain to quantify the levels of NK cells in patients with serve sepsis,11 there was a statistically significant difference between patients who died and those who survived regarding the admission cause (P<0.001), with septic shock being the most frequent cause among non-survivors. Respiratory tract infection was identified as the primary site of infection in more than half of the study population and this goes in accordance with another study conducted by Andaluz-Ojeda et al. (2011) who found the principal suspected source of infection to be the lower respiratory tract.11 The presence of a microorganism was documented in all cases, with a nearly balanced proportion of Gram-positive and Gram-negative bacteria. The most frequently isolated Gram-negative bacteria were Klebsiella, P. aeruginosa and E. coli, whereas, S. aureus and Acinetobacter were the most frequently isolated Gram-positive bacteria. However, in a study conducted in the Medical school of Athens University to detect early changes of NK cells in patients with Gram-negative sepsis, the most frequently encountered Gram-negative bacteria was E. coli followed by Klebsiella pneumonia and Acinetobacter.19
One of the strategies to improve medical care in intensive care units is to implement prognostic scoring systems to assess illness’ severity, triage planning, therapeutic options and clinical course and outcome. They are also used as tools for quality control measures and costs/benefit analysis. In the present study, the severity of patients’ illnesses assessed by PRISM and PELOD scores was significantly higher (P<0.001) among the patients who died compared to those who survived. Similarly, in a study conducted by Leteurtre et al., PELOD score was significantly higher in non-survivors (mean 31.0 [SE 1.2]) than survivors (9.4 [0.2]; P<0.0001).15 In another study conducted to evaluate the association of the PRISM III score with infant outcomes in the PICU, the PRISM III score had good sensitivity and specificity to predict mortality.16
In this study, levels of IgG and C4 in the first 24h following admission to the PICU were significantly higher among children with septic shock who finally died, when compared to those who survived. However, Andaluz-Ojeda et al. (2011)11 and Nakae et al. (1996)20 found that survivors had significantly higher levels of IgG and C4 at day 1 compared with patients who died. Moreover, Taccone et al. (2009) found that low concentrations of IgG in patients with community-acquired septic shock were associated with increased risk of acute lung injury/or acute respiratory distress and high mortality.21 The discrepancy between the results of the present study and others regarding the levels of immune parameters could be attributed to the study population: whereas the present study focused on children, the others focused on adults. Therefore, studies with larger numbers of patients are necessary to confirm the trend of IgG and C4 in this disease.
After one week of ICU admission, significantly higher levels of IgG, IgM, C4 and CD3+T were detected among survivors compared to non-survivors. The significantly lower levels of complement and other immune parameters in the patients who died could be attributed to their consumption over time. In agreement with the results of the current study, Wiśniewska-Ligier and colleagues (2002)8 emphasised that maintenance of T lymphocytes declining and lack of CD4 number stabilisation during10 days in septic patients were associated with disease severity and predict an unfavourable outcome of the disease.
NK cells have complex biological functions; they could have a beneficial role in fighting infection by sharing antigen presenting cells and T cells in the cellular response against pathogens.22 Moreover, NK cells serve as a bridge between innate and acquired immunity and thus should play a major role in the early stages of sepsis.23 However, they could have a deleterious effect by producing powerful inflammatory mediators and thus promoting tissue damage and interfering with the adaptive immune response against the causative microbial agents.22 The harmful effect of NK cells has been reported in different animal models after polymicrobial intra-abdominal sepsis,24E. coli intra-peritoneal injection,25Streptococcus pyogenes intravenous injection26 and Ehrlichia-induced toxic shock-like syndrome.27 As regards the role of NK cells in human sepsis, the available data derived from patients in the ICU are few and controversial.28 Although Giamarellos-Bourboulis et al. (2006)19 found improved survival in patients with severe Gram-negative sepsis and high NK counts, de Palbo and colleagues (2012) found that patients with septic shock who did not survive had increased NK cell counts at ICU admission.17 In the current study, the results highlighted significant higher levels of NK cells (P<0.001) among the non-survivor group compared to survivor group in the first 24h following admission to the ICU. A recent prospective study including more than 500 patients with sepsis suggested that the discrepancies involving the number and/or function of NK cells are probably due to the heterogeneity of patients as regards severity of the disease (severe sepsis or septic shock) or type of pathogens (Gram-positive versus Gram-negative bacteria).28
Significant predictive ability of the relative concentration NK cell (CD3-CD56/16+ %) at day 1 was illustrated by ROC curve analysis, where the most suitable cut-off point was mentioned to be ≥10% with 100% sensitivity and 86.0% specificity. Analysis of survival curves in the present study provided evidence that levels of NK cells (CD3-CD56/16+ %) ≥10% at day 1 were concomitant with mortality, this coincides with Andaluz-Ojeda et al. (2011)11 who highlighted the prognostic role of NK cells (CD3-CD16+CD56+ lymphocytes) in severe sepsis and septic shock, proving a direct association of early high blood counts of these cells with mortality.
Study limitationsThe current study did not include a subset of CD16-NK cells which account for a minor percentage of the total NK cells (5–10%). Generalisability of the findings of this study is hindered by the relatively small sample size and difficulty in obtaining blood samples due to the bad general condition of patients.
ConclusionThe results of this study demonstrated the prognostic role of certain immunological parameters, namely IgG, C4 and NK cells beside the well-established PELOD and PRISM III scores in severe sepsis and septic shock. The study revealed a significant association between early high blood counts of these cells and mortality. Therefore, it is of great importance to monitor the levels of these cells early in sepsis to identify patients at risk of mortality and to initiate early therapy which could markedly affect their prognosis and survival. Further studies with big sample size are needed to confirm the precise role of immunological parameters in the pathogenesis of sepsis in children.
Ethical disclosuresProtection of human subjectsThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients mentioned in the article. The author for correspondence is in possession of this document.
Conflict of interestThere are no conflicts of interest.
The authors are thankful to the Chairmen of the Pediatric and Clinical pathology Departments at Cairo University Hospitals for conducting this study. The authors also thank all the nursing staff in the PICUs at Cairo university Hospitals who helped the researchers during the data collection process. The research team also thanks all the patients’ relatives.