Food allergy results from an atypical response of the mucosal immune system to orally consumed allergens. Antacid medication inhibits the digestion of dietary proteins and causes food allergy. A decrease of the gastric pH might enhance the function of digestion and reduce the risk of food allergy.
ObjectiveTo test a possible decrease in the allergenicity of powerful food allergens (egg, chicken, lentils) with the addition of vinegar during the cooking process.
MethodsWe included seven patients who suffered from anaphylaxis due to egg, chicken and lentils. We added vinegar to egg, chicken and lentil processed extracts used for skin prick tests (SPT) and compared the wheal areas obtained with the same extracts sources and the same way but without vinegar addition. Immunodetection was performed with the different processed extracts and patients’ sera. Only one patient consented food challenge with vinegar-marinated-chicken.
ResultsWheal areas were significantly minor with the food extract with vinegar. Inmunodetection showed a decrease of the response with vinegar processed extracts.
ConclusionsVinegar addition during the cooking process may decrease lentil and chicken allergenicity.
Gastric digestion substantially decreases the potential of food proteins to bind IgE, which increases the threshold dose of allergens required to elicit symptoms in patients with food allergy1. The ability of food proteins to remain structurally intact after gastrointestinal digestion increases their potential to be allergens1. Impairment of gastrointestinal digestion with antacid medication represents a risk factor for food allergy induction1,2. By increasing the gastric pH, they interfere substantially with the digestive function of the stomach, leading to persistence of labile food proteins during gastric transit. On the contrary, a decrease in gastric pH perhaps might improve food digestibility and decrease their allergenicity.
Skin prick testing is a rapid screening method for IgE-mediated food hypersensitivity. Positive skin tests may be helpful especially when a clear-cut history of food reactivity is present3.
Most studies have used raw foods as starting material for skin prick testing, thus not considering potential changes in allergenic properties induced by the cooking process (dressing, marination) and other industrial processing to produce derived foodtuffs. Recent studies showed that the processing of wheat flour to obtain derived foodstuffs seems to decrease strongly the IgE binding capacity of the major salt-soluble wheat proteins. Moreover, simulated gastric fluid digestion could further inactivate some heat-resistant potential allergens4.
Vinegar is a wine-derived product widely used to improve food digestibility but its potential capacity to change the allergenicity of food has not been measured.
A young patient, who suffered from severe sensitisation to egg and chicken (anaphylaxis), maintained that she tolerated chicken marinated previously in vinegar (a usual practise in the Mediterranean area, where it is commonly added to legumes dishes). This prompted us to perform a study in patients who had suffered from anaphylaxis due to ingestion of powerful food antigens such as egg, chicken and lentils, using extracts of previously cooked food like those usually consumed by our patients.
Patients and methodsWe included seven patients who had suffered from anaphylaxis due to egg, chicken and lentils. These patients were selected from the database of food allergic patients (Hospital Rio Hortega, Valladolid, Spain). They had all been diagnosed by prick tests (SPT), specific IgE, and double-blind placebo-controlled food oral challenge to the food implicated.
Specific IgE antibodies to egg and egg allergens, chicken, lentil and other legumes and a battery of foods were determined using the Immuno-CAP 100 System (Phadia AB, Uppsala, Sweden). Demographic and clinical data of selected patients can be seen in Table 1.
Demographic and clinical data of selected patients
| Patient n. | Age/sex | Reported symptoms | Specific IgE | SPT | ||||||
| Lentil (kU/l) | Other foods (sIgE>0.35kU/l) | Lentil (mm2) | Lentil +vinegar (mm2) | Soy (mm2) | Green pea (mm2) | Peanut(mm2) | ||||
| 8742 | 3/M | Ad, U | 3.08 | egg, tuna | 28.26 | 8.2 | 28 | 78.5 | 32 | |
| 9750 | 2/M | Ad, An | 7.35 | egg, cod, | 78 | 26.6 | 19 | 78.5 | 68 | |
| 8949 | 3/F | As, An | 22.3 | ba, ri, co, | 62 | 15.6 | 65 | 42 | 48 | |
| 8816 | 3/F | As, An | 1.61 | ba, ry, co | 28,5 | 12.8 | 32 | 28 | 28 | |
| 8805 | 25/M | An, U | 0.82 | ba, ry, | 38 | 10.6 | 28 | 22 | 22 | |
| 6306 | 46/M | An | 27.01 | ba, ry | 69 | 19.9 | 60 | 62 | 62 | |
| 10168 | 30/F | An | 17.2 | ba, ry, soy | 62 | 12 | 43 | 12 | 36 | |
| Egg white | E. yolk, chick | Egg white | Egg white+vinegar | Chicken | Chicken+vinegar | |||||
| 10165 | 21/F | An | >100 | >100 | 92 | 142 | 41 | 99 | 15 | |
| 10162 | 9/M | An | 17.8 | 15 | 19.21 | 42 | 12 | 30 | 10 | |
As, asthma; An, anaphylaxis; U, urticaria, Atopic dermatitis; ba, barley; ry, rye; ri, rice; co, corn.
Informed consent was obtained from each patient and ethical approval from the Ethical Committee of the Hospital Rio Hortega.
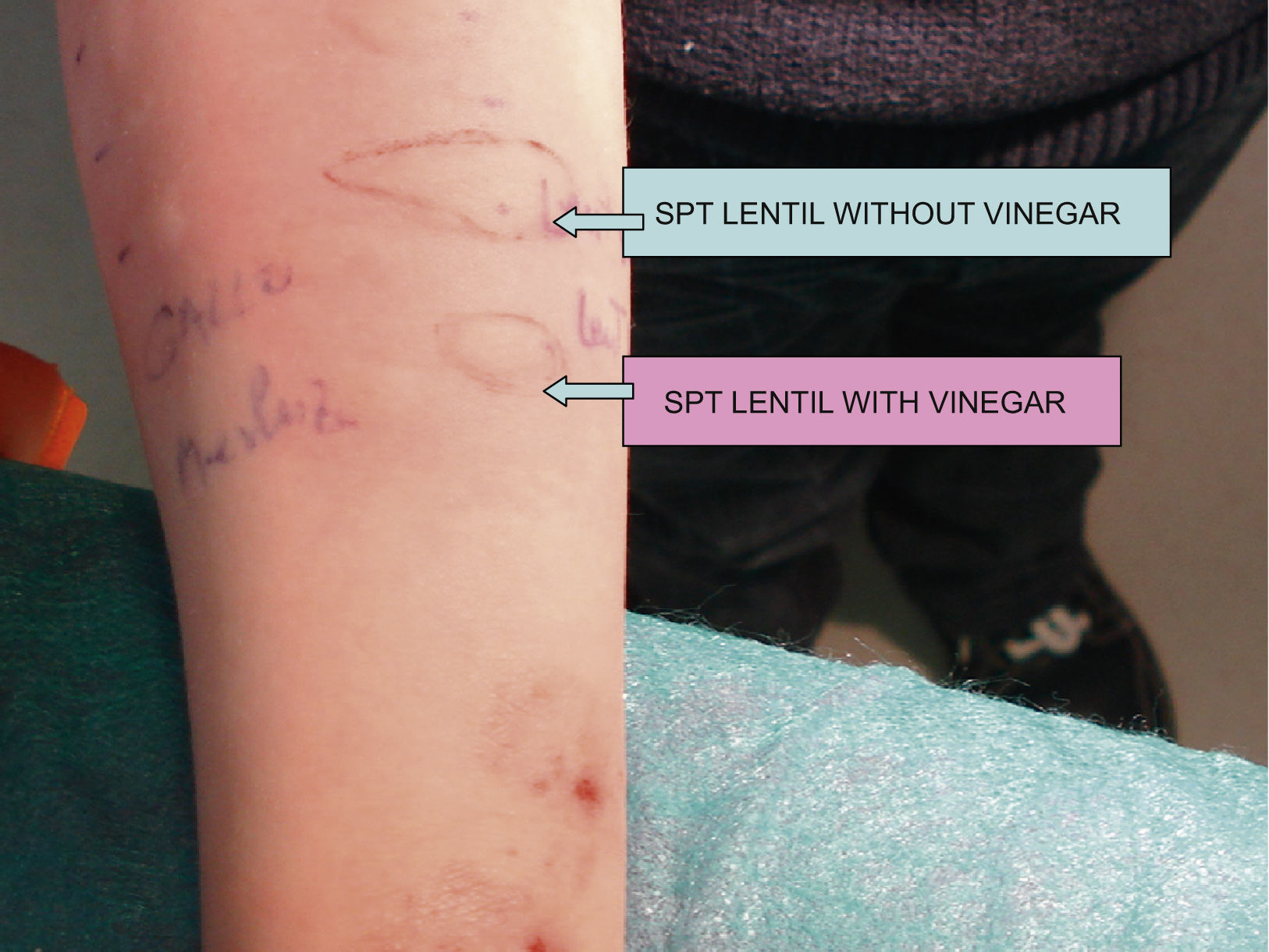
Skin prick testsSkin prick tests were performed with a commercial panel of food allergenic extracts (Bial-Aristegui Laboratories, Bilbao, Spain). In the cases of egg allergy we also tested egg allergens, (ovoalbumin, ovomucoid, connalbumin, chicken albumin) and in lentil also the lentil pets (Bruchus lentis). Additionally we boiled 100g of chicken meat during 30min in water and performed two extracts 1/10 w/v from the same preparation (3.4mg of protein/ml). In one of the extracts we added 5ml of vinegar from white wine. The pH of these extracts (measured with Acilit®, Merck, German) was 4.2 in the case of lentil and 4.5 in the case of egg. These extracts were tested in controls (10 atopic and 10 non-atopic) in order to exclude irritative responses to vinegar.
In the same way we boiled 200g of lentils with and without vinegar. The two extracts had 2.8mg of protein/ml.
SPT were done with all the extracts and wheals measured after 15min and outlined by tracing on adhesive paper. Saline solution 0.9% was used as negative and histamine (10mg/ml) as positive control. A wheal area greater than 7mm2 was considered positive.
Oral challenge testsOnly one patient (sensitised to chicken and egg) consented to double-blind placebo-controlled food challenge with vinegar-marinated-chicken. The possibility of oral challenge in all patients was difficult because of the severity of their symptoms.
In vitro testsIgE-Immunoblots were performed with and without vinegar added to the allergenic sources (lentils and chicken) under non-reduced conditions with a pool of sera from the patients, diluted 1:10.
ResultsMeasurements of SPT areas and IgE levels can be seen in Table 1 and Figure 1.
Double-blind placebo controlled challenge was negative with 10g of chicken with vinegar and positive (urticaria-angioedema) after eating 2g of chicken meat without vinegar.
Immunoblots revealed less specific IgE binding to the lentils and chicken with vinegar than to the lentils and chicken without vinegar. The vinegar added to lentil proteins reduced the IgE-binding to the proteins between 30 and 90kDa with respect to lentils without vinegar. No bands were recognised by the pool patients’ sera to chicken with vinegar with respect to chicken without vinegar Figure 2).
DiscussionDigestion assays with simulated gastric fluid have been performed in order to characterise food allergens. So, true food allergens (digestion-resistant class 1) trigger direct oral sensitisation, and labile class 2 allergens, that are heat labile and susceptible to digestive processes, are non-sensitising elicitors. Infants have augmented vulnerability because of decreased acid production and reduced pancreatic and intestinal enzymatic activity5. Consequently, they have an increased absorption of intact food proteins, which may cause stimulation of the immune system and generation of IgE antibodies.
Alterations in the gastric milieu are frequently experienced in the very young and in the elderly or as a result of gastrointestinal pathologies. Additionally, acid-suppression medications are frequently used for treatment of dyspeptic disorders. By increasing the gastric pH, they interfere substantially with the digestive function of the stomach, leading to persistence of labile food protein during gastric transit. Indeed, both murine and human studies reveal that antacid medications increase the risk of food allergy induction1,2. Gastric digestion substantially decreases the potential of food proteins to bind IgE, which increases the threshold dose of allergens required to elicit symptoms in patients with food allergy.
Vinegar added in processed food makes the pH more acid and may help the gastric labour. In popular medicine its digestive effects have been well-known for a long time. It is also known that at low pH levels, the acidic amino acid residues in the active digestive pepsin moieties undergo protonation. The electrostatic interactions between the N-terminal prosegment and the active pepsin are disrupted, which initiates a conformational change in both the prosegment and the active enzyme portion. Thus, the removal of the prosegment results in conversion into the enzymatically active form of pepsin6. Whereas at a pH above 5.0, limited pepsin is activated, the rate of active enzyme increases with decreasing gastric pH. An acidic milieu is required for the proteolitic activity of pepsins, with an activity optimum between pH 1.8 and 3.2. Here we have demonstrated a significant decrease both in skin response and ‘in vitro’ response to the same allergens after vinegar addition. Taking into account not only the rising number of food-allergic patients but also the severity of food-induced adverse reactions, a simple and reliable method like the addition of this popular food dressing may be useful in certain cases of food hypersensitivity.
Conflict of interestThe authors have no conflict of interest to declare.