Background/Aims: Patients with primary Sjögren’s syndrome may present liver involvement. Our goals were to establish the prevalence of abnormal hepatic biochemistries and clinical liver disease in patients with primary Sjögren’s syndrome and correlate their presence with other clinical and laboratory features. Methods: Ninety-five patients with diagnosis of primary Sjögren’s syndrome were studied. Data on gender, age, clinical features, liver biochemistries, tests of inflammation and autoimmunity, and concomitant diseases were collected. Results: Forty-two patients (44%) had abnormal hepatic biochemistries, and of these 19 patients (20%) had clinical liver disease. Patients with abnormal hepatic biochemistries had higher frequency of autoimmune hypotiroidism, arthritis, vasculitis, Raynaud’s phenomenon, higher sedimentation rate, and higher frequency of antinuclear and antimitochondrial antibodies than patients with normal liver biochemistries (P < 0.05 for each). Patients with clinical liver disease had higher frequency of arthritis, vasculitis, and higher frequency of antimitochondrial antibodies than patients without clinical liver disease (P < 0.05 for each). Twenty-one patients had diagnosis of a specific liver disease, such as hepatitis C virus infection (n = 11), autoimmune hepatitis (n = 2), primary biliary cirrhosis (n = 5), nonalcoholic fatty liver disease (n = 2), and hepatitis B virus infection (n = 1). In half of patients with liver involvement a definitive cause could not be identified. Conclusion: Liver involvement is frequently found in patients with primary Sjögren’s syndrome, and its presence is associated with clinical features of systemic disease, and markers of autoimmunity and inflammation. There may be a subgroup of patients with liver involvement secondary to primary Sjögren’s syndrome.
Primary Sjögren’s syndrome (primary SS) consists of the association of keratoconjunctivitis sicca, xerostomia, and swelling of the salivary glands in the absence of other rheumatologic condition.1 Although primary SS is an autoimmune exocrinopathy, the involvement of the liver has been reported.2-8 Liver diseases that have been associate with primary SS include primary biliary cirrhosis (PBC),9,10 autoimmune cholangitis11-13 autoimmune hepatitis (AIH),6,7,14 hepatitis B and C virus infection (HBV, HCV),15-25 primary sclerosing cholangitis,26 and nodular regenerative hyperplasia.27 Moreover, the pathological examinations of labial salivary glands in patients with different types of liver cirrhosis have been shown the presence of chronic lymphocytic sialadenitis in a considerable proportion of cases.20-24,26
In this retrospective analysis of prospectively acquired data, we evaluate the prevalence of abnormal hepatic biochemistries and clinical liver disease in patients with primary SS, and we correlate the presence of abnormal hepatic biochemistries and clinical liver disease with other systemic features and markers of autoimmunity and inflammation.
Patients and methodsStudy populationOne hundred fifty-six medical clinical charts of patients with diagnosis of primary SS attended in our Institution between January 1985 and December 2005 were reviewed. These patients were captured from the Rheumatology outpatient clinic, Rheumatology consults records, different out-patient services, and through our Institutional Biostatistics Database. Of the 156 patients with diagnosis of Sjögren syndrome evaluated, we included only 95 patients who satisfied the European Epidemiology Center Criteria (EECC) for primary SS.28,29 Since the EECC were developed in 1993, those patients attended before this year were evaluated retrospectively with their clinical charts to establish if they satisfied these criteria. We also excluded patients with evidence of other rheumatologic diseases, since this would make them to be considered as secondary Sjögren’s syndrome. Fifty patients (53%) had a salivary gland biopsy performed and the histopathological study confirmed the diagnosis of Sjögren’s syndrome. All patients had objective evidence of primary SS, including keratoconjunctivitis sicca, positive labial salivary gland biopsy, Schirmer test, autoantibodies (anti-Ro/SS-A, anti-LA/SS-B) and/or salivary gland hypofunction evidence by sialography or gammagram.
Ninety patients were women (95%), the mean age at presentation was 55 ± 2 years (median 56 years, range 19-84 years), and the mean length for duration of the disease was 4 ± 1 years (median 1 year, range 1-25 years).
Laboratory and other studies assessmentLaboratory studies reviewed were the serum levels of aspartate aminotransferase (AST), alanine aminotrans-ferase (ALT), alkaline phosphatase (AP), bilirubins, albumin, prothrombin time, antinuclear antibodies (ANA), rheumatoid factor (RF), antimitochondrial antibodies (AMA), anti-smooth muscle antibodies (ASMA), anti-Ro/ SS-A and anti-La/SS-B antibodies, viral hepatitis markers (B, C), liver ultrasound, and results of liver biopsies.
Markers of viral hepatitis including hepatitis B surface antigen (Abbott Laboratories, North Chicago, IL) and antibodies to hepatitis C virus (Abbott Laboratories, North Chicago, IL) by second-generation ELISA were done in 67 patients (71%), and all patients with positive serology for HCV had a confirmatory polymerase chain reaction test.
ANA were determinated in 60 patients (63%) by indirect immunofluorescence on HEp-2 cells (Binding site Ltd, Birmingham, UK) as described elsewhere,30 ASMA were determined in 42 patients (44%) by indirect immunofluorescence on murine tissue sections (Binding site Ltd, Birmingham, UK), as described somewhere else,31 AMA were determined in 42 patients (44%) by ELISA (Orgentec, Mainz, Germany), RF was determined in 32 patients (34%) by laser nephelometry (Beckman, Coulter) as described elsewhere,32and anti-Ro/SS-A and anti-La/ SS-B were determined by ELISA (Binding site Ltd, Birmingham, UK) in 81 patients (85%). Liver ultrasound was done in 29 patients (31%), and liver biopsies were performed in 15 patients (16%). Risk factors for liver disease that were investigated include blood transfusions, surgeries, and history of alcohol and hepatotoxic drugs consumption.
Operative definitionsAbnormal hepatic biochemistries were considered present when elevated abnormal levels of AST (nl, • • 31 U/L) and ALT (•• 29 U/L), or AP (nl, ••251 U/L) and bilirubin (nl, ••1.1 mg/dL) were detected in at least two determinations.
Clinical liver disease was considered when the following symptoms and signs were present in association with abnormal hepatic biochemistries: jaundice, ascites, spider angiomata and palmar erythema, gynecomastia and reduction in testicular size, evidence of collateral circulation, asterixis, hepatomegaly, or other signs of portal hypertension including esophageal or gastric varices and splenomegaly.
Systemic features evaluated include the presence of autoimmune hypotiroidism, Raynaud’s phenomenon, vasculitis, arthritis, pancreatitis, peripheral neuropathy, and glomerulopathy.
Autoimmunity and inflammation markers evaluated were ANA, ASMA, AMA, RF, anti-Ro/SS-A, anti-La/SS-B, and globular sedimentation rate. A serum titer of 1:40 or higher by indirect immunofluorescence was considered positive for ANA; a serum titer of 1:160 or higher was considered positive for ASMA by indirect immunofluorescence; a serum titer > 10 U/mL was considered positive for AMA by ELISA, and a serum titer > 10 U/mL was considered positive for anti-Ro/SS-A and anti-La/SS-B by ELISA.
Statistical analysisComparisons of patients with and without abnormal hepatic biochemistries and clinical liver disease were done using chi square and Fisher exact test for categoric measures and the Student t test was used for continuous variables in larges samples of similar variances. Nonparametric Mann-Whitney U test was used for small samples. Odds ratios (OR) with 95% confidence intervals (95% CI) were calculated by the equation (a x d)3(c x b), where a connoted patients who had abnormal hepatic biochemistries or clinical liver disease an had certain clinical features of systemic disease and/or markers of autoimmunity or inflammation, b connoted patients who did not have abnormal hepatic biochemistries or clinical liver disease an had certain clinical features of systemic disease and/or markers of autoimmunity or inflammation, c connoted patients who had abnormal hepatic biochemistries or clinical liver disease and did not have clinical features of systemic disease and/or markers of autoimmunity or inflammation, and d connoted patients who did not have abnormal hepatic biochemistries or clinical liver disease and did not have clinical features of systemic disease and/or markers of autoimmunity and inflammation. A P value ••0.05 was considered statistically significant. Data are presented as the mean ± standard error of the mean in tables and text.
ResultsFrequency and patterns of abnormal liver biochemistriesAbnormal hepatic biochemistries were found in 42 patients (44%). Eleven patients (11%) had abnormal liver biochemistries at baseline, and 31 (33%) had normal hepatic biochemistries at accession but presented abnormal hepatic biochemistries during a mean follow-up of 4 ± 3 years.
The pattern of biochemical liver abnormalities was mainly hepatocellular (defined as predominant increase of ALT and/or AST compared with AP and/or bilirubins) in 22 patients (52%); proceed by cholestatic (defined as predominant increase in AP and/or bilirubins compared with ALT and/or AST) in 13 patients (31%), and mixed (evidence of both cholestatic and hepatocellular patterns) in 7 patients (17%).
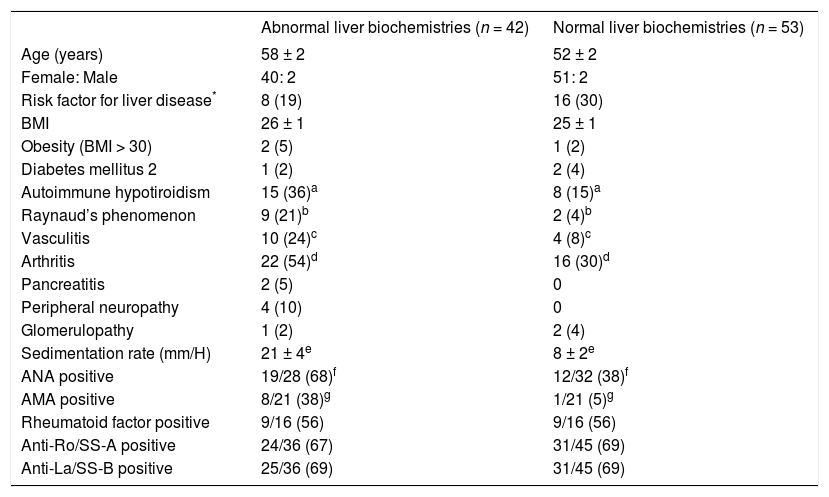
Clinical and laboratory features associated with abnormal liver biochemistriesPatients with abnormal hepatic biochemistries had a higher frequency of autoimmune hypotiroidism (36% vs 15%, P = 0.04), arthritis (54% vs 30%, P = 0.03), vasculitis (24% vs 8%, P = 0.04), and Raynaud’s phenomenon (21% vs 4%, P = 0.01) than patients with normal hepatic biochemistries. Also, patients with abnormal hepatic biochemistries had higher sedimentation rate (21 ± 4 mm/H vs 8 ± 2 mm/H, P = 0.005), greater frequency of ANA (70% vs 38%, P =0.02), and AMA (38% vs 5%, P =0.02) (Table I).
Clinical and laboratory features associated with abnormal LFT.
| Abnormal liver biochemistries (n = 42) | Normal liver biochemistries (n = 53) | |
|---|---|---|
| Age (years) | 58 ± 2 | 52 ± 2 |
| Female: Male | 40: 2 | 51: 2 |
| Risk factor for liver disease* | 8 (19) | 16 (30) |
| BMI | 26 ± 1 | 25 ± 1 |
| Obesity (BMI > 30) | 2 (5) | 1 (2) |
| Diabetes mellitus 2 | 1 (2) | 2 (4) |
| Autoimmune hypotiroidism | 15 (36)a | 8 (15)a |
| Raynaud’s phenomenon | 9 (21)b | 2 (4)b |
| Vasculitis | 10 (24)c | 4 (8)c |
| Arthritis | 22 (54)d | 16 (30)d |
| Pancreatitis | 2 (5) | 0 |
| Peripheral neuropathy | 4 (10) | 0 |
| Glomerulopathy | 1 (2) | 2 (4) |
| Sedimentation rate (mm/H) | 21 ± 4e | 8 ± 2e |
| ANA positive | 19/28 (68)f | 12/32 (38)f |
| AMA positive | 8/21 (38)g | 1/21 (5)g |
| Rheumatoid factor positive | 9/16 (56) | 9/16 (56) |
| Anti-Ro/SS-A positive | 24/36 (67) | 31/45 (69) |
| Anti-La/SS-B positive | 25/36 (69) | 31/45 (69) |
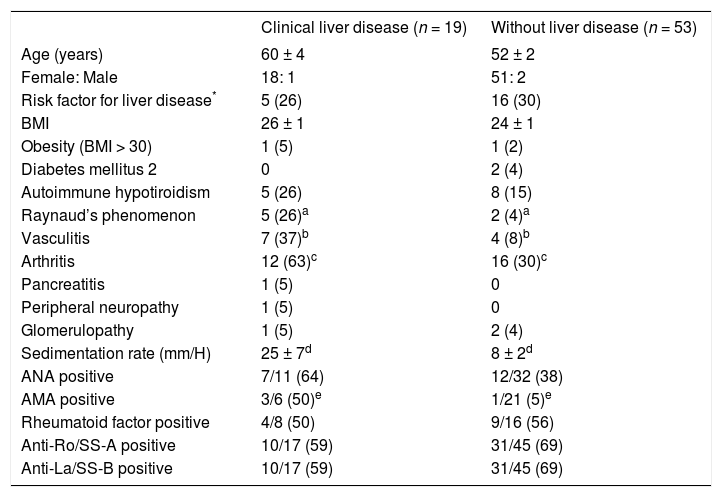
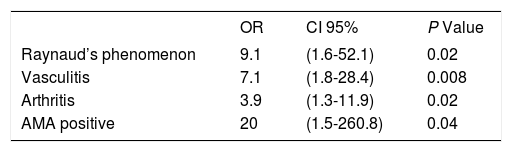
Clinical liver disease was found in 19 patients (20%), and in all cases the diagnosis of primary SS antedated the onset of the liver disease. Patients with clinical liver disease had a higher frequency of arthritis (63% vs 30%, P = 0.01), vasculitis (37% vs 8%, P = 0.006), Raynaud’s phenomenon (26% vs 4%, P = 0.01), elevated sedimentation rate (25 ± 7 mm/H vs 8 ± 2 mm/H, P = 0.006) and greater frequency of AMA (16% vs 5%, P = 0.02) than patients without clinical liver disease (Table II).
Clinical and laboratory features associated with clinical liver disease.
| Clinical liver disease (n = 19) | Without liver disease (n = 53) | |
|---|---|---|
| Age (years) | 60 ± 4 | 52 ± 2 |
| Female: Male | 18: 1 | 51: 2 |
| Risk factor for liver disease* | 5 (26) | 16 (30) |
| BMI | 26 ± 1 | 24 ± 1 |
| Obesity (BMI > 30) | 1 (5) | 1 (2) |
| Diabetes mellitus 2 | 0 | 2 (4) |
| Autoimmune hypotiroidism | 5 (26) | 8 (15) |
| Raynaud’s phenomenon | 5 (26)a | 2 (4)a |
| Vasculitis | 7 (37)b | 4 (8)b |
| Arthritis | 12 (63)c | 16 (30)c |
| Pancreatitis | 1 (5) | 0 |
| Peripheral neuropathy | 1 (5) | 0 |
| Glomerulopathy | 1 (5) | 2 (4) |
| Sedimentation rate (mm/H) | 25 ± 7d | 8 ± 2d |
| ANA positive | 7/11 (64) | 12/32 (38) |
| AMA positive | 3/6 (50)e | 1/21 (5)e |
| Rheumatoid factor positive | 4/8 (50) | 9/16 (56) |
| Anti-Ro/SS-A positive | 10/17 (59) | 31/45 (69) |
| Anti-La/SS-B positive | 10/17 (59) | 31/45 (69) |
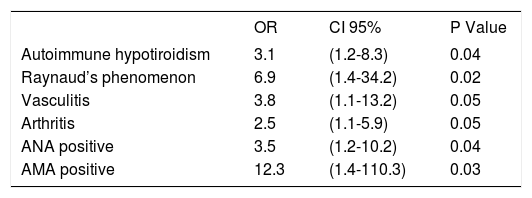
The OR for abnormal hepatic biochemistries in patients with features of systemic disease, such as autoimmune hypotiroidism, arthritis, vasculitis, Raynaud’s phenomenon, and the presence of ANA, AMA, anti-Ro/SS-A, and anti-La/SS-B were from 2.5 to 12.3, each with 95% CI above 1, and P values ••0.05(Table III). The OR for clinical liver disease in patients with features of systemic disease such as arthritis, vasculitis, Raynaud’s phenomenon and the presence of AMA were from 3.9 to 20, each with 95% CI above 1, and P values ••0.05 (Table IV).
Odds ratios for abnormal liver biochemistries.
| OR | CI 95% | P Value | |
|---|---|---|---|
| Autoimmune hypotiroidism | 3.1 | (1.2-8.3) | 0.04 |
| Raynaud’s phenomenon | 6.9 | (1.4-34.2) | 0.02 |
| Vasculitis | 3.8 | (1.1-13.2) | 0.05 |
| Arthritis | 2.5 | (1.1-5.9) | 0.05 |
| ANA positive | 3.5 | (1.2-10.2) | 0.04 |
| AMA positive | 12.3 | (1.4-110.3) | 0.03 |
OR = odds ratio. ANA = antinuclear antibodies. AMA = antimitochondrial antibodies. Numbers in parentheses are 95% confidence intervals (CI).
Odds ratios for clinical liver disease.
| OR | CI 95% | P Value | |
|---|---|---|---|
| Raynaud’s phenomenon | 9.1 | (1.6-52.1) | 0.02 |
| Vasculitis | 7.1 | (1.8-28.4) | 0.008 |
| Arthritis | 3.9 | (1.3-11.9) | 0.02 |
| AMA positive | 20 | (1.5-260.8) | 0.04 |
OR = odds ratio. AMA = antimitochondrial antibodies.
Numbers in parentheses are 95% confidence intervals (CI).
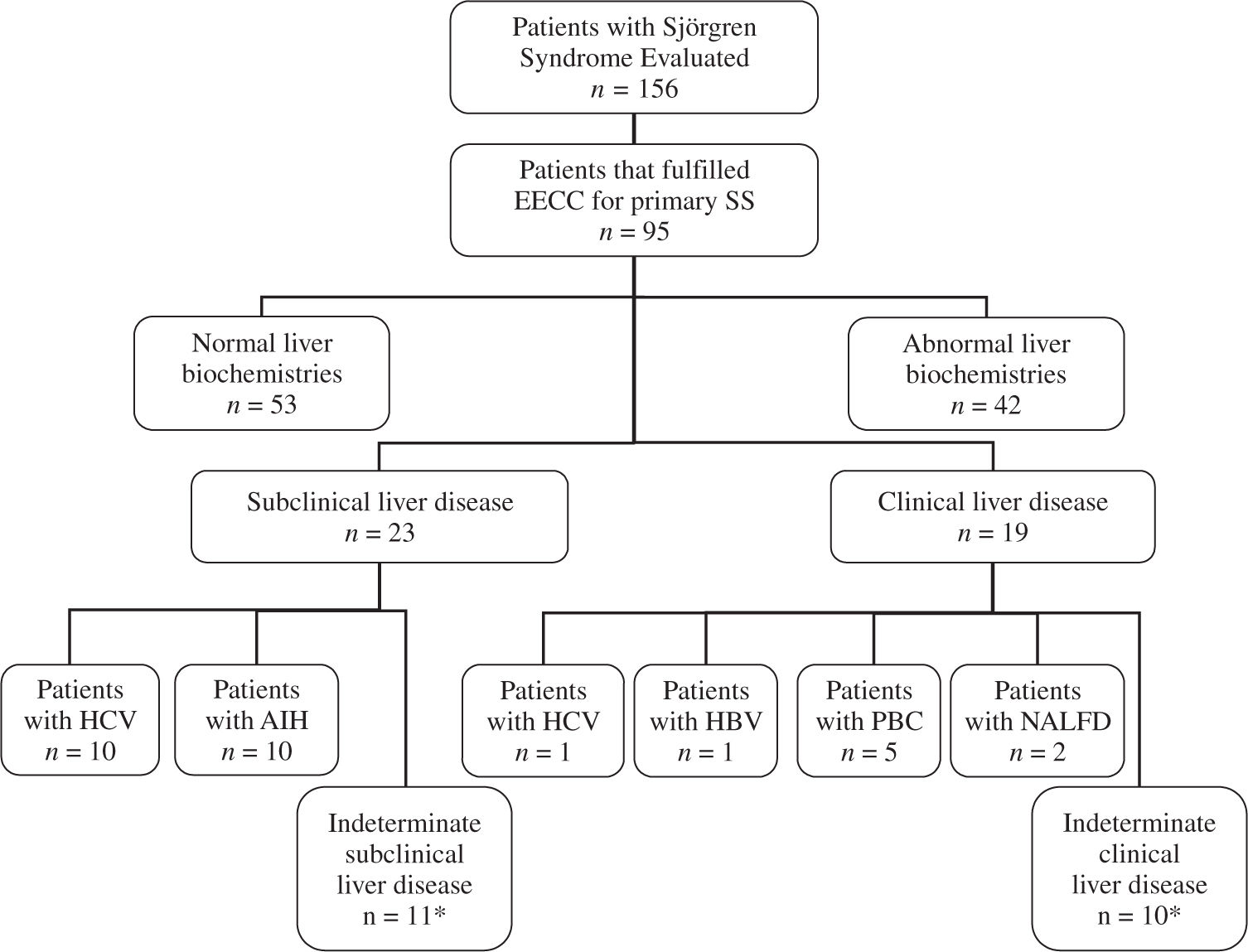
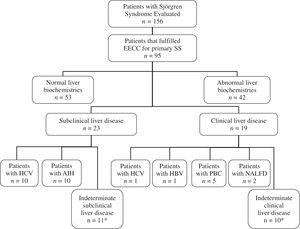
In 12 of the 23 patients with abnormal hepatic biochemistries a specific disease was determinate (10 HCV infection, and 2 AIH). In 9 of the 19 patients with clinical liver disease a specific disease was established (5 PBC, 2 nonalcoholic fatty liver disease, 1 HBV infection, and 1 HCV infection). Besides, 8 patients with abnormal liver biochemistries had history of risk factors for liver diseases such as transfusions, surgeries, alcohol or hepatotoxic drugs consumption (5 patients with indeterminate subclinical liver disease, and 3 patients with indeterminate clinical liver disease)(Figure 1); however, the frequency of these risk factors was not significantly higher than patients with normal liver biochemistries (19% vs 30%, P > 0.1)(Table I).
Flowchart of patients with primary Sjögren’s syndrome (primary SS) evaluated. EECC = European Epidemiology Center Criteria. HBV = Hepatitis B virus, HCV = Hepatitis C virus, AIH = Autoimmune hepatitis, NALFD = Non-alcoholic liver fatty disease. *5 patients with indeterminate subclinical liver disease, and 3 patients with indeterminate clinical liver disease had risk factors for liver disease (transfusions, surgeries, history of alcohol or hepatotoxic drugs).
Therefore, in 21 patients with primary SS (50%) with abnormal liver biochemistries with and without clinical evidence of liver disease, no clear explanation was found in their clinical charts(Figure 1).
DiscussionOur study indicates that liver involvement is common in patients with primary SS, and its presence correlates with clinical features of systemic disease and certain autoimmune and inflammatory markers. Specifically, we found an association between liver involvement and the presence of autoimmune hypotiroidism, arthritis, vasculitis, Raynaud’s phenomenon, elevated globular sedimentation rate, ANA and AMA.
The frequency of liver involvement in our study is higher compared with other reports2,4,5,7,35 and only one previous study had showed higher prevalence of abnormal liver biochemistries and clinical liver disease in patients with primary SS.8 These reports had showed a prevalence of liver affection in patients with primary SS from 6% to 58%; however, most of these studies had an unclear or subjective definition of liver involvement.2,4,5,36
Previously, the Sjögren’s complex has been associated with well established liver diseases such as PBC, AIH, and cryptogenic cirrhosis.35 The largest study of patients with primary SS that studied the presence of liver disease using clinical, biochemical, immunological, and histological data, found that 7% of patients had evidence of liver disease, and the same percentage of patients presented AMA detected by immunofluorescence.4 Also, they found that AMA-positive patients had features of chronic cholangitis similar to stage I PBC. However, this study used a higher cut-off value for define the presence of abnormal liver biochemistries, which may explained in part the lower frequency of subclinical liver involvement in their patients with primary SS.
A subsequent study by Lindgren et al.6 showed that the presence of abnormal liver biochemistries is present in 27% in patients with primary SS, and demonstrated an association with autoimmune liver diseases, such as PBC or AIH. However, similar to our study, they showed that a significant percentage of these patients had indeterminate abnormal liver biochemistries and even indeterminate clinical liver disease, suggesting that primary SS is the possible underlying cause of the liver involvement. Other small study37 analyzed 12 patients with primary SS, and abnormal liver function biochemistries were found in four. The liver biopsies of these four patients showed non-specific changes, suggesting that the histological changes may be interpreted as an independent liver disease, or as histological features associated with the main autoimmune disease.
Currently, it seems that the question of whether primary SS is a sign of a liver complaint, or vice versa can not be completely answer. An overlap between PBC and primary SS has been well recognized,13,36 and the exact prevalence of PBC in primary SS is unknown, but previous reports suggest that is approximately of 6%. Moreover, there is evidence of histological sialadenitis in 95% of patients with PBC and anti-La antibodies.13 In this study we found that 10% of patients expressed AMA (n = 9), and the diagnosis of PBC was established only in 5 patients; as a result there may a subgroup of patients with primary SS expressing AMA as an earlier marker of PBC or as a reflection of other indeterminate liver disease.
Interestingly, primary SS and PBC share common features. For example, in both conditions the inflammation starts around the ducts and both epithelial populations inappropriately express class II HLA molecules. Besides, infiltrates of CD4 T lymphocytes are common in bile ducts and salivary glands in patients with PBC and primary SS, respectively. Thus, it seems that in both diseases common pathogenetic mechanisms operate despite the fact that their autoantibody profiles are different.
In another hand, viral infection has long been suspected as a potential cause of primary SS, and more recently a possible relationship between primary SS and HCV has been postulated. There is evidence that shows an association of characteristic histological changes of Sjögren’s syndrome in the salivary glands and HCV infection.16 In this study we found a prevalence of 12% of HCV infection in our patients with primary SS, and there are reports that showed frequencies up to 19%,25 being significantly higher than the prevalence of infection reported in the general population. Also, patients with primary SS and HCV infection had higher prevalence of abnormal hepatic biochemistries and clinic liver disease.25
The tissular damage observed in some patients with primary SS could be related to an anomalous interaction between lymphocytes and different epithelial tissues, including the liver.1 Since it has been suggested that the target tissue involved in the autoimmune damage of primary SS might be the epithelium, is remarkable that we found a significant association between vasculitis and liver involvement, pointing out a possible link between the hepatic and vascular damage. Epithelial cells seems to be active participants rather than passive targets in the chronic immune response in primary SS, but further studies are required to establish the role of liver epithelial cells in the pathogenesis of this disease, maybe including analysis of HLA expression and cytokine profiles.
Finally, we found an important proportion of primary SS patients with liver involvement that had no evident explanation; consequently, it seems that this subgroup of patients may have an immunologic hepatic damage secondary to primary SS. However, one limitation of this study is that the true prevalence of liver involvement could not be precisely determinate because it’s retrospective nature, and that not all laboratorial, radiological and histological studies were done in all patients.
Future studies must expand these observations in prospective ways and correlate the association of liver involvement and primary SS syndrome. It will be important to establish if in patients with primary SS and indeterminate liver disease the liver affection is secondary to the activation of immunologic mechanisms of the primary disease, or the autoimmune exocrinopathy represents a secondary manifestation of the primary liver disease.
In summary, liver involvement is commonly present in patients with primary SS and its presence seems to correlate with certain clinical features of systemic disease, such as arthritis, vasculitis and Raynuad’s phenomenon, and some autoimmune and inflammatory markers, such as ANA, AMA, and globular sedimentation rate. In a considerable proportion of patients with primary SS with subclinic and clinic liver disease no other diagnosis explaining the liver involvement can be found.