The topic of this paper is to report an update on management of Budd-Chiari syndrome (BCS). Actually, the flow-chart of BCS management comes from experts opinion and is not evidence-based due to the rarity of BCS. Management of BCS follows a step-wise strategy. Anticoagulation and medical therapy should be the first line treatment. Revascularization or TIPS in case of no response to medical therapy. OLT as a rescue therapy. Surgery has limited but important space, especially in cases with high inferior vena cava obstruction not suitable for endovascular treatment. However, no clear indication can actually be given about the timing of different treatments. Moreover, there is some concern about treatment of some subgroup of patients, especially regarding the risk of recurrence after liver transplantation. This paper propose a new algorithm of BCS management suggesting an earlier therapeutic approach when clinical signs are evident.
The aim of this paper is to propose a new algorithm of BCS management. In fact, as it will be discussed, evidence is lacking about BCS due to the rarity of the syndrome. We will see that some data argue against the actual expectant strategy of BCS management and suggest a more interventional approach.
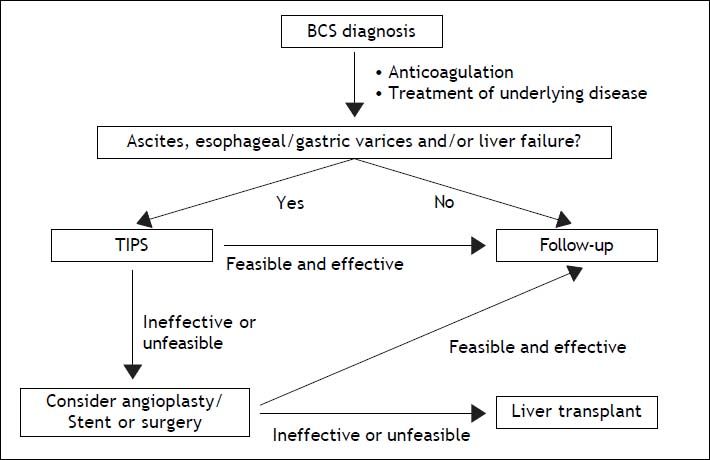
The topic of this paper is to report an update on management of Budd-Chiari syndrome (BCS). BCS is a rare disease with a generally worsening outcome without intervention. Following experts opinion coming from not evidence-based experiences, BCS management should follow a step-wise management. In fact, medical therapy (anticoagulation, treatment of underlying disease, symptomatic therapy of portal hypertension complications) should be the first-line treatment, angioplasty/stenting the second-line (in patients with short-length stenoses not responding to medical therapy), TIPS the next step (in patients not responding to medical therapy and in case of no response to, or stenoses unsuitable for, angioplasty/ stenting) and liver transplantation (LT) the last chance when TIPS is not effective.1–3 Step-wise management suggests moving forward when no response to therapy appears.1
A therapeutic approach limited to short-length stenoses is angioplasty/stenting, with a good medium term outcome reported in some experience.4–6 However, no data can argue against the use of TIPS also in the subgroup with short-length stenoses. In fact a prospective comparison between TIPS and angioplasty/stenting has not been performed. Probably, such a therapeutic choice in this subgroup of patients should be personalized and based on local expertise.
The most frequent treatment for BCS is surely TIPS.1–3 TIPS has been shown to be effective as BCS treatment in early experiences.7–9 Moreover, TIPS can be successful also in the technically difficult case of extension of thrombosis to the portal vein tree.10,11 Finally, a multi-center experience provided long-term data on TIPS treatment for 147 BCS patients not responding to medical treatment or recanalization. TIPS was successful in 124 BCS patients, who were followed for a median of 36.7 months. Overall, 16 (13%) died, 8 (6.5%) underwent OLT. Main complications were hepatic encephalopathy in 21% and TIPS dysfunction in 41%. The 10-year survival was 69%.12
Apart from LT, surgical treatment is generally not contemplated in the BCS management by recent guidelines.1 In fact, the most used treatment for BCS non responsive to medical therapy is TIPS, LT is used as a rescue therapy, while surgical treatments are limited to a strict minority of patients.1–3 Surgical shunts for BCS can be very successful, but have been associated with rapid decompensation and in-hospital mortality can be high (about 25%), primarily due to the patients’ poor general condition.13–16 In the past years, a surgical approach has been traditionally considered the first choice. In some series, an excellent outcome with long-term follow-up has been reported (95% survival, 3 to 28-year followup).15 However, in this series, the SSPCS was used, an approach that cannot be used in case of inferior vena cava (IVC) thrombosis or significant compression. In the same series, the mortality rates of patients with IVC involvement were very high after traditional surgery (mesoatrial shunts) and better results were described with another technique (SSPCS + cavoatrial shunt) in 18 patients, all surviving after a follow-up of 5-25 years.15 However, outcome after surgical portosystemic shunt is variable and worse results were reported by others.13,14,16 In most of the above series, patients with liver failure were not considered for surgery but for liver transplantation.13–16
Patients with BCS due to a IVC obstruction near to the atrium constitute a difficult to treat subgroup of BCS patients, whose management can benefit from endovascular dilatation/stenting or surgical treatment. Because of obvious reasons, TIPS does not by-pass IVC obstruction, resulting ineffective. Although some study describes the possibility of endovascular management for patients with IVC obstruction near to the atrium as safe and with good long-term patency, data are scanty and need confirmation on a larger scale.17
The widest experience with a surgical shunt approach for BCS due to a IVC obstruction was the above reported after a combination of SSPCS and cavo-atrial shunt: 18 patients, all surviving after a follow-up of 5-25 years.15 Other surgical techniques reported in that and other series either had unacceptable or non reported outcomes.13,14,16
We recently described a patient with IVC obstruction near to the atrium and a previous severe complication of a trial of endovascular management, who had an excellent outcome after the replacement of the obstructed segment of the IVC with a caval homograft.18
LT is the last chance for BCS syndrome non responsive to either medical therapy or recanalization/ decompression.19–24 A European multi-center study reported long-term data on 248 patients who underwent LT for BCS between 1988 and 1999. MPD was the underlying syndrome in 45%. LT was performed electively in 55%, in emergency in 21%. Hepatocellular carcinoma was incidentally found in explanted liver in 3. Before LT, 19% had portal vein thrombosis and 16% IVC thrombosis. Median follow-up was 48 months. Overall, 67 (27%) died (49% in the first month). Causes of death were sepsis in 47%, graft dysfunction or hepatic artery thrombosis in 19%, venous thrombosis in 12%, cardiac in 9% and brain damage in 5%. There was a significantly increased mortality if LT was shortly after SPSS or TIPS. Thirty-seven patients underwent re-LT (4 twice). The 10-year survival was 68%. After 1 year there were 9 deaths, seven of which were in MPD patients. Causes were: 4 BCS recurrence, 1 leukaemia (7 years post-LT), 1 ovarian cancer, 1 colangitis, 2 not known. Anticoagulation after LT was performed by 200/235 (18 heparin or aspirin), suspended in 10, all of which were believed to have a cause of BCS reversible after LT (antithrombin III and Protein C deficiency); all had an uneventful outcome but one who reported pulmonary embolization 1 year after, when anti-phospholipid syndrome was discovered. Complications post-OLT in the patients treated with anticoagulation were thrombosis in 27 (11%), 11 of whom (41%) died; recurrence of BCS in 6 (1 ReOLT, 1 TIPS, 4 death); bleeding in 27 (11%), 2 of whom died (intracranial bleeding). Prognostic Factors were pre-OLT renal function and pre-OLT SPSS/TIPS.23 However, the prognostic factor of a previous shunt before LT has to be weighed cautiously because it can only reflect the fact that patients who underwent TIPS before LT had the most severe liver disease. Moreover, a recent American multi-center study found no negative effect of TIPS on the following LT outcome.24 Finally, recent data show promising results of living donor LT for BCS.25
The possibility of BCS underlying disease progression is a concern, in particular the development of leukaemia in MPD after LT. Preliminary multicenter studies failed to draw conclusions on this topic, given that long-term outcome was not correlated to the type of underlying disease predisposing to BCS.23,24 However, although not statistically significant, 7 of the 9 patients who died after 1 year post LT in the European study had MPD.23 The impact of Jak2 and MPL mutations on prognosis of splanchnic vein thrombosis (either BCS or portal vein thrombosis) was recently reported in 241 cases.
In BCS, patients with the Jak2V617F mutation had a significantly more severe disease (Child-Pugh, Clichy PI, Rotterdam score). Moreover, event free survival tended to be decreased, but not significantly, in patients with Jak2V617F mutation and significantly decreased in MPD. However, at a median follow up of 3.9 years, overall survival was not influenced by either Jak2V617F mutation or MPD.26
The outcome of BCS with currently available treatment is described in a prospective multi-center study in which 163 patients were followed for a median of 17 mo (range 1-31 mo); 18% also had portal vein thrombosis, 84% had a thrombophilic syndrome, 46% of which a myeloproliferative disorder (MPD). Overall, 29 died, 8 for liver failure, 2 multiple organ failure, 2 bleeding. The 24 mo survival was 82%. Prognostic factors were sex (male), ascites and creatinine. Importantly, about 1/3 of the patients remained on medical therapy only.27 However, the follow-up was not long enough to eventually show the consequences of a slowly progressing disease, possibly prevented by early recanalization/decompression, and to draw any definitive conclusion about the exact timing of treatment.
The long-term outcome of the same multicenter experience was recently reported. Interestingly, 20 of the 69 who received only medical therapy died, a high rate considering the availability of further invasive treatments. The remaining 88 underwent invasive treatment, mostly TIPS, 20 LT, and only 3 surgical shunt. Overall survival was 74% at 5 years.28
One of the main unsolved issue of BCS management is probably timing of treatment. In fact, although step-wise management suggests moving forward when no response to therapy appears, definition for response to therapy was not stated1,2,29 and a proposal of such a definition needs validation.2 Moreover, because of the rarity of BCS, management guidelines come from expert opinion, are empirical, and are not evidence based. Given that benefit of treatments for BCS is not under debate, anticipating invasive treatments, namely TIPS, before no response to medical therapy appears, could perhaps decrease hepatic fibrosis development, disease progression, and finally improve outcome.29 In conclusion, a proposal of a new algorithm of BCS management suggesting early interventional therapy when clinical signs appear is reported (Figure 1).
Financial DisclosureI declare I have nothing to disclose