Non-alcoholic fatty liver disease (NAFLD) is defined as a spectrum of liver diseases ranging from simple steatosis to steatohepatitis (NASH). Alterations in intestinal microbiota and inflammatory response may play a key role in disease progression and development of complications in liver diseases, mainly in cirrhosis and NASH. The aim of this study was to perform a systematic review on randomized clinical trials (RCTs) testing probiotics, prebiotics or both (synbiotics) in the treatment of NAFLD in adult patients. After the screening process, 9 full-text articles were included in the review and 6 studies were excluded. Three randomized controlled trials were finally included in the qualitative synthesis. All patients in all the 3 studies were randomized to receive different formulations of probiotics, synbiotics or placebo. Reductions in aminotransferases were observed in the treated group in 2 of the studies. However, in one study reductions were also detected in the control group. In conclusion, the available evidence precludes, for the moment, recommendations on the use of pre and probiotics in clinical practice.
Non-alcoholic fatty liver disease (NAFLD) is defined as a spectrum of liver diseases ranging from simple steatosis to steatohepatitis (NASH), which in some cases can progress to fibrosis/cirrhosis1 and complications such as hepatocellular carcinoma.2 NAFLD has been shown to be the most common liver disease affecting children and adults worldwide with increasing incidence.3 Its true prevalence in the general population is unknown due to the high number of asymptomatic patients. It has been estimated that NAFLD affects 20% of the world’s population. Hepatic disease is the most prevalent in the United States of America (USA).4–6 Its prevalence can be much higher in several diseases and may reach 90% in morbid obese patients eligible for bariatric surgery, 69% in type 2 diabetes mellitus and 50% in dyslipidemic patients.7
Hepatic steatosis is accepted to be a relatively benign non-progressive process. It is different from NASH in which an evolving process might take place. Approximately 26 to 37% of NASH patients may present progression in the disease, and 9 to 20% of these cases can progress to cirrhosis. Many cases of cryptogenic cirrhosis as well as hepatocellular carcinoma result from NASH progression.8
NAFLD patients present higher mortality rates than the general population. The most common cause of death in NAFLD is the cardiovascular diseases. This is not true of patients presenting NASH in which mortality rates are due more often to hepatic causes.7
Considering that NAFLD progression may lead to cirrhosis or hepatocellular carcinoma, an effective treatment is necessary. However, there is no consensus in the treatment approach in the literature, and randomized clinical trials present several limitations. There is no proven effective therapy for NASH, although modification of risk factors, such as obesity, hyperlipidemia, and proper diabetic control is generally recommended. Hepatitis A and B vaccinations should be given after serologic testing for immunity.9 Weight loss and increased physical activity can lead to sustained improvement in liver enzymes, histology, serum insulin levels, and quality of life in patients with NASH.10–13 Multiple drugs have been studied, but most trials have been too short to determine the real impact on clinical outcomes.14
Recently, it has been suggested that the gut microbiota is implicated in obesity, diabetes, metabolic syndrome (MS) and NAFLD through effects on caloric salvage, host energy metabolism, proinflammatory signaling, and via direct hepatotoxicity of bacterial products, including ethanol and ammonia.15 Alterations in intestinal microbiota and inflammatory response may play a key role in disease progression and development of complications in liver diseases, mainly in cirrhosis and NASH.
Prebiotics are defined as a group of non-digestible carbohydrates that beneficially affects the host by altering the composition and activity of the gut microbiota.16 Probiotics are defined as live microorganisms, which when consumed in adequate amounts, confer health effects on the host.17 Synbiotics are the combination of both pre- and probiotics. They are potential treatments options for consideration based primarily on animal studies.18 Prebiotics and probiotics have been suggested to be likely useful in the delay of disease progression and prevention of complications development due to their ability to modulate intestinal flora, intestinal permeability and inflammatory response.16,19
The aim of this study was to perform a systematic review on randomized clinical trials in which probiotics, prebiotics or both (synbiotics) were used in the treatment of NAFLD in adult patients.
Material and MethodsProtocol and registrationThis systematic review was registered at the PROSPERO International prospective register of systematic reviews platform (http://www.crd.york.ac.uk/ NIHR_PROSPERO/), number CRD42013004592. This study followed the recommendation of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement.20
Eligibility criteriaRandomized clinical trials (RCTs) testing the use of probiotics, prebiotics or both (synbiotics) in the treatment of NAFLD in adult patients (over 18 years) were included in the study. The intervention with probiotics, prebiotics or both (synbiotics) should not have been combined with any other medication. Patients should present histology-proven NAFLD/NASH. Primary outcomes of interest to be evaluated in the pre and post treatment were; aminotransferases levels (AST, ALT); liver fibrosis and NASH activity index assessed by biopsy or non-invasive methods. Secondary outcome were the body mass index (BMI). Studies where one or more of these outcomes were assessed basally and at the end of treatment were included in this review.
Search and study selectionThe search for eligible studies was performed in PubMed and Cochrane in April, 2013. The search strategy included the following set of keywords: probiotics or prebiotics or synbiotics or gut microbiota and [additional keyword]. The last gap was changed at each search using the keywords NAFLD, NASH, liver steatosis, liver, steatosis, fibrosis, liver biopsy, inflammation, liver inflammation. The searches were performed with and without limiting the types of articles (ECR, clinical trial, comparative study). The selection of eligible studies was performed by title and abstract reading. When abstracts regarding subjects or outcomes of interest were not clear, the full text of the article was read.
Data collection processData was collected by two independent investigators for the following variables: age of participants, basal and final data regarding AST, ALT, liver activity/fibrosis grade, intervention, treatment duration, and weight change. Authors of studies were contacted whenever raw data was needed.
The methodological quality assessment criteria considered were- randomized sequence generation, allocation concealment, blinding of outcome assessors, intention-to-treat analysis and description of losses and exclusions.
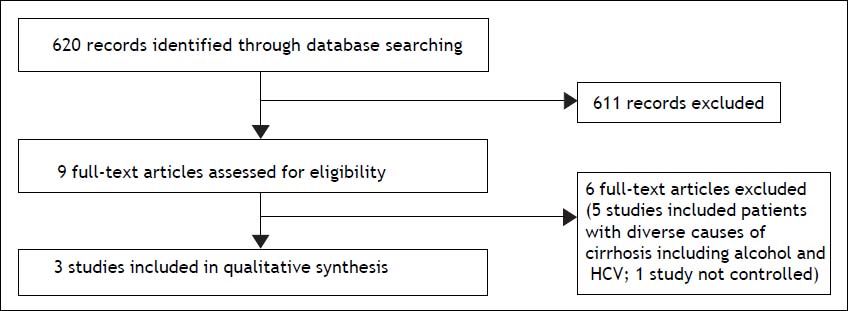
ResultsAfter the screening process (Figure 1), 9 full-text articles were included in the review and 6 studies were excluded. Three randomized controlled trials were finally included. The authors attempted a systematic review, but due to the variance amongst the three trials and small sample size, a qualitative synthesis was provided.
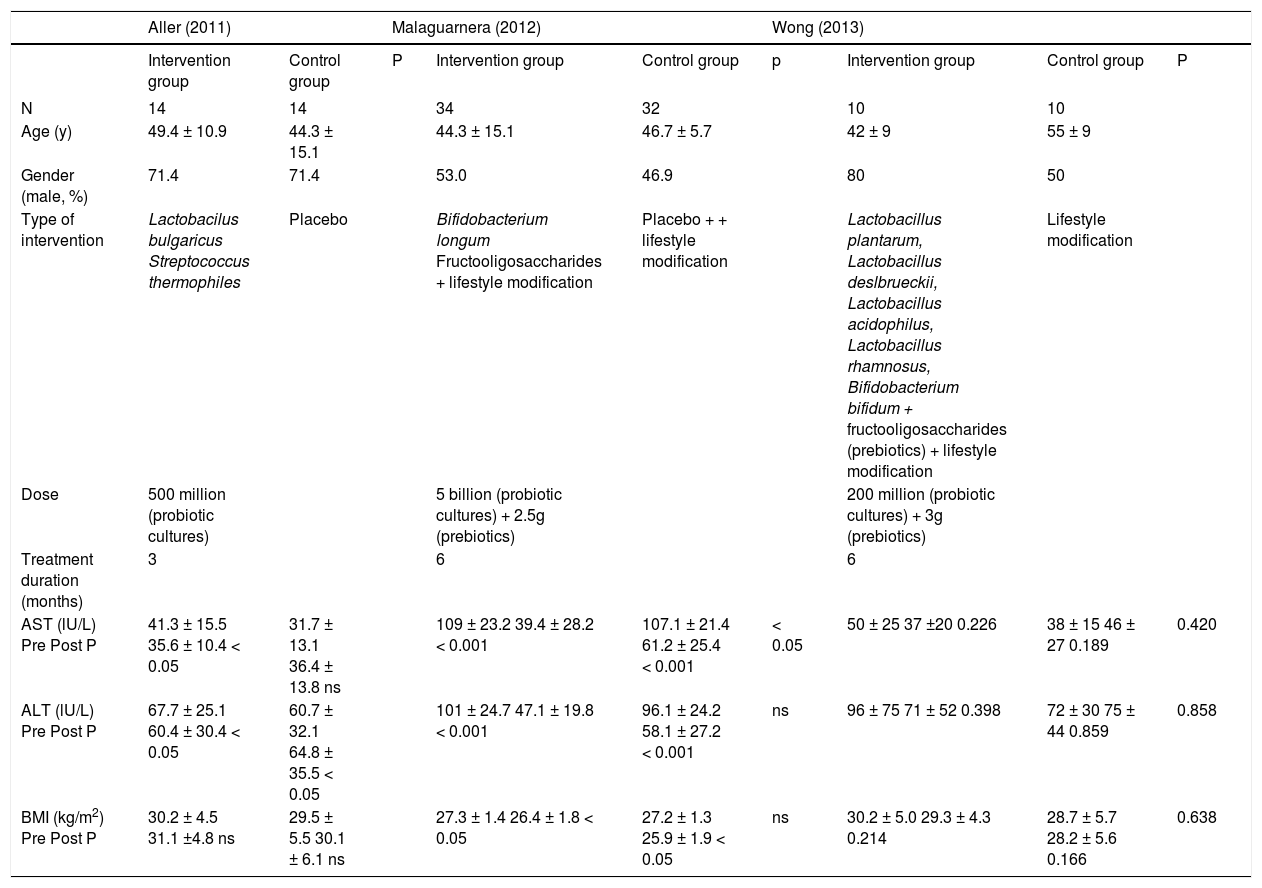
Results of individual studiesPre and post treatment aminotransferases levels and body weight change were the only outcomes assessed that were common to all the 3 studies. The results of the studies with respective comparisons within groups and between the groups (when available) are shown in table 1. All patients in the three studies were randomized to receive different formulations of probiotics or synbiotics or placebo. Also, lifestyle modifications were suggested in the studies by Malaguarnera, et al.21 and Wong, et al.22 but not in the study by Aller, et al.23 Reductions in ALT and AST were observed in the treated group in two studies.21,23 However in the study by Malaguarnera, et al.,21 reductions were also detected in the control group. Reduction in BMI was also observed in the same study21 in both treated and control groups, but not in the other 2 studies.22,23
Sociodemographic, clinical characteristics and outcomes in included studies.
| Aller (2011) | Malaguarnera (2012) | Wong (2013) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Intervention group | Control group | P | Intervention group | Control group | p | Intervention group | Control group | P | |
| N | 14 | 14 | 34 | 32 | 10 | 10 | |||
| Age (y) | 49.4 ± 10.9 | 44.3 ± 15.1 | 44.3 ± 15.1 | 46.7 ± 5.7 | 42 ± 9 | 55 ± 9 | |||
| Gender (male, %) | 71.4 | 71.4 | 53.0 | 46.9 | 80 | 50 | |||
| Type of intervention | Lactobacilus bulgaricus Streptococcus thermophiles | Placebo | Bifidobacterium longum Fructooligosaccharides + lifestyle modification | Placebo + + lifestyle modification | Lactobacillus plantarum, Lactobacillus deslbrueckii, Lactobacillus acidophilus, Lactobacillus rhamnosus, Bifidobacterium bifidum + fructooligosaccharides (prebiotics) + lifestyle modification | Lifestyle modification | |||
| Dose | 500 million (probiotic cultures) | 5 billion (probiotic cultures) + 2.5g (prebiotics) | 200 million (probiotic cultures) + 3g (prebiotics) | ||||||
| Treatment duration (months) | 3 | 6 | 6 | ||||||
| AST (lU/L) Pre Post P | 41.3 ± 15.5 35.6 ± 10.4 < 0.05 | 31.7 ± 13.1 36.4 ± 13.8 ns | 109 ± 23.2 39.4 ± 28.2 < 0.001 | 107.1 ± 21.4 61.2 ± 25.4 < 0.001 | < 0.05 | 50 ± 25 37 ±20 0.226 | 38 ± 15 46 ± 27 0.189 | 0.420 | |
| ALT (lU/L) Pre Post P | 67.7 ± 25.1 60.4 ± 30.4 < 0.05 | 60.7 ± 32.1 64.8 ± 35.5 < 0.05 | 101 ± 24.7 47.1 ± 19.8 < 0.001 | 96.1 ± 24.2 58.1 ± 27.2 < 0.001 | ns | 96 ± 75 71 ± 52 0.398 | 72 ± 30 75 ± 44 0.859 | 0.858 | |
| BMI (kg/m2) Pre Post P | 30.2 ± 4.5 31.1 ±4.8 ns | 29.5 ± 5.5 30.1 ± 6.1 ns | 27.3 ± 1.4 26.4 ± 1.8 < 0.05 | 27.2 ± 1.3 25.9 ± 1.9 < 0.05 | ns | 30.2 ± 5.0 29.3 ± 4.3 0.214 | 28.7 ± 5.7 28.2 ± 5.6 0.166 | 0.638 | |
IG: intervention group. CG: control group. ns: presented in original study as non significant.
Aller, et al.23 included patients with NAFLD diagnosed by liver biopsy. The study methods declared using randomized sequence generation, blinding of outcome assessors and description of losses and exclusions. There was no information regarding allocation concealment. The analysis was not carried out based on intention to treat.
The study of Malaguarnera, et al.21 was the only one that performed liver biopsies at entry and after 24 weeks of treatment. In 67% of patients, favorable histological response (reduction of steatosis and reduction in the NASH activity index by at least 3 points) was observed. The study method mentioned using randomized sequence generation, blinding of outcome assessors, and description of losses and exclusions. There was no information regarding allocation concealment and intention-to-treat analysis.
Wong, et al.22 included patients with histologyproven NASH by liver biopsy performed within 6 months before inclusion. In this particular study, intrahepatic triglyceride content and liver stiffness (using the Fibroscan device) were evaluated pre and post treatment. No change was observed after treatment in either groups in the aminotransferases levels and BMI, but there was a decrease in the intrahepatic trygliceride content. The authors stated that randomized sequence generation, allocation concealment were employed in the study protocol and losses and exclusions were reported. The study was open-label.
DiscussionMost studies reported increased risk of progression of NAFLD to cirrhosis, mainly in the presence of more severe histology at baseline.24,25 The relative risk-benefit of different interventions remains one of the most challenging aspects of treating NAFLD. Correcting dietary habits and increasing physical activity are considered the cornerstones and these lifestyle changes are typically part of the standard recommendations.26
In order to emphasize uncertainties regarding the management of NAFLD, the most recent practice Guideline by the American Association for the Study of Liver Diseases (AASLD), American College of Gastroenterology (ACG), and the American Gastroenterological Association (AGA)7 recommends weight loss that can be achieved either by hypocaloric diet alone or in conjunction with increased physical activity. The guideline states that: ursodesoxycholic acid is not recommended for the treatment of NAFLD or NASH; that metformin is not recommended as a specific treatment for liver disease in adults with NASH, because it has no significant effect on liver histology; that pioglitazone can be used to treat steatohepatitis in patients with biopsy-proven NASH, but long term safety and efficacy is not established; that vitamin E (α-tocopherol) should be considered as a first-line pharmacotherapy for nondiabetic adults because it has been shown to improves liver histology with biopsy-proven NASH (although it is not recommended to treat NASH in diabetic patients, NAFLD without liver biopsy, NASH cirrhosis or cryptogenic cirrhosis). However, it must be noted that vitamin E interventions present safety issues among which are increased mortality,27 vascular events28 and prostate cancer29 as observed in some studies.
Recently, it has been suggested that some bacterial bioproducts may be hepatotoxic, as ammonia, phenols and ethanol. Increased intestinal production of ethanol, due to alterations in the gut microbiota, has been described in NASH and obese patients.30 However, the main bacterial bioproduct involved in NAFLD/NASH pathogenesis is the lipopolysaccharide (LPS), the active component of endotoxin.31,32 It has been suggested that increased LPS are related to increased inflammation and NASH progression.
Therefore, considering that hepatic steatosis and NASH are associated with bacterial proliferation and increased intestinal permeability, it could be expected that interventions that modulate intestinal microbiota may be beneficial.34
Probiotics, prebiotics or both (synbiotics) have been suggested to be used in the treatment of NASH.35 Despite numerous articles published in this area, it is difficult to assess the true effect of probiotics on NAFLD prevention or treatment, since the experiments used different animal models and different bacterial strains were employed in different experimental setups.34 A Cochrane Collaboration Systematic Review published in 200736 did not identify any RCTs and suggested that although probiotics may be well accepted and ameliorate liver function tests, the lack of randomized clinical trials makes it impossible to support or refute probiotics in the case of NAFLD.
The three studies analyzed in this systematic review presented different results concerning prebiotics and/or probiotics supplementation for liver aminotransferases levels improvement in NAFLD patients. The studies with the highest doses and combined treatment21,23 showed amelioration of aminotransferases in the treated group. From a clinical standpoint, aminotransferases improvement cannot be directly correlated to NAFLD/NASH improvement.37 However, given the lack of post-intervention liver biopsies and quantification of inflammatory markers in the included studies, this present analysis focused on aminotransferases since it was the only parameter evaluated in all the three studies that presented changes after the interventions.
Increased amelioration of aminotransferases was observable when treatment was combined with lifestyle intervention, with the resulting reduction in body mass index (BMI). However the doses of pre-and probiotics and combination of treatment differed in each study and thus hindered adequate comparisons.
In the study of Aller, et al.23 there was no combined treatment and the intervention used only pro-biotics. A reduction in AST and ALT was observed in the treatment group, without any change in BMI. The study of Malaguarnera, et al.21 employed an intervention combining a 10-fold greater dose of probiotics with prebiotics and lifestyle intervention. The reduction in the aminotransferases was much higher and there was a BMI reduction in both groups. One could attribute the amelioration to the effect of lifestyle intervention and not to the synbiotic treatment. Nevertheless, a significant difference between groups after treatment in AST levels was demonstrated, which could support the effect of the synbiotic treatment.
The study of Wong, et al.22 employed the lowest probiotic dose associated with a higher dose (compared to the study of Malaguarnera, et al.21) of prebiotic also in combination with lifestyle intervention. In spite of having similar treatment duration it did not demonstrate a significant change in any of the parameters.
It is important to highlight that treatment duration was too short in all of the studies to be able to reflect histological improvement for clinical benefit and morbimortality reduction. However, the study of Aller, et al.,23 which had the shortest treatment duration, presented significant results. This may imply a dose-response effect of probiotics treatment, which should be tested in future trials. An additional recommendation for upcoming studies would be the assessment of liver biopsy pre- and post-treatment, since only one of the trials included in this review presented such analysis. The studies also presented small sample sizes and different probiotic species combinations, which make comparisons difficult to be made.
It would be interesting to also document alterations in gut microbiota subsequent to treatment, which was not reported in any of the studies analyzed in this review. The use of molecular techniques for analysis of microbiota constituents in health and disease are now available.38
In conclusion, a central role for the microbiota in the precipitation of complications of liver diseases has been established and evidence for a more fundamental role in the etiology of certain liver diseases, such as NAFLD and NASH, continues to accumulate. Unfortunately, the paucity of high-quality clinical evidence precludes, for the moment, recommendations on the use of probiotics in clinical practice. The safety of the interventions with probiotics and synbiotics in NAFLD should be tested in longer term studies. The beneficial effects of probiotics and synbiotics on NALFD have been demonstrated in limited human studies. However, since there is sufficiently strong rationale for the use of strategies that involve modulation of the gut microbiota in the management of liver diseases, the therapeutic potential of probiotics in NAFLD should be tested in larger and high-quality studies.