Non-alcoholic fatty liver disease (NAFLD) is the most common chronic liver disease in Western countries, and its prevalence is increasing worldwide. It currently affects approximately 30% of adults and 10% of children and adolescents. The resulting increase in the number of patients with NAFLD is expected to translate into increased numbers of patients with liver cirrhosis, and hepatocellular carcinoma. In this context, it is particularly important to identify patients at risk for progressive chronic liver disease. Currently, liver biopsy is the gold standard to diagnose non-alcoholic steatohepatitis (NASH) and to establish the presence and stage of fibrosis. Due to the remarkable increase in the prevalence of NAFLD and the concomitant efforts in developing novel therapies for patients with NASH, non-invasive, simple, reproducible, and reliable noninvasive methodologies are needed. This paper provides a concise overview of the role of non-invasive diagnostic tools for the determination of presence and extent of fibrosis in NAFLD patients, with particular emphasis on the methods currently available in clinical practice.
NAFLD, non-alcoholic fatty liver disease;
NASH, non-alcoholic steatohepatitis;
CLD, chronic liver disease;
LSM, liver stiffness measurement;
ALT, alanine aminotransferase;
AST, aspartate aminotransferase;
TE, transient elastography;
BMI, body mass index;
Z-BMI, Z-score of BMI;
OGTT, oral glucose tolerance test
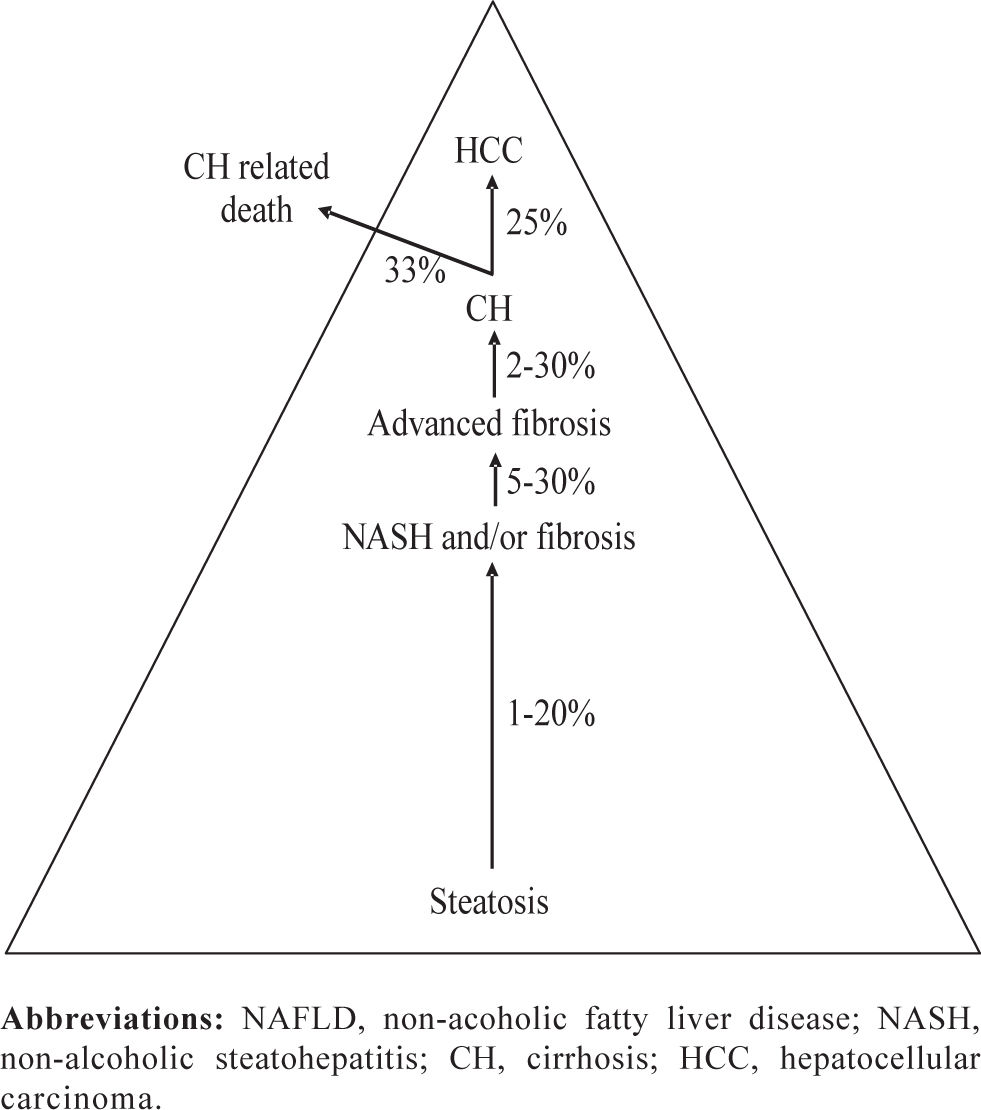
IntroductionNon-alcoholic fatty liver disease (NAFLD) is the hepatic manifestation of the metabolic syndrome, a cluster of abnormalities related to insulin resistance, frequently associated with obesity. The high prevalence of NAFLD, and the likelihood of evolution to cirrhosis and its complications warrant increased attention toward this disorder.1,2 Disease progression depends on the presence of hepatocellular damage, inflammation and fibrogenesis which define a pathological entity called non-alcoholic steatohepatitis (NASH). The increasing number of patients with NAFLD is expected to translate into increased prevalence of liver cirrhosis, and hepatocellular carcinoma3 (Figure 1). Currently, histopathological analysis of liver tissue represents the only mean to assess fibrosis in NAFLD4 and to disclose other histological finding typical of NASH. In the past decade, major efforts have been directed at identifying non-invasive methods for the assessment of liver fibrosis in different chronic liver diseases (CLD) including NAFLD. Several non-invasive approaches — serum markers, transient elastography (i.e. liver stiffness measurement; LSM) and a re-visitation of classical imaging techniques — have been proposed as a replacement for, or to be used in combination with, histopathological analysis of liver biopsies.5 This article provides a concise overview on the non-invasive diagnostic methodologies proposed to differentiate simple fatty liver from possible fibrogenic evolution typical of NASH and to stage fibrosis in NASH. Given the considerable increase in the prevalence of overweight among children and adolescents in Western countries, NAFLD represents an emerging clinical problem affecting also a substantial proportion of these subjects (2.6 to 9.8%).6-9 Therefore, a concise analysis of non-invasive approaches in the pediatric setting is also included.
Non-invasive methodologies: An overviewWith the current epidemic of obesity, type 2 diabetes, and other abnormalities associated with the metabolic syndrome, NAFLD has become the most frequent liver disease in Western countries, while its prevalence in the developing world is increasing at a worrying pace. As already mentioned, NAFLD encompasses a spectrum of diseases ranging from simple steatosis with or without inflammation, to a more severe entity, NASH that is associated with fibrosis and carries a significant risk to progress to cirrhosis and its complications, including hepatocellular carcinoma (HCC).10 While simple steatosis will probably never progress in the majority of patients,11 patients with NASH and fibrosis require a close follow-up and enrolment in clinical trials for testing novel pharmacological approaches, and may also have to undergo periodical screening for HCC. Thus, given the extremely high prevalence of this condition in the general population (up to 30% in Western countries)12 it is critical to define non-invasive methods that could allow to focus attention on those with the higher likelihood to progress.
It should be noted that fibrosis developing in the context of NASH shows several differences when compared to fibrosis developing in patients with chronic viral hepatitis. First, fibrosis develops in a liver where hepatocytes are laden with fat, an event associated with changes in the biochemical and biophysical properties of the tissue. Second, the pattern of fibrosis development involves predominantly zone 3, and leads to formation of pericellular bundles (chicken-wire pattern). This aspect is also associated with the involvement of fibrogenic cells that are believed to be implicated in the process, with activated hepatic stellate cells playing a key role.13 The lack of a clear-cut biomarker that easily allows identification of patients with NAFLD, together with the fact that metabolic abnormalities and cardiovascular disease may overshadow the hepatic disturbances and delay or prevent referral to a hepatologist, have delayed the recognition of the factors associated with fibrosis. As a result, sophisticated and extensively tested diagnostic algorithms have yet to be developed. Nonetheless, a number of clinical studies that have cross-sectionally evaluated the patients with NASH and fibrosis have allowed the identification of factors associated with a greater risk of fibrosis.4,14-25 A list of the main clinical and laboratory parameters related with advanced stages of disease is shown in Table 1 In particular, the risk conveyed by the clinical features of the metabolic syndrome indicates that the severity of the underlying pathophysiological abnormality has a significant impact on disease progression. Several factors, such as age, AST/ALT ratio, extracellular matrix proteins, and thrombocytopenia have been implicated in other types of liver diseases, including hepatitis C, indicating the role of matrix turnover and/or portal hypertension. Finally, the role of autoantibodies recognizing adducts with oxidative stress-related products recalls data previously described in alcoholic liver disease,26 while an increase in ferritin plasma levels has been interpreted as a proxy of inflammatory activity rather than a marker of iron overload. Interestingly, age and insulin-resistance, that almost invariably emerge as risk factors in cross-sectional studies,14-16,19 appear to be poorly correlated with fibrosis progression in longitudinal studies,4,18,26 reflecting the complexity of understanding fibrosis dynamics in this disease.
Main predictive factors of advanced fibrosis in patients with NASH (data from references 4, 14-25).
| Clinically determinable factors | Laboratory tests |
|---|---|
| • Older age | • Low platelets count |
| • Gender | • ALT, AST/ALT ratio |
| • Elevated BMI | • Elevated ferritin levels |
| • Diabetes mellitus | • Indexes of insulin resistance |
| • Visceral obesity | (HOMA, QUICKI, OGIS) |
| • Metabolic syndrome | • Elevated HA |
| • Systemic hypertension | • Anti-MDA antibodies |
Abbreviations: NASH, non-alcoholic steatohepatitis; BMI, body mass index; ALT, alanine aminotransferase; AST, aspartate aminotransferase; HOMA, homeostatic model assessment; OGIS, oral glucose insulin sensitivity index; QUICKI, quantitative insulin-sensitivity check index; HA, hyaluronic acid; MDA, malondialdehyde.
A small number of studies have provided performance data for tests that identify fibrosis in patients with NAFLD (Table II). It is important to realize that only in few studies22,27-29 the results of the training set were confirmed in an independent, validation cohort. Moreover, interpretation of the available data is not always easy, particularly because these series often report on small numbers of patients. In addition, evaluation of fibrosis stage varies across studies. While the Brunt scoring system (or its recent modification)33 has been used more often, other studies have employed different systems, making comparisons difficult except for extreme stages. Additionally, the fact that performance of the tests varies based on the prevalence of the severity of fibrosis in the population tested makes difficult to extrapolate the results in clinical practice. This is particularly important when considering that patients with NAFLD may be seen in settings (e.g. diabetes or obesity clinics) where the prevalence of advanced fibrosis is largely lower than in a Hepatology tertiary referral center.
Serum markers of fibrosis in non-alcoholic fatty liver disease.
| Index | N. of pts | Staging system | Factors | Fibrosis stage | Cut off | PPVa (%) | NPVa (%) | AUC |
|---|---|---|---|---|---|---|---|---|
| BAAT15 | 93b | METAVIR | Age, BMI, serum ALT, triglycerides | F • 2 vs • 1 | 2 | 61 | 86 | 0.84 |
| OELF27 | 61c | Modified | Age, HA, TIMP-1, PIIINP | F • 3 vs • 2 | 0.375 | 80 | 98 | 0.87 |
| Scheuer | 0.462 | 87 | 96 | |||||
| ELF plus simple | 192c | Kleiner | HA, TIMP-1, PIIINP plus NFS | F • 1 | -5.002 | 86 | 66 | 0.84 |
| clinical markers28 | F • 2 | -0.995 | 94 | 75 | 0.93 | |||
| F • 3 | -0.2826 | 77 | 99 | 0.98 | ||||
| HA 30 | 79 b | Brunt | HA | F • 3 vs • 2 | 46.1 | 51 | 96 | 0.92 |
| NFS 22 | 480b | Brunt | Hyperglycemia, BMI, platelet | F • 3 vs • 2 | >-1.455 | 56 | 93 | 0.88 |
| 253d | count, albumin, AST:ALT, age | > 0.676 | 90 | 85 | 0.82 | |||
| NS39 | 112b | Brunt | Type IV collagen, HA | F • 3 vs • 2 | Coll • 5 or HA • 50 | 66 | 95 | NS |
| FT31,32 | 170b | Brunt/ | Total bilirubin, GGT, gender, age, | F • 2 vs • 1 | 0.30 | 54 | 90 | 0.81 |
| 97d | Kleiner | • 2-macroglobulin, apolipoprotein | 0.70 | 98 | 76 | |||
| A1, haptoglobin | F • 3 vs • 2 | 0.30 | 71 | 98 | 0.88 | |||
| 0.70 | 97 | 89 | ||||||
| BARD29 | 827b | Kleiner | Diabetes mellitus, BMI, ALT:AST | F • 3 | 2-4 | 43 | 96 | 0.81 |
| 160d |
Abbreviations: ALT, alanine aminotransferase; AST aspartate aminotransferase; GGT gamma-glutamyl transpeptidase; AUC, area under receiver operator curve; BAAT, BMI, ALT, Age, Triglycerides; BMI, body mass index; OELF, original european liver fibrosis; ELF, enhanced liver fibrosis; FT, FibroTest; HA, hyaluronan; PIIINP, N-terminal propeptide of type III procollagen; NFS, NAFLD fibrosis score; PPV, positive predictive value; NPV, negative predictive value; NS, not stated; TIMP, tissue inhibitors of metalloproteinases; BARD, BMI, AAR (AST/ALT ratio), Diabetes mellitus.
It is interesting to note that the available non-invasive markers of fibrosis in NASH not only include single serum markers, or combination thereof, but algorithms that have been developed to accommodate clinical parameters, such as the presence or absence of diabetes.22,34 In general, performance of these tests will allow identifying or excluding patients with severe fibrosis, although a significant proportion of the population is likely to fall in an undetermined area. Clearly, also in this case, a dynamic test that allows monitoring of changes in fibrosis or fibrogenesis would greatly improve our ability to follow these patients.
Recently, transient elastography (TE) has been proposed also for the assessment of fibrosis in patients with different forms of chronic liver disease.5,35 TE is based on a non-invasive medical device (Fibroscan®, Echosens SA, Paris, France). This system has received a great attention in the past 5 years as a measurement of liver stiffness, which is considered a direct consequence of the fibrotic evolution of CLD.36 The major limitation of this technique in patients with NASH is represented by high prevalence of obesity, considering that a BMI ≥ 28 is independently associated with failure of TE examination.37 Moreover, the inter-observer agreement was found to be lower in the presence of moderate or severe steatosis.38 Yoneda et al39 recently reported that TE was successfully used in 67 patients with NAFLD, demonstrating progressive increases in liver stiffness along the stages of fibrosis, and excellent sensitivity and specificity in the identification of patients with cirrhosis. An additional problem that may arise in patients with NAFLD and metabolic syndrome is related to the possible presence of congestive heart failure that has been recently shown to influence liver stiffness measurement.40
Although several tests (Table II) are sufficiently valid to identify patients with advanced fibrosis caused by NASH, a critical point is whether this is the most useful determination for a correct management of these patients. The sole recognition of bridging fibrosis and cirrhosis would overlook patients with lower degrees of fibrosis, which nonetheless are at risk to progress, especially if they are young. For this reason, a number of tests have been proposed to differentiate the presence of NASH from bland steatosis.41,42 Not surprisingly, some of the biomarkers tested were similar to those used for the identification of fibrosis.16,43 A combination of biochemical markers was recently used to develop the NashTest, that was evaluated in a training set and a validation set.44 Another interesting approach is represented by the evaluation of plasma caspase-3-generated cytokeratin-18 fragments, a biomarker of hepatocytes apoptosis.45 Levels of cytokeratin-18 fragments were able to identify patients with NASH as compared to those with bland steatosis with remarkably high specificity and acceptable sensitivity. Larger studies are awaited to test the usefulness of this novel biomarker (for an extensive review see 41-42).
Other non-invasive test may be of use in patients with fatty liver. The SteatoTest, a derivation of FibroTest/ActiTest46 has been proposed as a simple quantitative estimate of liver steatosis. An algorithm based on body mass index (BMI), waist circumference, triglycerides and gamma-glutamyl transpeptidase was used to develop the fatty liver index47 that may be helpful in selecting subjects for liver ultrasonography and lifestyle counseling. Finally, the ASH/NASH index (ANI) has been found to represent a useful tool for detecting alcohol abuse in patients with steatohepatis.48
The pediatric settingGiven the strong association of NAFLD with increased BMI and the considerable increase in the prevalence of overweight among children and adolescents,49 NAFLD represents an emerging clinical problem affecting a substantial proportion of these subjects (2.6 to 9.8%),6,7 especially in the presence of obesity.8 Efforts in identifying non-invasive methods for predicting fibrosis assume particular relevance in the pediatric setting, where the use of liver biopsy is perceived as bearing higher risks and is less acceptable than in adults.
Considering routine laboratory variables, the NASH Clinical Research Network recently50 failed to identify tests with an adequate discriminating power to replace liver biopsy in evaluating NAFLD pattern and fibrosis severity in children and adolescents. However, other crosssectional studies evaluating children with NASH and fibrosis have allowed the identification of clinical and biochemical parameters associated with advanced stages of fibrosis in patients with NAFLD. Sartorio et al51 showed that the Z-score of BMI (Z-BMI), ALT, uric acid, glucose during oral glucose tolerance test (OGTT), and insulin during OGTT are independent predictors of NAFLD in obese children, with most of the prediction explained by ALT and Z-BMI. Abdominal rather than generalized obesity contributes to liver fibrosis in children with NAFLD, and accordingly, waist circumference seems to be associated with fibrosis. Therefore, the presence of abdominal obesity is an additional criterion for the selection of children and adolescents who should undergo extensive investigation, including liver biopsy.52-53
We recently evaluated the diagnostic accuracy of TE54 and enhanced liver fibrosis test (ELF)55 in predicting fibrosis in a cohort of NAFLD pediatric patients. These authors demonstrated that TE is an accurate and reproducible methodology to identify, in children and adolescents affected by NAFLD, those without any degree of fibrosis or significant fibrosis, or with advanced fibrosis. Similarly, the ELF test seems to predict fibrosis stages in pediatric NAFLD patients with a high degree of sensitivity and specificity, and, interestingly, the results were superior to those reported for adult patients with NAFLD.28 Information obtained through these methodologies may be relevant for identifying subjects with progressive fibrogenic liver disease that require further histopathological analysis or therapeutic follow-up.
ConclusionThe large number of publications on non-invasive methodologies confirms the interest in, and need for, this type of innovation in the setting of NAFLD; however, the complexity of these surrogate non-invasive measures of disease progression need further investigation and guidance on their use and caution on results interpretation.
Some major considerations have arisen from the experience accumulated so far. First, the majority of non-invasive methodologies have sufficient to excellent diagnostic accuracy for the detection (or exclusion) of advanced fibrosis and cirrhosis, but none allow a follow-up of the fibrogenic evolution of NAFLD in a stepwise fashion. In other words, due to the absence of a true gold standard, achieving 90% diagnostic accuracy remains a goal for the future. Therefore, non-invasive methodologies must be integrated with histology in indefinite cases and/or to confirm fibrotic evolution and discern other histological features of NASH.
Hopefully, new approaches employing high-throughput technologies including genomics, proteomics, metabolomics and glycomics may identify biomarkers that may help in categorizing patients, enhancing or replacing currently available non-invasive methodologies. Up to now, only single-center studies have employed these technologies in NAFLD.56,57 These types of studies must be encouraged and clearly require a multicenter design in order to gather a large number of well-characterized cases and controls.