Oxidative stress is importantly involved in the pathophysiology of various liver diseases. The redox state participates on the course of the inflammatory, metabolic and proliferative liver diseases. The main sources of the reactive oxygen species (ROS) are represented by the mitochondria and cytochrome P450 enzymes in the hepatocyte, Kupffer cells and neutrophils. Cells are provided with efficient molecular strategies to strictly control the intracellular ROS level and to maintain the balance between oxidant and antioxidant molecules. Hepatocyte’s proteins, lipids and DNA are among the cellular structures to be affected primarily by ROS and reactive nitrogen species (RNS). This process disrupts at cellular and molecular level the structure-function relationship on liver cells at different sites. Therefore, further studies on the molecular mechanisms of the oxidative stress pathways on liver diseases are urgently required, because they could explain the pathogenesis of various liver disorders. Moreover, new methods to evaluate oxidative stress like the oxidative markers among hepatocytes offers the potential to diagnose the degree of liver injury and ultimately to assess the response to pharmacological therapies. In this review, we discuss the molecular, metabolic and aging aspects of the oxidative stress, and the methods to evaluate oxidative stress on liver damage.
It is well known that oxidative stress plays an important role in the pathophysiology of various liver diseases, such as alcoholic liver disease (ALD),1 nonalcoholic stea-tohepatitis (NASH),2 and hepatitis type C,3 where free radicals attack to cell membranes, lipids and proteins leading to tissue injury, initially for cirrhosis and finally to hepatocellular carcinoma (HCC). However, the exact mechanisms by which oxidative stress produces hepatic injury is not yet well understood, and further studies on this topic are needed. In addition, there exist several methods to assess oxidative and nitrosative stress which deserve revision in depth to facilitate the understanding of these processes in hepatic injury. Therefore, the main objectives of this review are to discuss some aspects which play role on the generation of free radicals, and to monitor and evaluate oxidative stress by markers on liver disease. Although studies about the molecular pathology of the oxidative stress have been carried out on small animals and isolated cells, these findings strongly suggest that similar mechanisms are present in the hepatopathology of human beings, and the methods to evaluate oxidative stress on patients will be a valuable tool for diagnosis, better treatment focus and better control of the illness evolution.
OxygenReactive oxygen species (ROS) are produced naturally in aerobic life and participate in the regulation of cellular functions such as signal transduction pathways, defense against invading microorganisms and gene expression for growth or death promotion.4 The liver plays a central and important role in metabolic homeostasis, since it is the main tissue in charge of the body’s metabolism, synthesis, storage and redistribution of carbohydrates, vitamins and lipids. Therefore, the high metabolic activity in this organ is an important place for free radicals generation. There are several enzymes-induced free radicals in the liver, including diamine oxidase, aldehyde dehydrogenase, tryptophan dual oxidase, liver dehydrogenase and the cytochrome P450 enzyme system.5 Mitochondria and cy-tochrome P450 enzymes in the hepatocyte, Kupffer cells, and neutrophils are responsible for ROS production.6 ROS may act either positively or negatively on cell functioning depending on the intensity and duration of the oxidative stress produced on the cell. For example, ROS can induce apoptosis or cell proliferation, depending on the cell type and on the intensity of the stress produced.5
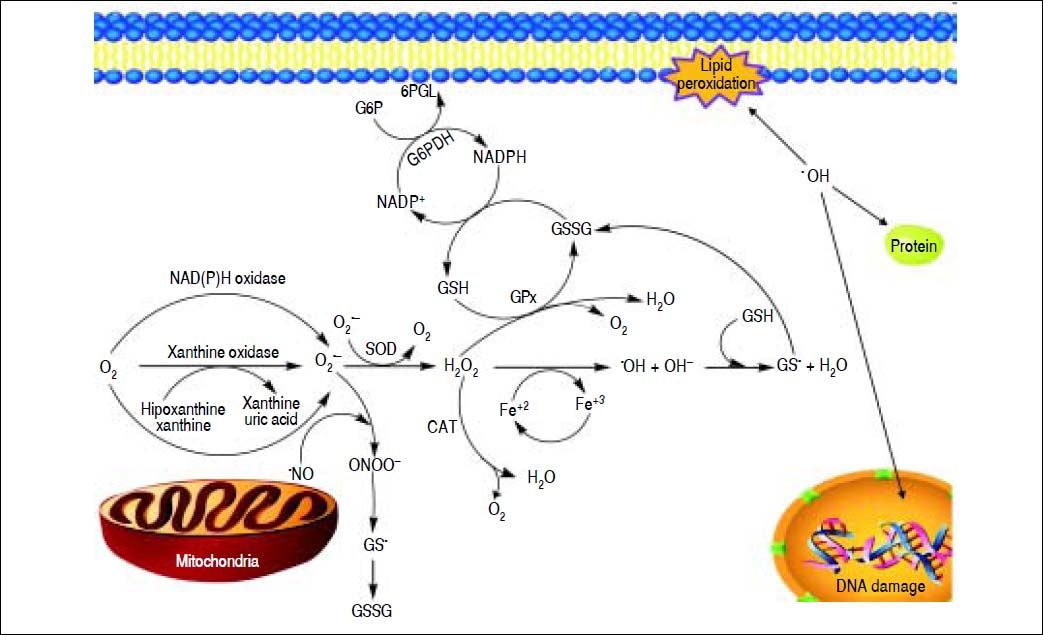
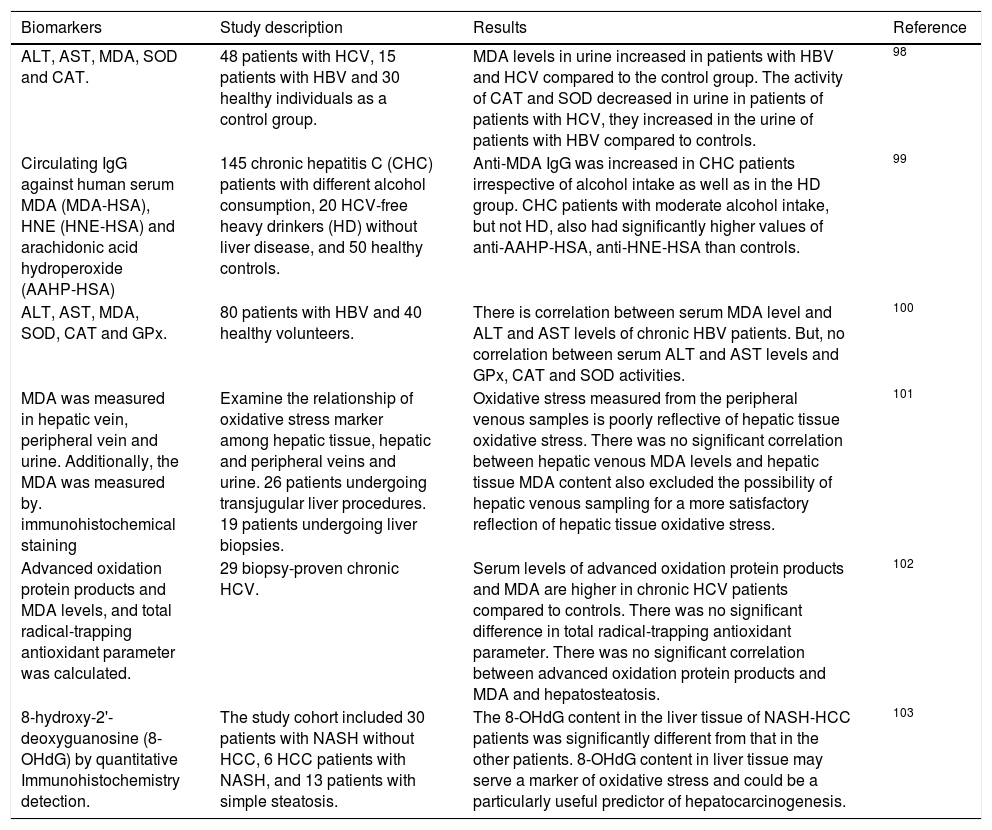
Free RadicalsFree radicals contain unpaired electrons in atoms which confer a considerable degree of reactivity to other molecules like proteins, lipids and DNA. Molecular oxygen has a unique electronic structure that can accept a total of four electrons, and frequently evolve into free radicals formation. ROS and RNS are the most important group of radical species produced in living systems. Unfortunately, an excessive amount of ROS or RNS are highly toxic to cells.6 Superoxide radical (O2•-),hydroxyl radical (OH-•) and hydrogen peroxide (H2O2) are the main forms of ROS. Reaction of O2•- with NO• produces peroxynitrite (ONOO−). ONOO− and its protonated form, peroxynitrous acid (ONOOH), react with cellular nucleophiles or oxidize hemeproteins to ferryl-oxo derivatives. Both ONOOH and ferryl-oxo complexes are strong oxidants.6 H2O2 and O2•- may react with ferric ion or nitric oxide to generate more potent oxidants (Figure 1). Ferric ion produces ferrous ion by means of a reduction reaction which, in turn, converts H2O2 to hydroxide ion and OH-*. Hydroxyl radical (which possess a redox potential of 2.8 Volts) is an extremely strong oxidant able to oxidize several molecules.7
A schematic model for the formation of ROS and NOS in the mitochondria. Electron flow leads to the formation of superoxide anion (O2•-) that is generated by the univalent reduction of molecular oxygen (O2). This process may also be mediated by enzymes such as NADPH oxidase and xanthine oxidase. Superoxide dismutase (SOD) catalyzes the dismutation of two superoxde anions into hydrogen peroxide (H2O2) and oxygen. Alternatively, H2O2 could be converted into water and molecular oxygen by enzymes catalase (CAT) and glutathione peroxidase (GPX). H2O2 can react with reduced transition metals, via Fenton’s reaction, to produce the highly reactive hydroxyl radica (OH), which, could damage lipids, DNA and proteins leading to cell death.
Defense mechanisms were developed by nature against ROS and RNS, and they are produced by several physiological or environmental activities, and these systems can prevent, repair, provide physical and antioxidant defenses. Antioxidants are substances that in low concentrations, delay or inhibit oxidation, and may decrease the concentration of oxidants, avoid the initiation of the chain reaction, join metal ions to prevent the formation of reactive species, transform peroxides into less reactive products or stop the formation and propagation of free radicals.
Oxidative stress, which is the result of an excess of ROS over the antioxidant defenses of the organism, and the free radicals attack lipids, proteins and DNA. These reactions are mainly produced through abstraction of hydrogen atom from target molecules resulting frequently in initiation of chain processes. Control of ROS steady-state level is provided not only via their production, but also via elimination. Organisms possess antioxidant system useful to inactivate ROS, or to eliminate them, minimizing or preventing their negative effects. Efficient molecular strategies were evolved in cells that allow to keep the intracellular ROS level under control, and to maintain the balance between oxidant and antioxidant molecules.8 The effects of ROS/RNS are counteracted by enzymatic and non-enzymatic antioxidant mechanisms. Antioxidant molecules eliminate free radicals from the body. There are two types of antioxidants:
- •
Low-molecular weight (LMW) compounds like ascorbic acid (vitamin C), α-tocopherol (vitamin E), glutathione (GSH), carotenoids, flavonoids and other antioxidants, and
- •
Enzymes with antioxidant activity, such as superoxide dismutase (SOD), glutathione peroxidase (GPx) and catalase (CAT) (Figure 2).8
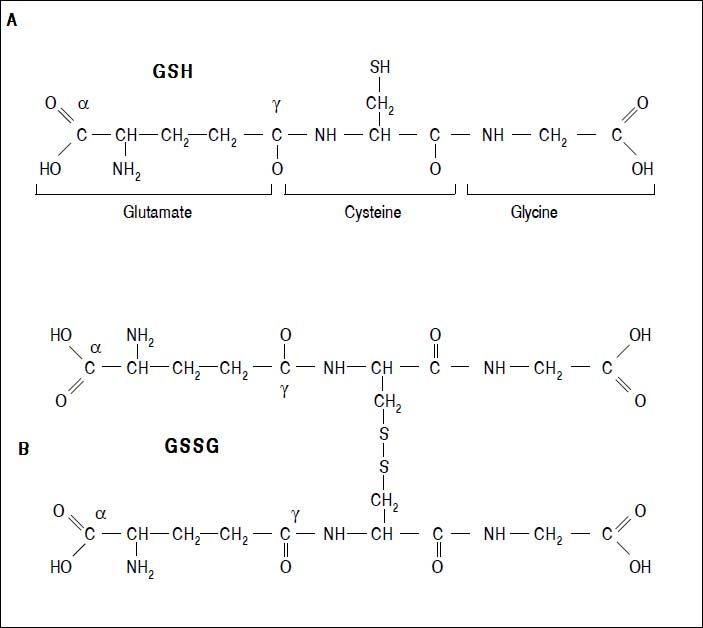
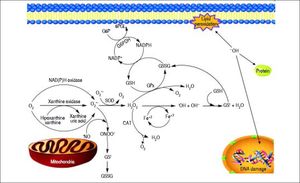
Figure 2.Glutathione is a tripeptide: L-γ-glutamyl-L-cysteinylglycine. In its reduced form (A) the N-terminal glutamate and cysteine are linked by the γ-carboxyl group of glutamate, preventing cleavage by common cellular peptidases and restricting cleavage to γ-glutamyl transpeptidase. The cysteine residue is the key functional component of glutathione, providing a reactive thiol group that plays an essential role in its functions. Furthermore, cysteine residues form the intermolecular dipeptide bond in the oxidized glutathione molecule (B).
(0.03MB).
Two forms of SOD are known. SOD-1 contains copper and zinc and is also known as Cu-ZnSOD, is primary located in the cytosol but also in the nucleus, is a homodimeric protein with a molecular weight of 32.5 kDa. Copper is essential for the catalytic reaction, while zinc is important for maintaining the structure of the protein. SOD-2, also known as manganese-dependent superoxide dismutase MnSOD, is found in the mitochondrial matrix.9 There are two isoforms that have a common mechanism: the dismutation of superoxide radical into hydrogen peroxide as shown in the following equation:
2O2•- + 2H+ + SOD → H2O2 + O2
CAT is a homotetrameric molecule with a molecular weight of 240 kDa whose principal function is to convert H2O2 to water and molecular oxygen according to the following reaction:
H2O2→ 2H2O +O2
GPx is an important enzyme in cellular antioxidant defense systems, detoxifying peroxides and hydroperoxides. As a component of the glutathione cycle, it protects the liver from reactive oxygen metabolites. Seleno-cysteine is present at the catalytic site of glutathione peroxidase, and selenium availability regulates glutathione peroxidase enzyme activity. GPx can be divided into two groups: cellular and extracelular.10 In general, GPx is a tetrameric protein with a molecular weight of 85 kDa; it requires four selenium atoms bonded cysteines within its functional structure for catalytic activity. Its function is to reduce H2O2 to water, oxidizing two molecules of GSH:
H2O2+2GSH→ GSSG2+2H2O
The reduction of GSSG is catalyzed by glutathione reductase, as shown in the following equation:
GSSG+NADPH+2GSH+H+→ 2GSH+NADP+
The GSH system functions via GPx enzymes, which inactivate H2O2 and other hydroperoxides (including alkyl and lipid peroxides) by conversion of GSH to glutathione disulphide (GSSG), which is converted back to GSH by glutathione reductase (GR) using NADPH11 (Figure 1).
GlutathioneThe tripeptide γ-glutamylcysteinylglycine or GSH is the major non enzymatic regulation of intracellular redox homeostasis, ubiquitously present in all cells, it is highly abundant in cytosol (1-11 mM), in nuclei (3-15 mM), and in mitochondria (5-11 mM), and it is considered the most abundant soluble antioxidant in these compartments. Cysteine-containing tripeptide exists either in reduced (GSH) or oxidized (GSSG) form and participates in redox reactions by the reversible oxidation of its active thiol.12 GSSG consists of two residues of GSH which have been oxidized in such a fashion as to be connected by an intermolecular disulphide bond (Figure 2).
The reaction of glutathione with a free radical can be described as follows:
GSH + R• → GS• + RH
Radicals generated could dimerize and form the oxidized glutathione as follows:
GS• + GS•→ GSSG
The main protective roles of glutathione against oxidative stress are:13
- •
Glutathione is a cofactor of several detoxifying enzymes against oxidative stress, e.g. GPx, glutathione transferase and others.
- •
GSH participates in amino acid transport through the plasma membrane.
- •
GSH scavenges hydroxyl radical (HO•) or ONOO- (Figure 1),14 singlet oxygen directly and direct oxidation leads to the production of thiylradicals,15 detoxifying hydrogen peroxide and lipid peroxides by the catalytic action of glutathionper-oxidase.
- •
Glutathione is able to regenerate the most important antioxidants, vitamins C and E, back to their active forms; glutathione can reduce the tocopherol radical of vitamin E directly, or indirectly, via reduction from semidehydroascorbate to ascorbate.
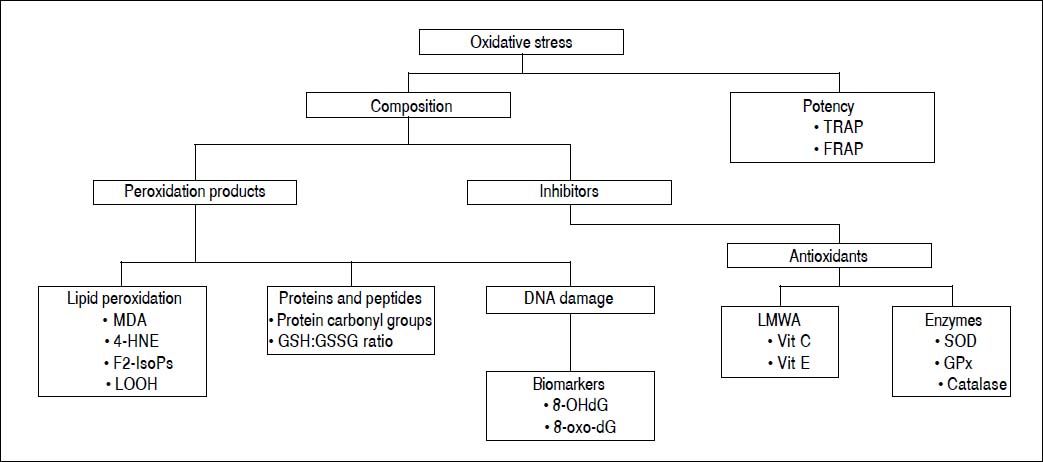
Oxidative stress can be measured by direct and/or indirect methods. Each of these criteria can be evaluated in terms of various indices, using diverse methods, as summarized in figure 3.
Electron paramagnetic resonanceROS and RNS possess a short life time and thus are difficult to measure. Electron spin resonance (ESR)-also known as electron paramagnetic resonance (EPR), is a suitable method to measure these unstable molecules,16 and EPR allows quantification of various free radical species.17 There are two types of spin traps, nitrone and nitroso compounds. Nitrone spin traps are by far the most utilized in aqueous solutions, and one example extensively used is the 5, 5-dimethyl-1-pyrroline N-oxide (DMPO).18
The oxidative status was monitored by EPR on liver samples from patients with hepatitis B, hepatitis C and non-viral diseases. The results showed that level of oxidative stress in pathologic liver is significantly higher in compare with healthy controls, and marked differences among the different conditions of damage.19 The major limitation of this method is the lack of selectivity for detecting one particular radical species; these findings for now seems to have no clinical relevance, because a particular stage is not evident in the pathophysiology of human liver disease.
Trapping methods are useful in vitro and in animal studies, but their usefulness in human studies have been limited, and unfortunately these are a very expensive techniques due to the special and pricy equipment required.16
Peroxidation productsSeveral methods to evaluate oxidative stress are based on determination of peroxidation products that are more stable than free radicals. These products are: lipid peroxidation products, oxidized proteins and fragmented DNA or DNA oxidation biomarkers.
Lipid peroxidation productsPolyunsaturated fatty acids (PUFAs) and their metabolites have a variety of physiological roles including: energy provision, membrane structure, cell signaling and regulation of gene expression. Lipids containing PUFAs are susceptible to free radical-initiated oxidation and can participate in chain reactions that increase damage to biomolecules.20
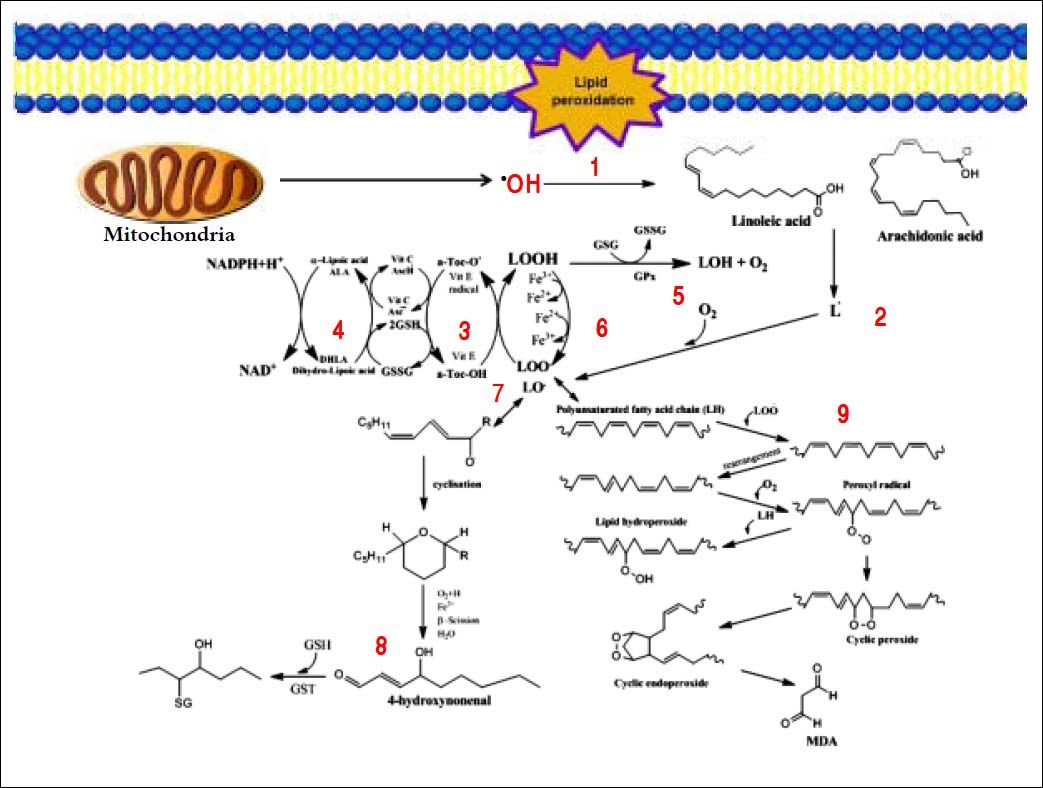
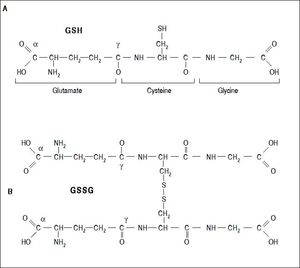
Several aldehydes can be formed as by-products of the lipid peroxidation process; these include malondialdehyde (MDA), propanal, hexanal and 4-hydroxynonenal (4-HNE), which were extensively studied on rodents’ liver by Esterbauer, et al.21–22(Figure 4).
Generation of lipid peroxidation products. Lipid peroxidation process and the role of glutathione (GSH) and other ntioxidants (vitamin E, vitamin C, lipoic acid) in the management of oxdative stress. 1. In Initiation, the hydroxyl radical can abstract the allylic hydrogen forming the carbon-cantered lipid radical; the carbon radical tends to be stabilized by a molecular rearrangement to give rise to a carbon-centered lipid radical (L•). 2. The lipid radical (L•) can further interact with molecular oxygen to give a lipid peroxyl radical (LOO•), which is reduced within the membrane by the reduced form of vitamin E (T-OH) resulting in the formation of a lipid hydroperoxide and a radica of vitamin E (a-Toc-O•). 3. The regeneration of vitamin E by vitamin C: the vitamin E radical (a-Toc-O•) is reduced back to vitamin E (a-Toc-OH) by ascorbic acid (the physiological form of ascorbate is ascorbatemonoanion, AscH-) leaving behind the ascorbyl radical (Asc•-). The regeneration of vitamin E by GSH: the oxidized vitamin E radical (aToc-O•) is reduced by GSH. 4. The oxidized glutathione (GSSG) and the ascorbyl radical (Asc•-) are reduced back to GSH and ascorbatemonoanion, AscH-, respectively, by the dihydrolipoic acid (DHLA) which is itself converted to -lipoic acid (ALA). The regeneration of DHLA from ALA using NADPH. 5. Lipid hydroperoxides are reduced to acohols and dioxygen by GPx using GSH as the electron donor. 6. Lipid peroxidation process: lipid hydroperoxides can react fast with Fe2+ to form lipid alkoxyl radicals (LO•), or much slower with Fe3+ to form lipid peroxyl radicals (LOO•). 7. Lipid alkoxyl radical (LO) derived for example from arachidonic acid undergoes cyclisation reaction to form a six-membered ring hydroperoxide. Six-membered ring hydroperoxide undergoes further reactions (involving β-scission) to from 4-hydroxy-nonenal. 8. 4-hydroxynonenal is rendered into an innocuous glutathiyl adduct (GST, glutathione S-transferase). 9. A peroxyl radical located in the internal position of the fatty acid can react by cyclisation to produce cyclic peroxide adjacent to a carbon-centered radical. This radical can then either be reduced to form a hydroperoxide or it can undergo a second cyclisation to form bicyclic peroxide which after coupling to dioxygen and reduction yields a molecule structurally analogous to the endoperoxde. Formed compound is an intermediate product for the production of malondialdehyde.
Various products from lipid hydroperoxide (LPO) process can be measured in blood and tissue by several techniques in animals and humans,23 such as gas chromatography-mass spectrometry (GC-MS) or liquid chromatography-tandem mass spectrometry (LC-MS/MS), which are methods with high sensitivity and specificity.24,25
Thiobarbituric acid reactive substances (TBARS) and MDA are the most utilized LPO markers.26 Colorimetri-cally MDA and other aldehydes are measured by reacting with TBA to give a pink chromogen that can be measured at 532 nm or by fluorimetry with excitation at 515 nm and emission at 553 nm.26
The TBA method can be improved by using HPLC with UV/VIS or fluorescence detection, in addition, MDA can be measured by GC-MS after derivatization.27
4-Hydroxynonenal (4-HNE) is a principal final-product of lipid peroxidation of membrane n-6-polyunsaturated fatty acids. 4-HNE is probably the main end product in the process of peroxidation of lipids and it is a very toxic compound capable of eliciting apoptosis in various cell types.28,29
4-HNE-protein adducts are good markers of lipid oxidation in hepatic damage.30 Free 4-HNE can be quite easily quantified by high performance liquid chromatography (HPLC).31 At present times, mass spectrometry (MS) methods are very useful in elucidating protein covalent adduction by 4-HNE, stoichiometry and sites of modification.32,33 1, 4-Dihydroxynonane mercapturic acid (DHN-MA), is a non-invasive biomarker of oxidative stress, and is easily assesses in human urine.34 Rapid and simple kits with monoclonal antibodies are finally on the market.35,36
Nonalcoholic fatty liver disease (NAFLD) is emerging as an important cause of chronic liver disease with a spectrum ranging to life-threatening complications of cirrhosis and hepatocellular carcinoma. Serum markers of lipid peroxidation have been extensively studied in patients with NAFLD.37 Oxidant stress in fatty liver arises as a result of reactive oxygen species which are produced as a result of excessive fatty acid oxidation by peroxisomes and mitochondria and as a result of overexpression of CYP2E1 enzymes.38
Several studies show high plasma concentration of products of lipid peroxidation in patients with NAFLD as compared to patients with either chronic viral hepatitis or healthy controls. In similar way, high levels of malondialdehyde were also demonstrated among patients with NAFLD.39,40 Recent studies indicate that HNE and MDA react with cellular proteins and the aldehyde-modified proteins serve as a biomarker for alcoholic liver disease (ALD).41
IsoprostanesF2-isoprostanes is a group of bioactive prostaglandin F2-like compounds generated by oxidatively catalyzed reaction of arachidonic acid, are considered as the reliable marker of lipid peroxidation in vivo.42 Moreover, isoprostanes are less reactive than others lipid peroxidation products such as lipoperoxides and aldehydes and can be easily detected in plasma and urine,43 usually quantified in urine instead of plasma for practical use because of the short half-life of plasma F2-isoprostane.44
Elevated levels of plasma and/or urinary 8-isoprostane have been reported in alcoholic liver disease. Meagher, et al.45they found that levels of isoprostanes were significantly higher in subjects with liver cirrhosis induced by former alcohol consumption than in subjects suffering from this disease induced by hepatitis C virus infection.
Two methods were developed to quantify isoprostanes in biological fluids: mass spectrometry and immunological techniques. The former is the reference method and the most accurate and sensitive.46 It utilizes gas chromatography and negative ion chemical ionization mass spectrometric detection (NICI MS); unfortunately, this method requires specialized equipment and is not adapted to routine determination. Therefore, radiometric and immunological methods were developed. Determination of 8-epi-PGF2a in biological fluids by immunoassays47 requires extraction and purification of the molecule before to their assessment because of interference by related metabolites.44
Protein carbonyl groupsOxidative modification of proteins in vivo may potentially be important, because a variety of cellular functions are affected including signal transduction mechanisms, transport, and enzymatic systems. Also, secondary damage to other biomolecules, such as inactivation of DNA repair enzymes, malfunction of DNA polymerases during DNA replication, and the development of new antigens provoking auto immune responses may also occur.48 Increased plasma protein carbonyls were found in patients with chronic alcohol dependence as compared with the control group.49 Interestingly, the increase of carbonyl groups in patients with alcoholic hepatitis is a potential biomarker of oxidative stress in alcoholic hepatitis, which will aid the physicians in determining the extent of oxidative damage in alcoholic liver disease.50
The most common method of quantifying oxidation of proteins is the carbonyl assay. It was developed as a “general assay” of oxidative protein damage and is based on the fact that several oxygen-derived species can attack amino acid residues (particularly histidine, arginine, lysine and proline) to produce carbonyl groups, which can be measured after reaction with 2,4-dinitrophenylhydrazine. It has become the most widely utilized determination of protein oxidation in several human diseases;51,52 this technique can be coupled to protein fractionation by HPLC leading to greater sensitivity and specificity than assessing total carbonyls in protein mixtures.53
DNA damageWhen DNA is attacked by ROS, stable covalent bonds are produced, leading to theformationof base modifications, including the formation of thymine and thymidine glycol, 5-hydroxylmethyluracil and 8-hydroxy-deoxyguanosine (8-OHdG). 8-OHdG is the main ROS-induced DNA adducts which generates a mutation in the DNA daughter strands. 54
Chronic viral hepatitis leads to cirrhosis, which in turn, is frequently associated to the development of HCC, which is characterized by increasing the production of 8-OHdG, and this is one of the main mutagenic modifications of DNA by oxidative stress.55 Li, et al.56 reported that 8-OHdG is a novel prognostic factor in HCC. The strong association between 8-OHdG expression and the poor patient survival together with the correlation between clinical staging, Child classification, tumor size and venous invasion, suggests a significant role for oxidative stress in HCC carcinogenesis and tumor behavior.
Mutations of DNA are assessed by direct methods such as HPLC and GC/MS.57 Coulometric electrochemical detection is an excellent choice for the determination of the electrochemical guanine adducts and is more sensitive than ultra-violet detection.58 Markers of DNA damage, such as 8-hydroxy-deoxyguanosine (8-OHdG), and lipid peroxidation, such as 4-HNE and MDA, are commonly elevated in the liver from patients with chronic HCV infection and correlate well with the degree of viral infection and inflammation.59
Measurement of GSHThe ratio of GSH to GSSG is a sensitive marker of oxidative stress. GSH is produced in the liver and is maintained in high concentration in most tissues. GSH is oxidized to GSSG acting as an antioxidant; thus, the GSH/ GSSG ratio is a reliable indicator of tissue and blood oxidative stress.60 When cells are exposed to damage caused by nitrosative or oxidative free radicals, the molar GSH: GSSG decreases from 100:1 to even 1:1.61
Hepatocytes represent the primary site of ethanol metabolism, and alcohol for long duration intake leads to oxidative stress and decreases the content of glutathione and additionally causes lipid peroxidation. The GSH/GSSG ratio can be used as a potential biomarker to assess oxidative stress in alcoholic hepatitis.50
To measure glutathione, HPLC with various detection techniques including ultraviolet (UV) absorbance and fluorescence detection, mass spectrometry and/or electrochemical detection (ED) are very commonly used,62,63 because of its advantages of simplicity, high sensitivity and relatively low cost.
Determination of antioxidant enzymesAntioxidant enzymes such as SOD-1 and CAT are closely linked with cellular responses to oxidative stress. SOD-1 (CuZnSOD) is ubiquitously expressed and is a cytosolic scavenger of oxygen free radicals by facilitating the dismutation of oxygen radicals to molecular oxygen and hydrogen peroxide, which in turn is metabolized to harmless water and oxygen by CAT and glutathione peroxidase (GSH-Px).9
Studies have demonstrated that enzymatic and non-enzymatic systems (which maintain cellular homeostasis) are remarkably affected by alcohol in diverse models. In particular, the activities of SOD, CAT, GSH-Px and the level of lipid peroxidation were modified in animals treated with alcohol.64,65
Chrobot, et al.66 demonstrated that SOD-1 and CAT activities decreased both in groups of children with CHC, and CHB. Ciragil, et al.67showed that antioxidant enzyme activities (SOD-1, CAT) decreased in the serum of patients with HCV compared to the control group. Osman, et al.68 reported that an increase in oxidative stress markers (MDA, nitric oxide) and a decrease in antioxidant enzyme activities (SOD-1, GSH and GSH-Px) were observed in the serum of patients with viral hepatitis.
CAT activity can be measured by examining the decrease in hydrogen peroxide concentration or the appearance of oxygen; and this decrease of hydrogen peroxide is measured at 240 nm.69 This method is useful to determine activity of CAT activity in whole blood or erythrocytes.
The methods to assay SOD activity are indirect approaches. Superoxide radical is generated either chemically or enzymatically with a constant flux and is allowed to react with a detector molecule which scavenges the radical. Several indirect methods have been reported using various detector molecules such as cytochrome c, nitrobluetetrazolium, luminol, lucigen, pyrogallol, nitrite, adrenalin or piodonitrotetnazolium.70
The most widely used method to measure GPx is based on specific determination of GSSG glutathione reductase and nicotinamide adenine dinucleotide phosphate. This assay is based in the conversion of GSSG to GSH with concomitant oxidation of NADPH to NADP+. The decrease in absorbance at 340 nm is measured.71 GPx activity has been used in several hepatic diseases in order to determine intracellular oxidative stress.72,73
Measurement of antioxidant moleculesOxidative stress and antioxidant molecules were measured in patients with ALD; it was found that as the severity of the disease increased, followed by elevation of serum level of lipid peroxidation indicator MDA and the concentrations of serum vitamins E and C, which act as indexes of antioxidant status, were decreased in ALD patients.74
Cankurtaran, et al.75measured levels of serum vitamin E in nonalcoholic fatty liver disease. They found a correlation between low vitamin-E levels, high triglyceride levels, as well as sonographic findings, both of which are negative prognostic factors causing progression of fatty liver to steatohepatitis.
Ascorbic acid (vitamin C), a water-soluble chainbreaking antioxidant, is able to scavenge essentially all physiologically relevant free radicals, in addition, is the most potent intra- and extracellular antioxidant. It scavenges superoxide, hydroxyl and peroxyl radicals, and inactivates hypochlorite and singlet oxygen.76 Vitamin C is commonly assessed spectrophotometric procedures, these include the reducing properties of the 1,2-enediol group that lead to absorbance changes in indicator dyes (2,6-dichlorophenol-indolphenol), the ketone derivatization method with 2,4-dinitrophenylhydrazine.77 However, HPLC methods coupled to UV-Vis,78 fluorometric,79 or electrochemical80 detectors are commonly utilized and possesses the advantage of allowing quantification of both vitamin C and its end-products.
Vitamin E (α-tocopherol) is the principal liposoluble antioxidant in the body; it has been reported that it decreases in chronic liver diseases of different origin;81 it acts by breaking the propagation chains occurring during lipid peroxidation of PUFAs.82 Vitamin E scavenges peroxyl radicals produced during lipid peroxidation, forming the tocopheroxyl radical. Reduced glutathione, vitamin C and ubiquinol regenerate α-tocopherol.83 Vitamin E determination in plasma, serum and tissues performed on reverse phase HPLC is commonly used. Detection of tocopherols can be performed by ultraviolet spectrophotometry,84,85 by spectro-fluorimetry.86
Ubiquinone or CoQ10 is an essential component of the electron transport chain in mitochondria; therefore, ubiquinol/ubiquinone ratio is a good marker of oxidative stress.87 The antioxidant form is ubiquinol (CoQ10H2), that is the reduction product of ubiquinone and whose redox state is controlled by an enzymatic mechanism. Patients with hepatitis, cirrhosis, and HCC have showed to have a reduction on the ubiquinol/ubiquinone ratio.88 HPLC methods are utilized to determine plasma ubiquinol.88
Clinical Relevance of Oxidative Stress ParametersThe increasing interest of Scientists and Physicians on free radicals pathophysiology in human diseases has led to widespread attempts to develop suitable techniques to measure free radicals and their reactions in vivo, especially in clinical pathology. The localization and effects of oxidative stress, as well as the information regarding the nature etiology of the ROS/RNS, may be revealed from the analysis of discrete biomarkers of oxidative/nitrosative stress/ damage on isolated tissue and biological fluids. Biomarkers are quali-quantitative indicators of normal and pathological biochemical processes.89 Since oxidative stress is a common pathogenetic event occurring in several liver disorders, ranging from hepatitis to cancer, the identification of a pattern for molecular alterations present at early stages of oxidative damage would be of great help in its early recognition and in monitoring the evolution of the disease.89
Unfortunately, the actual role of ROS/RNS in human inflammation, in connection with both acute and chronic disease has never been clearly defined due to the difficulty of quantifying ROS/RNS or oxidative stress in humans.
The development of noninvasive biomarkers in liver diseases has become a major focus of interest, since liver biopsy often requires admission of patients to hospital, a sedation protocol, and there exist the risk of surgical bleeding.90
How to Measure Oxidative DamageThe first major problem to be faced is the quick reactivity of free radicals reaction close to their biochemical source. On the other hand, the redox state at cellular, tissue and organism level is complex, which cannot be measured or defined by a single parameter isolated. There is no standardized method for measuring oxidative status in humans, and none of the so-called biomarkers of oxidative stress allows accurately assessing and defining oxidative stress, which can be directly applied to human disease.91
Nevertheless, antioxidant status in biological samples is regarded as an indicator of oxidative stress, and in many cases low antioxidant capacity of tissue and body fluids is a consequence of increased oxidative processes, which can be assessed either on the basis of the overall oxidative/reductive potency of a given specimen (e.g. blood, urine or tissue) or on the basis of the susceptibility of the various oxidizable components of body fluids to ex vivo peroxidation.92 Consequently, free radicals are not amenable to direct assay and free radical activity is usually assessed by indirect methods such as measurement of the various end products of reactions with lipids, proteins and DNA.89
Oxidative Stress at PresentThe liver is an organ with very intensive metabolic and synthetic functions. Many proteins and metabolites in human blood and other tissues are derived from the liver. Serum concentrations of many of these substances change under different physiological conditions, as well as in liver disorders.93
Liver pathology is very complex, and differential diagnosis is usually difficult to achieve, thus, the assessment of the stage of liver disease is important for diagnosis, evolution and treatment, and the follow-up for both: during treatment and after cessation of treatment. Ultrasonographically guided percutaneous liver biopsies an important diagnostic option in the routine of therapeutics in clinical practice, and this represents a valuable tool in many hepatic diseases, either for diagnostic or therapeutic purposes. Although ultrasonography, computed tomography, and magnetic resonant imaging are useful in investigation of liver disease, the liver biopsy followed by histological examination is still the gold standard for the assessment of liver fibrosis in the majority of patients.94
The percutaneous liver biopsy is considered a relatively safe procedure. Nevertheless, there is a risk of complications arising from liver biopsy, and they can vary from mild symptoms, such as mild abdominal pain, to severe hemorrhage and injury to the biliary system. In some cases in particular it is not possible to perform liver biopsy and, on the other hand, if tumor lesions are very small, the result of liver biopsy may be falsely negative.90,94 Therefore, besides imaging techniques and biopsy, blood markers have an important role in diagnosis and monitoring of the course of liver disease.
Oxidative stress has been considered as a conjoint pathological mechanism, and it contributes to initiation and progression of liver injury, which has been demonstrated in animal and human studies.95 Risk factors include abuse alcohol, drugs, viral infections and environmental pollutants, may induce oxidative stress in liver, which in turn results in severe liver diseases, such as alcoholic liver disease, non-alcoholic steatohepatitis and HCC.96
Therefore, the identification of a pattern of molecular alterations present at early stages of oxidative damage would be of great help in monitoring the progression of the disease and in its early recognition, through the monitoring of endogenous and exogenous antioxidants, and enzymes involved in free radical control. The increasing interest in the role of free radicals in the liver disease has led to widespread attempts to develop techniques suitable to measure free radicals and their reactions in vivo, specifically, in clinical pathology.97
Several in vitro markers of oxidative/nitrosative stress are available, including ROS/RNS themselves, but most are of limited value in vivo because they lack sensitivity and/or specificity or require invasive methods,89 further, the repeated liver sampling is not ethical and/or feasible in human studies, a better approach in clinic is the extrapolation of peripheral markers of oxidative stress, such as urine or plasma.
There are several limitations in identifying oxidative markers. The first major problem to be faced is the quick reactivity of free radicals reaction close to their biochemical source. On the other hand, the redox state at the cellular level, tissues and organisms is complex, which cannot be measured or defined by a single parameter isolated.91 There is no standardized method for measuring oxidative status in humans, and none of the so-called biomarkers of oxidative stress, allows us assess accurately and definitively oxidative stress, which can be directly applied to human clinic.
Another issue is the identification of fibrosis stage, the clinical importance lies with being able to differentiate between no/minimal fibrosis (F0/F1), significant fibrosis (F2), severe fibrosis (F3) and cirrhosis (F4). Since the main determinant of prognosis and knowledge the extent of fibrosis is useful in making treatment decisions, in patient selection for treatment studies and in monitoring progression/regression.
There has been much focus on developing the ideal biomarkers, and on the validation of noninvasive methods as biomarkers for liver diseases in recent years. However, there has been lacking of definitive evidence for this association due to the shortcomings recognition with the available methods to assess oxidative stress status in vivo, especially in humans (Table 1).
Previous studies investigating serum biomarkers in liver disease.
| Biomarkers | Study description | Results | Reference |
|---|---|---|---|
| ALT, AST, MDA, SOD and CAT. | 48 patients with HCV, 15 patients with HBV and 30 healthy individuals as a control group. | MDA levels in urine increased in patients with HBV and HCV compared to the control group. The activity of CAT and SOD decreased in urine in patients of patients with HCV, they increased in the urine of patients with HBV compared to controls. | 98 |
| Circulating IgG against human serum MDA (MDA-HSA), HNE (HNE-HSA) and arachidonic acid hydroperoxide (AAHP-HSA) | 145 chronic hepatitis C (CHC) patients with different alcohol consumption, 20 HCV-free heavy drinkers (HD) without liver disease, and 50 healthy controls. | Anti-MDA IgG was increased in CHC patients irrespective of alcohol intake as well as in the HD group. CHC patients with moderate alcohol intake, but not HD, also had significantly higher values of anti-AAHP-HSA, anti-HNE-HSA than controls. | 99 |
| ALT, AST, MDA, SOD, CAT and GPx. | 80 patients with HBV and 40 healthy volunteers. | There is correlation between serum MDA level and ALT and AST levels of chronic HBV patients. But, no correlation between serum ALT and AST levels and GPx, CAT and SOD activities. | 100 |
| MDA was measured in hepatic vein, peripheral vein and urine. Additionally, the MDA was measured by. immunohistochemical staining | Examine the relationship of oxidative stress marker among hepatic tissue, hepatic and peripheral veins and urine. 26 patients undergoing transjugular liver procedures. 19 patients undergoing liver biopsies. | Oxidative stress measured from the peripheral venous samples is poorly reflective of hepatic tissue oxidative stress. There was no significant correlation between hepatic venous MDA levels and hepatic tissue MDA content also excluded the possibility of hepatic venous sampling for a more satisfactory reflection of hepatic tissue oxidative stress. | 101 |
| Advanced oxidation protein products and MDA levels, and total radical-trapping antioxidant parameter was calculated. | 29 biopsy-proven chronic HCV. | Serum levels of advanced oxidation protein products and MDA are higher in chronic HCV patients compared to controls. There was no significant difference in total radical-trapping antioxidant parameter. There was no significant correlation between advanced oxidation protein products and MDA and hepatosteatosis. | 102 |
| 8-hydroxy-2'-deoxyguanosine (8-OHdG) by quantitative Immunohistochemistry detection. | The study cohort included 30 patients with NASH without HCC, 6 HCC patients with NASH, and 13 patients with simple steatosis. | The 8-OHdG content in the liver tissue of NASH-HCC patients was significantly different from that in the other patients. 8-OHdG content in liver tissue may serve a marker of oxidative stress and could be a particularly useful predictor of hepatocarcinogenesis. | 103 |
However, anti-oxidative therapy using mainly natural and synthetic antioxidants represents a reasonable therapeutic approach for the prevention and treatment of liver diseases due to the role of oxidative stress on contributing to the initiation and progression of hepatic damage.104 Regarding the important role of oxidative stress on liver diseases, various anti-oxidative therapies and antioxidants are proposed to prevent and treat liver diseases.105
A series of studies have tested the effectiveness of some antioxidants (e.g. vitamin E) on the treatment of patients with various liver diseases, such as chronic HCV infection, alcoholic hepatitis or cirrhosis, and NAFLD liver disease.106,107 Therefore, the clinical application of the antioxidants signifies a rational curative strategy to prevent and cure liver diseases involved with oxidative stress. However, despite some positive results were obtained, it cannot reach the conclusion that antioxidants are efficient therapeutic agents in all cases of liver diseases, partially due to the sample scale and treatment duration.
Although some promising results have been shown, in several cases antioxidants failed to improve the prognosis of patients. Nevertheless, on clinical trials, a close monitoring of the alteration of biomarker levels in biological samples may provide important information about the oxidative/nitrosative damage and about the dose of antioxidants treatment. We hope that these aims should reduced oxidative stress damage in study subjects and it will help finding a more safe and reliable antioxidant treatment.
Conclusions and PerspectivesOxidative stress is a pathogenetic event that plays a causative role in various hepatic diseases, including hepatitis, NASH, fibrosis, cirrhosis and HCC, thus monitoring endogenous and exogenous antioxidants and enzymes involved in free radical control, can make an important contribution on the establishment and progression of the disease, and could be considered a good adjuvant in antioxidant therapies.
To provide a more integral attention and stratified care to patients with liver disease, we urgently need noninvasive tools that can effectively phenotype patients based on their degree of liver injury, natural history of the disease and clinical outcomes; and in the same way, to improve the antioxidant therapies.
Conflict of InterestsThe authors declare that there is no conflict of interests regarding the publication of this paper.
AcknowledgementsThis work was partially supported by a grant (UABC-PTC-464) of PRODEP, Mexico (Dr. Jonathan Arauz). I would like to thank one of my colleagues, Physician/ Scientist, Dr. Eleobardo Castro-Luque for his valuable help on revising this paper and his contribution with some ideas on the structure of this review-article.