Background. Liver transplantation is the only therapy for end-stage liver disease. Cirrhosis secondary to autoimmune hepatitis (AIH) is an indication in 4-6% of adult transplants. Aims. To describe the outcomes and recurrence of AIH in liver transplant patients.
Material and methods. Twenty patients were retrospectively studied. Results. The female/male ratio was 3:1, the median age was 36.7 years (range, 16 to 39 years), and the median MELD score was 18.5. According to serological analysis, 19 patients were AIH type 1 and one patient was AIH type 2. AIH was associated with human leukocyte antigen (HLA) DR13+ and DR4+. The overall 5-year patient and graft survival rates were 94 and 85%, respectively. Three (15.7%) cases of recurrent AIH were diagnosed based on histological evidence. Clinical and histological features of acute and chronic rejection were present in four (20%) and three (16.6%) patients, respectively. Conclusion. AIH frequently affected young women, was the most frequent indication for liver transplantation. Rejection and recurrence were commonly associated with AIH, but did not affect patient survival. No significant relationship between HLA-DR type and recurrence was found. Rapid progression to cirrhosis should be considered in severe recurrences.
Autoimmune hepatitis (AIH) is a chronic inflammatory liver disease of unknown etiology characterized by interface hepatitis, hypergammaglobulinemia and autoantibodies; it occurs most frequently in women.1 It has been reported that 29% of patients with AIH are cirrhotic at the time of diagnosis.2 Liver transplantation is the only available therapy for end-stage liver disease with a 5-year survival rate of 80-90%.3,4 Liver transplantation is indicated for patients with AIH that progress to cirrhosis, hepatocellular carcinoma (HCC) and liver failure. Recurrence of AIH after LT may vary from 20-30% according to the applied diagnostic criteria. Low levels of immunosu-ppression and human leukocyte antigen (HLA) status have been related to recurrence.3,5
ObjectiveThe aim of this study was to determine the epide-miological, clinical, biochemical, pathological and histocompatibility features of patients who underwent LT at our institution and to determine the associations between these factors and survival and rejection rate.
Materials and MethodsWe conducted a retrospective review of patients who underwent LT for AIH at the Transplant Department of the Guillermo Almenara National Hospital (Lima, Peru) between March 2000 and December 2010. We reviewed the medical records of 20 patients who met AIH diagnostic criteria based on the original scoring system of the International Autoimmune Hepatitis Group.6 All patients were tested for autoantibodies: antinuclear antibodies (ANA), anti smooth muscle antibodies (ASMA) and anti liver kidney microsomal-1 antibodies (antiLKM-1). The indication for LT was end-stage chronic liver disease in all cases, including one case HCC within Milan Criteria.7 According to the protocol of the Liver Transplant Service, post-transplant follow-up consisted of clinical and laboratory evaluation on a weekly basis during the first and second months, biweekly during the third month and monthly from the fourth month onward.
Patients who presented with abnormal liver function tests with or without auto antibodies underwent a percutaneous liver biopsy under fluoroscopic guidance. AIH recurrence after LT was established by liver biopsy with histological typical pattern.
Epidemiological, clinical, biochemical, immunolo-gical and histocompatibility data were collected before the transplant and biochemical and pathological data were collected after the transplant. We used Fisher's test to evaluate relationships between these variables. Actuarial patient survival was computed using the Kaplan-Meier estimate.
ResultsWe performed LT in 78 patients during the study period. The indication of LT was AIH cirrhosis in twenty cases (25.6%). Nineteen patients had AIH type I (ANA or ASMA positive) and one had AIH type II (anti LKM-1 positive). Fifteen patients were women (75%), the average age was 36.7 years (range, 16-59 years) and most were of mixed racial origin. The average duration of disease before transplantation was 4.6 years (range, 2-8 years). The clinical presentation was predominantly cholestatic disease (80% had jaundice and hyperbilirubinemia); ascites and encephalopathy were the most frequent complications (60%). The clinical, epidemiological and follow-up characteristics of the 20 patients are shown in tables 1 and 2. The biochemical profile of our series of cases at the time of transplantation is presented in table 3. During the pretransplant period, four patients (20%) received prednisone, two of them also received azathioprine.
Demographic and clinical characteristics of patients before transplantation.
| Variable | n = 20 |
|---|---|
| • Age | 36.75 (16-59) |
| 20 | 4 (20%) |
| 20-29 | 3 (15%) |
| 30-39 | 5 (25%) |
| 40-49 | 3 (15%) |
| 50-59 | 5 (25%) |
| • Race | |
| Mixed race | 19 (95%) |
| Black | 1 (5%) |
| • Sex (M:F) | 3:1 (15:5) |
| • Complications of ESLD | |
| Ascites | 12/20 (60%) |
| Encephalopathy | 12/20 (60%) |
| Variceal bleeding | 5/20 (25%) |
| SBP | 2/20 (10%) |
| Hepatopulmonary syndrome | 1/20 (5%) |
| Hepatorenal syndrome | 1/20 (5%) |
| • MELD score | 18.5(15-40) |
| • Child-Pugh | |
| A | 0 (0%) |
| B | 11 (55%) |
| C | 9 (45%) |
ESLD: end-stage liver disease. SBP: spontaneous bacterial peritonitis.
Number of patients who received a liver transplant because of AIH.
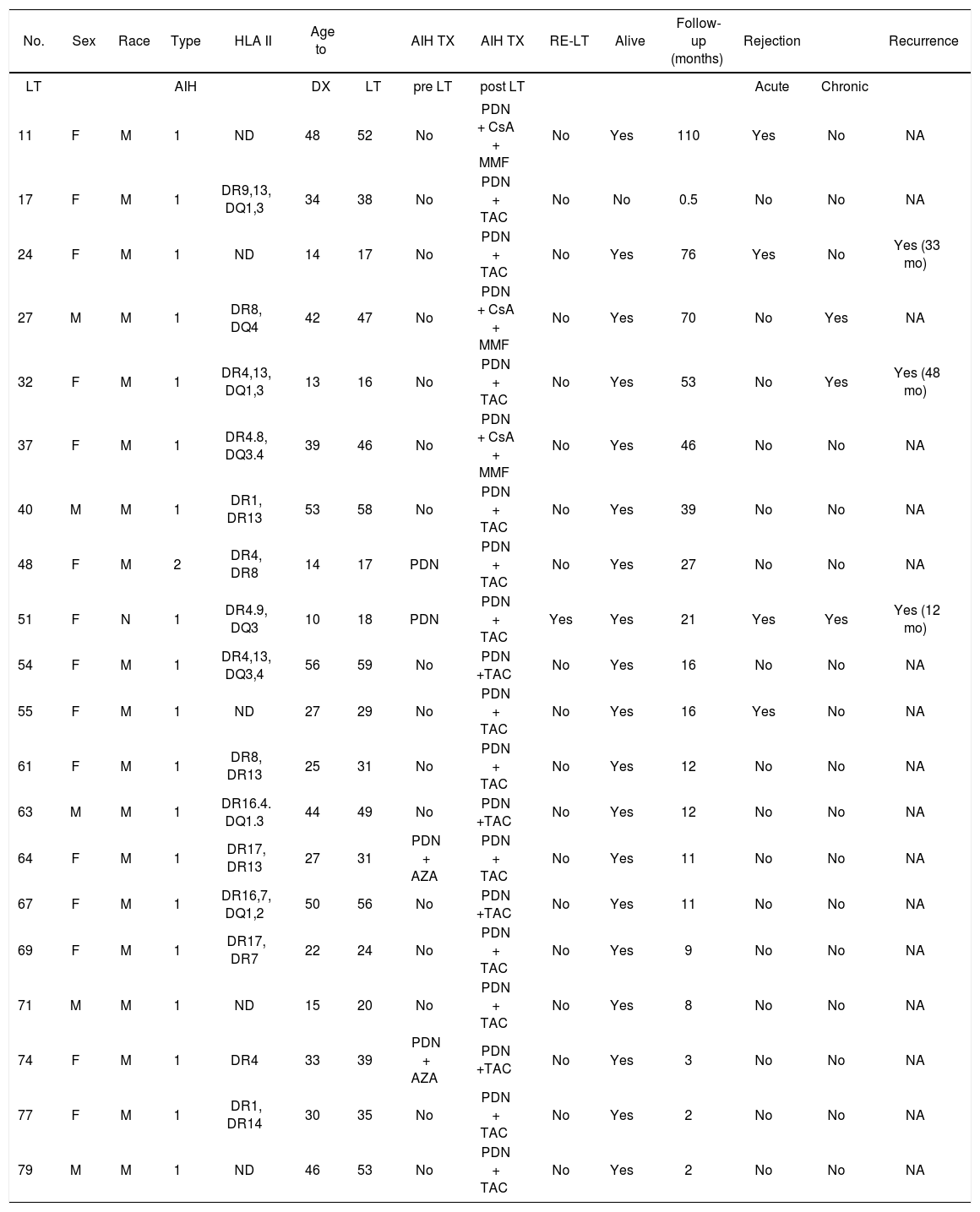
| No. | Sex | Race | Type | HLA II | Age to | AIH TX | AIH TX | RE-LT | Alive | Follow-up (months) | Rejection | Recurrence | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LT | AIH | DX | LT | pre LT | post LT | Acute | Chronic | |||||||
| 11 | F | M | 1 | ND | 48 | 52 | No | PDN + CsA + MMF | No | Yes | 110 | Yes | No | NA |
| 17 | F | M | 1 | DR9,13, DQ1,3 | 34 | 38 | No | PDN + TAC | No | No | 0.5 | No | No | NA |
| 24 | F | M | 1 | ND | 14 | 17 | No | PDN + TAC | No | Yes | 76 | Yes | No | Yes (33 mo) |
| 27 | M | M | 1 | DR8, DQ4 | 42 | 47 | No | PDN + CsA + MMF | No | Yes | 70 | No | Yes | NA |
| 32 | F | M | 1 | DR4,13, DQ1,3 | 13 | 16 | No | PDN + TAC | No | Yes | 53 | No | Yes | Yes (48 mo) |
| 37 | F | M | 1 | DR4.8, DQ3.4 | 39 | 46 | No | PDN + CsA + MMF | No | Yes | 46 | No | No | NA |
| 40 | M | M | 1 | DR1, DR13 | 53 | 58 | No | PDN + TAC | No | Yes | 39 | No | No | NA |
| 48 | F | M | 2 | DR4, DR8 | 14 | 17 | PDN | PDN + TAC | No | Yes | 27 | No | No | NA |
| 51 | F | N | 1 | DR4.9, DQ3 | 10 | 18 | PDN | PDN + TAC | Yes | Yes | 21 | Yes | Yes | Yes (12 mo) |
| 54 | F | M | 1 | DR4,13, DQ3,4 | 56 | 59 | No | PDN +TAC | No | Yes | 16 | No | No | NA |
| 55 | F | M | 1 | ND | 27 | 29 | No | PDN + TAC | No | Yes | 16 | Yes | No | NA |
| 61 | F | M | 1 | DR8, DR13 | 25 | 31 | No | PDN + TAC | No | Yes | 12 | No | No | NA |
| 63 | M | M | 1 | DR16.4. DQ1.3 | 44 | 49 | No | PDN +TAC | No | Yes | 12 | No | No | NA |
| 64 | F | M | 1 | DR17, DR13 | 27 | 31 | PDN + AZA | PDN + TAC | No | Yes | 11 | No | No | NA |
| 67 | F | M | 1 | DR16,7, DQ1,2 | 50 | 56 | No | PDN +TAC | No | Yes | 11 | No | No | NA |
| 69 | F | M | 1 | DR17, DR7 | 22 | 24 | No | PDN + TAC | No | Yes | 9 | No | No | NA |
| 71 | M | M | 1 | ND | 15 | 20 | No | PDN + TAC | No | Yes | 8 | No | No | NA |
| 74 | F | M | 1 | DR4 | 33 | 39 | PDN + AZA | PDN +TAC | No | Yes | 3 | No | No | NA |
| 77 | F | M | 1 | DR1, DR14 | 30 | 35 | No | PDN + TAC | No | Yes | 2 | No | No | NA |
| 79 | M | M | 1 | ND | 46 | 53 | No | PDN + TAC | No | Yes | 2 | No | No | NA |
*LT: liver transplantation. F: female. M: male. HLA: human leukocyte antigen. DX: diagnostic. PDN: prednisone. AZA: azathioprine. TAC: tacrolimus. MMF: mycophenolate mofetil. NA: not applicable.
ND: not determined.
Results of laboratory analysis of samples taken at the time of liver transplantation.
| n = 20 | |
|---|---|
| ALT (U/L) | 98 (21-561, σ 146.2) |
| AST (U/L) | 148.5 (45-658, σ 187.9) |
| ALKP (U/L) | 266 (47-622, σ 177.9) |
| TB (mg/dL) | 15.65 (1.5-84, σ 21.58) |
| Prothrombin time (s) | 23.1 (11.9-120, σ 28.14) |
| Albumin (g/dL) | 3.1 (1.5-3.8, σ 0.5) |
| Globulin (g/dL) | 4.12 (1.1-5.4, σ 1.1) |
Pathological analysis of liver explants confirmed the presence of liver cirrhosis in all cases (Table 4). One of the patients had a single 2.5 cm diameter tumor that corresponded with the appearance of a well-differentiated fibrolamellar HCC.
Immunosuppressive post-transplant therapy consisted of prednisone in all cases and a calcineurin inhibitor (17 patients received tacrolimus and three patients received a combination of cyclosporine and mycophenolate mofetil) (Table 2).
The average duration of follow-up was 27.2 ± 28 months (range, 0.5-110 months). During this time, three cases (3/19, 15.7%) of recurrence of AIH were diagnosed based on abnormal liver function tests, liver biopsy, with or without autoantibodies (ANA, ASMA, antiLKM-1) (Table 2), two of them showed no inflammatory activity and the other one had mild inflammatory activity. Recurrences of AIH were treated with triple therapy; two patients received prednisone, tacrolimus and azathioprine, and one patient received prednisone, tacrolimus and mycophenolate mofetil. The response to treatment was good in the first two cases, but the third case developed rapidly progressive cirrhosis 12 months after transplantation and required retransplantation (MELD 34), during which micronodular cirrhosis with severe AIH inflammatory activity was observed. Six months after the retransplant, the patient presented with recurrence of AIH in the second graft with moderate inflammatory activity but no fibrosis. The patient received 20 mg of basiliximab intravenously on days 0 and 4, and is currently being treated with triple therapy (prednisone + tacrolimus + azathioprine). Data on HLA type II are shown in tables 2 and 5. According to Fisher's test, there was no correlation between HLA and AIH or its recurrence.
Class II HLA and recurrence in AIH liver transplant patients (n = 16).*
| Class II HLA | HAI recurrence (n = 2) | No recurrence (n = 14) | Fisher | Significance |
|---|---|---|---|---|
| DR13 | 0 | 5 (35%) | 0.94 | NS |
| DR4 | 1 (50%) | 4 (28%) | 0.95 | NS |
| DR13/DR4 | 1 (50%) | 1 (7%) | 0.89 | NS |
| DR8 | 0 | 5 (35%) | 0.94 | NS |
| DR9 | 1(50%) | 2 (14%) | 0.91 | NS |
| DR17 | 0 | 2 (14%) | 0.96 | NS |
| DR3 | 0 | 0 | - | - |
Four patients (20%) experienced episodes of acute cellular rejection and three patients (16.6%) developed chronic rejection. All cases of rejection had a Banff score of mild and exhibited good biochemical responses to treatment with corticosteroid boluses and to optimization of immunosuppression.
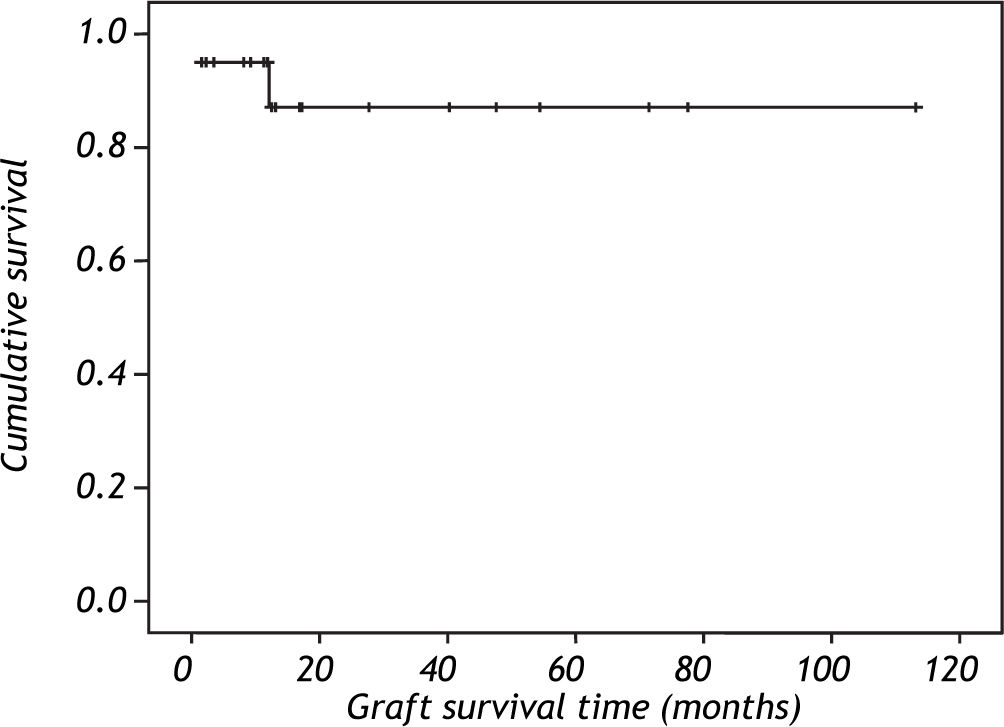
Survival was 94% after the first year and 85% after the second year according to Kaplan Meier curve (Figure 1). One patient (5%) died 14 days after the transplant because the primary non function of the graft.
DiscussionThe prevalence of AIH is 11.6-16.9 per 100,000 inhabitants in Europe8 and 0.1-1.9 per 100,000 inhabitants in the USA. There are no epidemiological data on this entity for Peru.
Cirrhosis secondary to AIH is an indication for LT in 2-3% of pediatric transplants and in 4-6% of adult transplants in the USA and Europe. LT is indicated in cases of acute liver failure and decompensated cirrhosis with a MELD score ≥ 15 and for patients with HCC who fulfill the Milan criteria.7-10 AIH cirrhosis was the most prevalent indication for LT from 2000 to 2010 (25.6%) at our institution; this contrasts with data from transplant centers in other parts of the world in that the main indications for LT elsewhere are cirrhosis caused by HCV or alcohol consumption.10-13 The reason for this disparity is that, in our study, strict selection criteria were applied at the beginning of this period because of the shortage of donors; this gave priority to young patients without major comorbidities, which skewed the sample population. However, the MELD score has been included in the LT selection criteria since 2009, which should avoid this disparity in indications for LT in the future.
In all of our cases, the indication for transplantation was cirrhosis with a MELD score ≥ 15, including one case with HCC. No cases of acute liver failure occurred in our series, in contrast to other centers, at which up to 25% of cases consist of acute liver failure.14
In our series, there was a female predominance (75%) and 95% of the participants were of mixed racial origin. The age distribution was bimodal, with peaks at 30-39 years and 50-59 years. Phenotypic differences in the manifestation of this disease occur between geographic areas; it has been reported that the age of onset is lower in South America than in other parts of the world and that the cholestatic form predominates in South America, which is consistent with the findings of our study.2,15,16 Some authors have reported that an initial presentation of cirrhosis is more frequent in black race AIH populations than in Caucasian AIH populations. In our series, a black race patient had a poor outcome; the patient developed a severe form of recurrence that required retransplantation, a year later AIH developed again.16
Susceptibility to AIH has been associated with specific alleles of the HLA complex, especially the HLA-DR serotypes. AIH has been associated with HLA-DR3+ and HLA-DR4+ in US and European populations and with HLA-DR13 in South American populations (Argentina and Brazil), and HLA-DR3+ has been associated with a higher incidence of recurrence after LT.1,3,17-20 In our series, AIH was more frequent in patients with HLA-DR13+ and HLA-DR4+, which agrees with the results of other South American authors.20,21 None of our patients had HLA-DR3+, in contrast to other studies 5,17,18,22,23
The recurrence of AIH in our series was 15.7%, similar to the reported by other centers (10-82%) (Table 6).4,9,14,22,24-29 The wide range of recurrence reported in the literature may be caused by differences in protocols, biopsy techniques or diagnostic criteria (e.g., elevation in alanine aminotransferase or aspartate aminotransferase level, persistence of autoantibodies, hypergammaglobulinemia, elevation of IgG level, his-tological findings, exclusion of alternative etiologies, response to steroids) because a standard score has not been developed for the diagnosis of recurrent AIH.29 We believe that including a liver time-guided biopsy protocol could improve the diagnosis of recurrent AIH, given that histological recurrence could precede clinical and biochemical recurrence, as has been reported in a small case series.23
Comparison of AIH recurrence at 11 liver transplant centers.
| Liver transplant center | Recurrence (%) | Follow-up interval (months) | Recurrence interval (months) | ReOLT |
|---|---|---|---|---|
| Spanish Coop. Group, 199925 Spain. | 9/27 (33) | 44 ± 28 (8-108) | 31 ± 18 (8-53) | No |
| H. Paul Brousse, 199924 France | 3/15 (20) | 58 (24-102) | 24 ± 12 (12-36) | 2 |
| U. Birmingham, 199926 UK | 13/47 (28) | 50 | 29 (6-63) | 3 |
| U. Humboldt, 199922 Germany | 18/22 (82) | 47 (20-101) | - | No |
| U. Ca. San Francisco, 199918 USA | 8/40 (20) | 42 | 18 ± 3 | No |
| Mt. Sinai Hospital, 200014 USA | 6/24 (25) | 27 ± 14 (6-52) | 15 ± 2 (12-18) | 4 |
| Mayo Clinic, 200127 USA | 7/41 (17) | 114 ± 19 | 55 ± 12 | 1 |
| U. Baylor, 20024 USA | 11/55 (20) | 28 (1-132) | - | No |
| Hannover Medical School, 200428 Germany | 9/28 (32) | 43-190 | 6-80 | 2 |
| H. King Faisal, 200710 Saudi Arabia | 3/16 (18.7) | 17 (1-67) | - | No |
| H. Almenara, 2010 Peru | 3/19 (15.7) | 28.2 ± 28 (2-110) | 31 ± 18 (12-48) | 1 |
Risk factors for recurrence include discontinuation of prednisone after transplantation, AIH type 1 receptor, positivity to HLA-DR3+, a long post-transplant interval; a high aspartate aminotrans-ferase, alanine aminotransferase and IgG before the transplant, or moderate to severe inflammatory activity or plasma cell infiltration in the liver explant.3,9,24,30 In our series, three cases of recurrence occurred in patients less than 20 years of age with type 1 AIH. Two of the three cases of recurrence were HLA-DR4+, including the case that required retransplantation. This finding has not been described in other studies.5,17,18,22,23 In one patient in our study, AIH recurred after a second liver graft. Others have also observed a high incidence of recurrence (33%) after a second trans-plant.14
In patients who receive LT because of AIH, the frequency of rejection episodes is 70%, patients experience more than one episode of rejection and the frequency of steroid-resistant rejection is higher than for other etiologies.14,18 We observed seven (36.8%) cases of rejection, which is less than that reported by other authors. Our patients all responded to corticosteroid boluses and optimization of immu-nosuppression. The patients with recurrent AIH had at least one episode of acute cellular rejection, and one patient also experienced ductopenic rejection. This relationship has been observed by other au-thors.14
ConclusionIn conclusion, AIH was the predominant indication for LT during the first 10 years of our center. The presentation of AIH was predominantly choles-tatic in young women and was associated with HLA-DR13 and HLA-DR4. The post-transplant survival rate was satisfactory, but not without complications such as rejection and recurrence; the latter may represent a risk factor for graft loss and retransplan-tation.
AcknowledgmentsThe authors thank Jorge Tarrillo M.D. and Julia Sumire M.D. for statistical and histopathological support.
Abbreviations- •
AIH: Autoimmune hepatitis.
- •
HLA: Human leukocyte antigen.
- •
MELD: Mayo end-stage liver disease.
None.
Conflict of InterestThe authors of this manuscript have no conflicts of interest to disclose as described by the Annals of Hepatology.