Flutamide is a non-steroidal anti-androgenic drug, commonly used in the treatment of advanced prostate cancer, acne and hirsutism. This drug may induce various degrees of liver injury, including acute liver failure (ALF), with further need for liver transplantation. Here, we present a report of 10 consecutive patients seen in a period of 14 years, with acute liver toxicity induced by flutamide (in most cases severe hepatotoxicity): 3 men and 7 women, with a mean age of 75 and 29 years old, respectively. All men received flutamide as treatment of advanced prostate carcinoma and they developed hepatotoxicity without ALF, and three months after withdrawal of the drug, they recovered completely. In contrast, in 7 young female with liver toxicity caused by flutamide as treatment of various hyperandrogenic conditions (acne and hirsutism), ALF was observed in 5 patients, all of them requiring urgent liver transplantation, with excellent outcome and survival in 4 of them. Based on the above, we believe that flutamide treatment should be preferentially avoided in young female patients with benign pathologies, or if it is used, patients should be warned of its potential severe complications. Also, serial liver tests should be closely monitored and, in case of elevations, the drug should be immediately withdrawn.
Flutamide (4’-nitro-3’ - fluoromethylisobutyra-milide) is a non-steroid anti-androgenic drug, used since 1989 in USA to treat advanced prostate carcinoma. This drug acts preventing the binding of circulating suprarenal androgens to androgenic receptors located in the prostate and in prostate cancer cells.1 More recently, it has been also used in young women presenting diverse forms of hyperan-drogenism (acne, hirsutism, alopecia).2,3 Side effects of this drug may include gastrointestinal problems (mainly diarrhea), hematological alterations, gynecomastia and muscle cramps.4 However, the most severe clinical side effect is acute hepatitis, which may evolve into a fulminant form (acute liver failure: ALF), associated with high mortality rate and eventually requiring liver transplantation.5
This current report presents the data of a 10 consecutive patients with hepatotoxicity induced by flutamide, including severe forms of the disease, evaluated by the authors during the last fourteen years.
Patients and MethodsRetrospective analysis of 10 consecutive patients with severe hepatotoxicity associated with flutamide intake, seen between July 1996 and May 2010. The history and clinical charts, laboratory tests, clinical evolution and histopathological studies when available (liver explants) in the cases of ALF with liver transplantation, were reviewed.
Patients included in the study were 3 men and 7 women, with a mean age of 75 (range 67-80) and 29 (range 21-44) years old, respectively. Flutamide was used in 3 men for advanced prostate carcinoma treatment, and in 7 young women due to hirsutism (in four cases), acne (in two cases) and alopecia (in one case) (Tables 1 and 2).
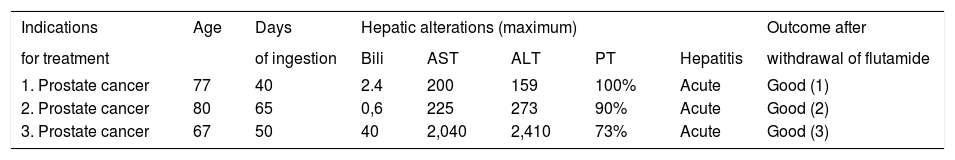
Hepatotoxicity induced by flutamide in men (n = 3).
| Indications | Age | Days | Hepatic alterations (maximum) | Outcome after | ||||
|---|---|---|---|---|---|---|---|---|
| for treatment | of ingestion | Bili | AST | ALT | PT | Hepatitis | withdrawal of flutamide | |
| 1. Prostate cancer | 77 | 40 | 2.4 | 200 | 159 | 100% | Acute | Good (1) |
| 2. Prostate cancer | 80 | 65 | 0,6 | 225 | 273 | 90% | Acute | Good (2) |
| 3. Prostate cancer | 67 | 50 | 40 | 2,040 | 2,410 | 73% | Acute | Good (3) |
PT: Prothrombin time (%). Bili: Bilirrubin (mg/dL). AST: Aspartate aminotransferase (IU/ml). ALT: Alanine aminotransferase (IU/ ml). (1) 60 days; (2) 30 days; (3) 90 days.
Hepatotoxicity induced by flutamide in women (n = 7).
| Indications | Age | Days | Hepatic alterations (maximum) | Outcome after | ||||
|---|---|---|---|---|---|---|---|---|
| for treatment | of ingestion | Bili | AST | ALT | PT | Hepatitis | withdrawal of flutamide | |
| 1. Hirsutism | 44 | 90 | 4.2 | 390 | 605 | 90% | Acute | Good (1) |
| 2. Hirsutism | 27 | 60 | 27 | 1064 | 1803 | 10% | Fulminant | Good (2) |
| 3. Hirsutism | 32 | 60 | 25 | 1189 | 925 | 38% | Acute | Good (3) |
| 4. Hirsutism | 20 | 180 | 20 | 850 | 590 | 15% | Fulminant | Good (4) |
| 5. Acne | 22 | 180 | 25 | 1,576 | 1,116 | 14% | Fulminant | Good (5) |
| 6. Acne | 21 | 65 | 29 | 2,778 | 3,360 | 10% | Fulminant | Good (6) |
| 7. Alopecia | 38 | 90 | 44 | 2,130 | 1,430 | 24% | Fulminant | Died (7) |
PT: Prothrombin time (%). Bili: Bilirrubin (mg/dL). AST: Aspartate aminotransferase (IU/ml). ALT: Alanine aminotransferase (IU/ml). (1) 1 month. (2) 4 months post transplant. (3) 3 months. (4) 7 months post transplant. (5) 53 months post transplant. (6) 53 months post transplant. (7) Post operative period.
In all our cases, other causes for acute or chronic hepatitis were excluded through viral markers (for Hepatitis A, B, C), other viral markers in selected cases (Cytomegalovirus, Epstein Barr virus, Hepatitis E virus, etc.), auto-antibodies (antinuclear antibodies and anti-smooth muscle antibodies), quantification of serum immunoglobulin (IgA, IgG and IgM), ceruloplasmine level, lack of alcohol intake in the history, or the concomitant use of other medications potentially hepatotoxic. Images were performed in all cases, and abdominal ultrasound or computed tomography of the abdomen provided no further relevant findings.
Clinical and laboratory data are presented in tables as means (range)
ResultsConsidering the whole group of patients (n = 10), the liver tests abnormalities were detected after a mean of 88 days (range 40-180 days) of initiation of flutamide treatment.
When considering only men (n = 3), the mean oral dose used was 750 mg/day. The maximum means of abnormal liver tests were: Bilirubin 14.3 mg/dL (range 0.6-40), AST 821 UI/mL (range 2002040), ALT 947 UI/mL (range 159-2410) and prothrombin time 88% (range 73-199) (Table 1). Laboratory tests abnormalities returned to normal values in all patients within a 90-day period after drug withdrawal.
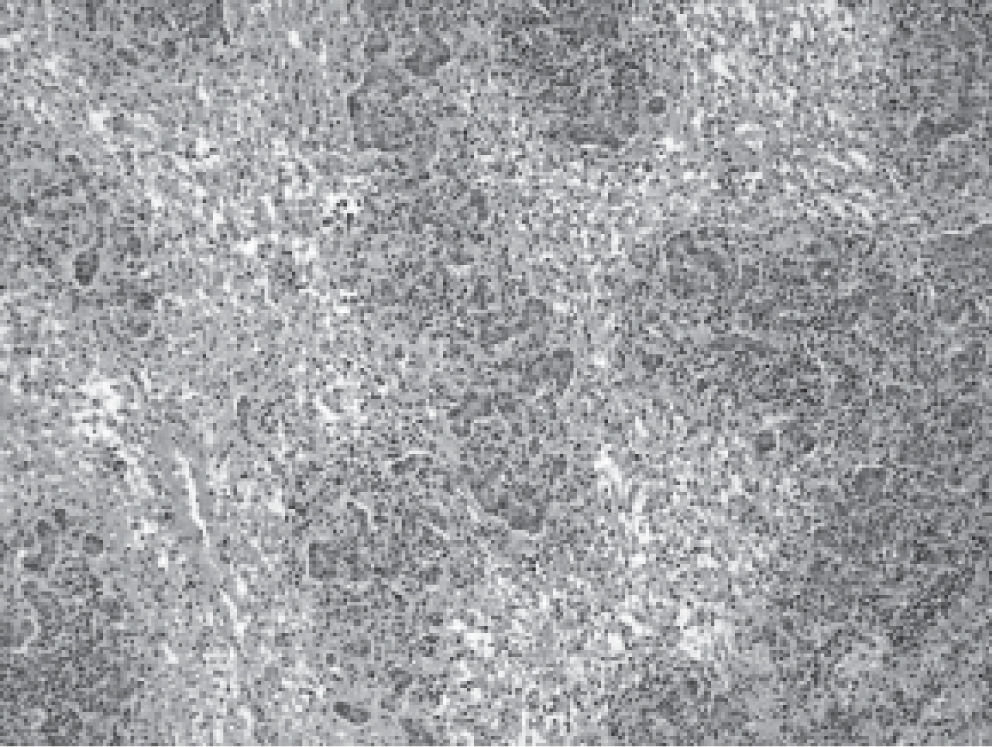
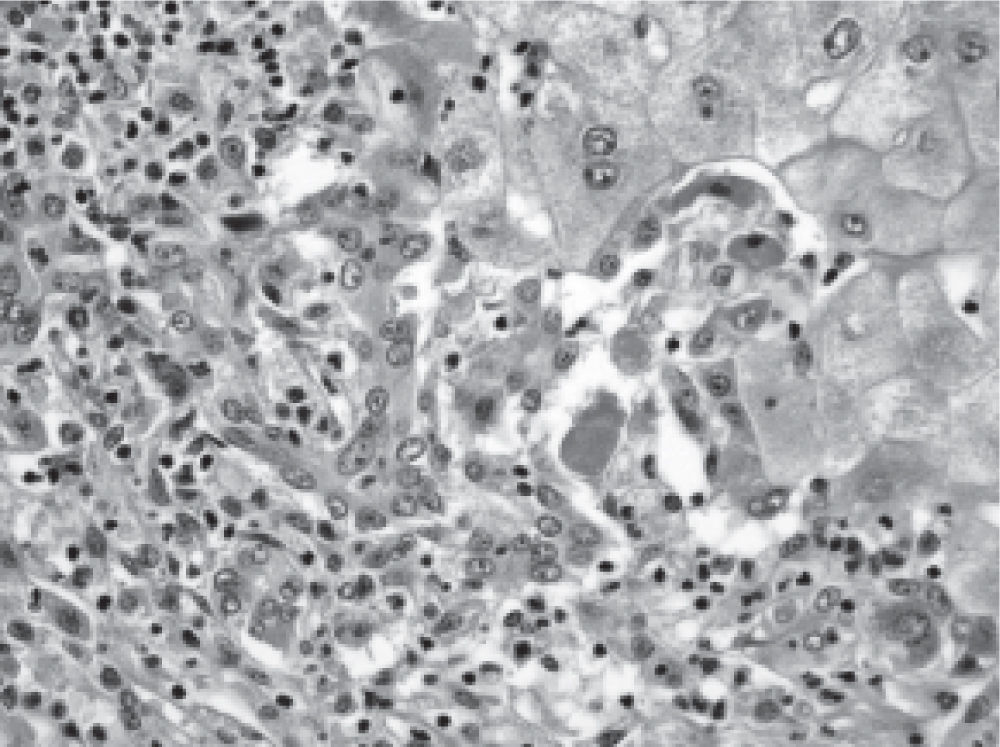
In contrast, doses used in women (n = 7) were lower and between 125 to 250 mg/day, but mean laboratory tests abnormalities were significantly more intense: Bilirubin 24.8 mg/dL (range 4.2-44), AST 1425 UI/mL (range 390-2778), ALT 1404 UI/ mL (range 590-3360) and prothrombin time: 28.7% (range 10-90). Five women (5 out of 7: 71%), evolved into ALF and required liver transplantation urgently. The other two cases normalized liver test abnormalities within a period of 90 days after flutamide withdrawal (Table 2). The histopathological evaluation of the explanted livers showed massive or sub-massive necrosis (Figures 1 and 2) and the mean weight of their explanted livers was 515 g (range 337-856). Four of the five liver transplanted women have had a good outcome with a mean survival follow-up of 29 months post-transplantation (range 4-53) at the date of presentation of this report (Table 3). One woman died due to postoperative complications in the immediate post transplant period.
Flutamide is an anti-androgenic drug, usually well tolerated in most patients and completely absorbed in the digestive tract. Its androgenic effect is mediated by blockade of androgenic receptors. Among its side effects, diarrhea is commonly mild, but its liver toxicity may be severe and potentially lethal. This drug has been traditionally used in patients with advanced prostate cancer (in a usual dose of 250 mg/8 hours, orally), and during the last decade in young female patients with hyperandrogenism (hirsutism and acne). Doses used in hirsutism are usually lower than in prostate cancer patients (between 250-500 mg/day). However, even lower doses (62.5-125 mg/daily) used in young women with acne may also show beneficial effects. This drug is metabolized in the liver, resulting in 2-3 small metabolites that are excreted in the urine.7
Diagnosis of liver induced damage by flutamide and other drugs is still based upon clinical criteria, considering a temporal relation between the initiation of the potentially hepatotoxic drug, and the development of various degrees of liver injury and its full recovery upon withdrawal of the medication.8-10
In our study, we found a clear temporal relation between flutamide ingestion and liver damage, with the exclusion of other known causes for acute and chronic liver diseases (including viral infections and other potentially hepatotoxic drugs). Clinical recovery without liver transplantation after drug withdrawal was observed in all the 3 male patients, but in only 2 out of 7 women, as previously reported, that hepatotoxicity may be more severe in women than in men.9,10 Women are more susceptible than men to acute liver injury from drugs and other xenobiotics. The biological mechanisms for this sex difference are unknown, but known sex differences in steroid hormone levels and immune response could play a role.
In general, it is considered that the incidence of flutamide-induced liver toxicity is low, with the warning that this drug should be avoided in patients with previous hepatic diseases.6 The first clinical reports on hepatotoxicity induced by flutamide were published between 1989 and 1991, as sporadic cases.11-15 Hepatic toxicity induced by flutamide was systematically studied for the first time in 1992, observing liver test alterations (defined as AST and ALT increases at least four times over the upper limit of normal) in 4 out of 1,091 (0.36%) consecutive patients with advanced prostate cancer exposed to flutamide. They received oral flutamide in a dose of 750 mg/daily for long periods of time and with a serial control of liver tests.16 Afterwards, complementary studies have allowed to estimate an incidence of 3 cases of hepatotoxicity per 10,000 treated patients, although other authors have suggested an incidence of up to 6-9% of significant liver toxicity.5,17 From the time of marketing of flutamide in the USA (February 1989) through March of 1991, the US Food and Drug Administration (FDA) received reports of 19 patients who developed serious hepatotoxicity while using flutamide. All of them received flutamide for prostate cancer or benign prostatic hypertrophy. Fourteen patients had resolution of liver test abnormalities after discontinuing or decreasing the dose of flutamide, but five patients died of progressive liver disease and autopsies showed hepatocellular necrosis and variable degrees of cholestasis.17
During the last decade, attempts have been made to reduce the potential risk of hepatotoxicity induced by flutamide through the administration of reduced doses, particularly in the case of women with hirsutism,18 and in adolescents and young women with hyperinsulinemic hyperandrogenism (a variant of polycystic ovarian syndrome).19-21 Addition of mini-doses of flutamide combined with metformin and oral contraceptives, with a clinical and laboratory follow-up of 3 to 9 months was associated to additional clinical benefits without reports of hepatotoxicity. In a recent communication, Ibañez et al.22 did not find any hepatotoxicity in a group of 190 young women with hyperandrogenism that received low doses of flutamide for up to 54 months.
The pathogenesis of drug-induced liver injury by flutamide encompasses probably a wide spectrum of mechanisms, some of which are still poorly understood. Most drug induced liver injuries, are unpredictable or idiosyncratic (like in flutamide), which may be allergic (with fever, rush, eosinophilia) or non-allergic (without allergic features). Liver damage in flutamide hepatotoxicity, is usually expressed as a mixed injury with an increase in serum level of both transaminases (ALT and AST) and the ALT/alkaline phosphatase ratio is usually between 2 and 5.102324 Moller et al.12 have suggested an idiosyncratic damage produced by interference of metabolic processes in hepatocytes, cell destruction by direct toxic effect upon essential structures or induction of an immunological reaction that would determine necrosis and cholestasis. In the majority cases reported of flutamide hepatotoxicity, neither rash nor fever has been clinically observed. In a few cases, eosinophilia has been described as the only sign of hypersensibility.
Both environmental factors and genetic differences in cellular responses to intracellular stress (oxidative or organelle specific) and the innate immune response may explain the individual susceptibilities to drug-induced liver injury.25 A study performed in rats suggested that these alterations were mediated by metabolites derived from the P450 system which induced mitochondrial damage.26
In this clinical case series report, we have found an important number of patients with flutamide hepatotoxicity. In Chile we have no accumulative data of the number of patients receiving flutamide each year. It is our impression that since the Chilean population has a strong Amerindian genetic component, it may have a different susceptibility to drug induced liver injury as well. It has been shown for other diseases like intrahepatic cholestasis of pregnancy (ICP), that Chile has one of the highest incidences of ICP in the World, and that multiple factors probably interact with an increased genetic predisposition to alter the membrane composition of bile ducts and hepatocytes and increase their sensitivity to sex steroids and other medications.27 ICP and drug-induced cholestasis are two clinically important forms of acquired cholestatic liver disease. The understanding of the underlying mechanisms of acquired cholestasis has recently made significan progress by the identification of canalicular ATP-binding cassette (ABC) transporters as likely targets for these forms of cholestasis. Genetic variants (and mutants) of the genes encoding for the phosphatidylcholine translocator MDR3 and BSEP and for the farnesoid X receptor have been associated with ICP.27 More recently keratin variants (KRT8 and KRT18) have been shown to represent important susceptibility genes for ALF development. Presence of K8/K18 variants predisposes to adverse ALF outcome, and some variants segregate with unique ethnic and race backgrounds.28
Recent data has described in more detail some risk factors for drug induced liver injury (DILI), including the association of several candidate genes and the development of DILI in the susceptible patient. Human leukocyte antigen-B*5701 is closely linked to the hypersensitivity reaction with abacavir, and this screening has been incorporated into HIV treatment. Other candidate genes conferring specific susceptibility to DILI have been identified for medications such as: flucloxacillin, amoxicillinclavulanate, ximelagatran and isoniazid, but others are under study.29 Preventing DILI through the identification of genetic markers will soon be incorporated in clinical practice.30
Reports in the literature have described a latency period between flutamide administration and the appearance of hepatic alterations between 5 days and 10 months, with a mean of 3 months.17,31 In our patients, the situation was highly variable, with a mean of 88 days (range 40-180). The majority of hepatic toxicity reported corresponds to subclinical, mild, and asymptomatic cases. However, various cases of ALF have been reported, particularly in older patients, who were treated for prostate cancer.12,17,32
It is noteworthy that in our clinical series, all severe cases correspond to women (women 5/7 vs. men 0/3), which may be associated with the influence of host factors. Regarding this, it is necessary to add that in our study all women were young (mean age 29, range: 20-44). This could be ascribed to the fact that in young women, exposure to the drug resulted in a hyper-acute inflammatory response probably through immunologic mechanisms, whose clinical manifestation would be an ALF. The histopathological study of 5 cases (through the explanted liver) confirmed the diagnosis of ALF (with massive or submassive hepatic necrosis), allowing the exclusion of other conditions and chronic pathologies, particularly autoimmune diseases. The required treatment in severe cases was liver transplantation, with a favorable evolution in four of the 5 cases. Fulminant hepatitis cases have been reported in the literature as isolated cases, mainly in men receiving flutamide due to prostate cancer,12,17,32-35 usually without need for liver transplantation, as in the present series.
In summary, the data presented in this report suggests that serial liver test monitoring should be done in all patients with prostate cancer under flutamide therapy. Furthermore, because of the possibility of severe liver injury (fulminant hepatitis needing hepatic transplant) in young women using flutamide for benign pathologies (acne, hirsutism, alopecia), its use should be critically reconsidered because of the potential risk of a lethal complication
Abbreviations- •
PT: Prothrombin time (%).
- •
Bili: Bilirrubin (mg/dL).
- •
AST: Aspartate aminotransferase (IU/mL).
- •
ALT: Alanine aminotransferase (IU/mL).