Zinc is an essential trace element playing fundamental roles in cellular metabolism. It acts mostly by binding a wide range of proteins, thus affecting a broad spectrum of biological processes, which include cell division, growth and differentiation. Zinc is critical to a large number of structural proteins, enzymatic processes, and transcription factors. Zinc deficiency can result in a spectrum of clinical manifestations, such as poor of appetite, loss of body hair, altered taste and smell, testicular atrophy, cerebral and immune dysfunction, and diminished drug elimination capacity. These are common symptoms in patients with chronic liver diseases, especially liver cirrhosis. The liver is the main organ responsible for the zinc metabolism which can be affected by liver diseases. On the other hand, zinc deficiency may alter hepatocyte functions and also immune responses in inflammatory liver diseases. Liver cirrhosis represents the most advanced stage of chronic liver diseases and is the common outcome of chronic liver injury. It is associated with energy malnutrition, with numerous metabolic disorders, such as hypoalbuminemia, with imbalance between branched-chain amino acids and aromatic amino acids, and with reduced zinc serum concentrations. All these processes can influence the clinical outcome of patients, such ascites, hepatic encephalopathy and hepatocellular carcinoma. In the present review, we summarize the emerging evidence on the pitoval role of zinc in the pathogenesis of liver cirrhosis.
Zinc is an essential trace element exerting important antioxidant, anti-inflammatory, and apoptotic effects. It is an essential micronutrient playing fundamental roles in cellular metabolism, acting mostly through binding a wide range of proteins and thus affecting a broad spectrum of biological processes.1
Influencing the molecular functions of many proteins in cell metabolism and signal transduction, zinc is involved in proliferation, differentiation, and apoptosis and of cells with profound implications for healthy growth, renewal, and repair of cells.2 Zinc-binding proteins represent about 10% of the human proteome, with more than 300 enzymes having zinc ions within their catalytic domains.1
In addition, zinc has extensive roles in both the adaptive (specific) and the innate (non-specific) immune response at multiple levels, including gene expression as well as differentiation and development of immune cells that either affects the cells.3
The implications of zinc biology for human health are enormous as about half of the world’s population is believed to be at risk for zinc deficiency.4 The World Health Organization (WHO) has identified zinc deficiency as the fifth most important risk factor for morbidity and mortality in developing countries.5
Zinc deficiency can result in a spectrum of clinical manifestations. The liver is the main organ responsible for the zinc metabolism, and various liver diseases may be influenced by zinc deficiency. Symptoms, such as poor appetite, loss of body hair, altered taste and smell, delayed wound healing, testicular atrophy, immune dysfunction and diminished drug elimination capacity are common in patients with chronic liver diseases, especially cirrhosis.6
Cirrhosis and its complications, ascites, hepatic encephalopathy (HE), variceal hemorrhage, infections and not at least the hepatocellular carcinoma (HCC) represent the end in the spectrum of chronic liver diseases, irrespective of aetiology.
In the present review, data on zinc homeostasis, its implication in the pathogenesis of liver cirrhosis and complications, especially ascites with malnutrition, HE, and HCC, and its therapeutic effects are summarized.
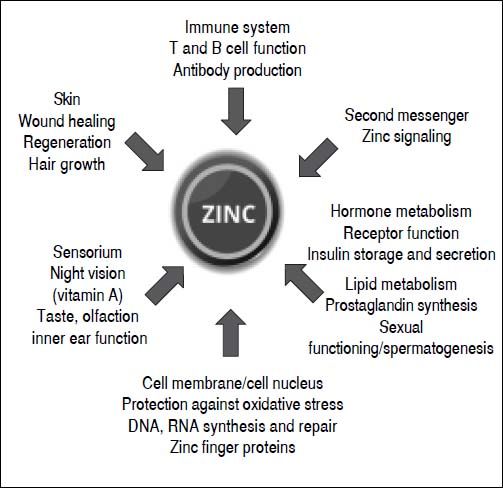
Roles and functions of zincZinc plays a key role in numerous biochemical and physiological processes. It is an essential component of more than 300 different enzymes and owes its catalytic effect to its direct involvement in substrate conversion and the stabilization of enzyme structure. Zinc exerts structural effects on several transcription factors and regulates hormone, hormone receptor and gene expression. It also has a proven influence on metabolism, growth and development.7 Moreover, zinc plays an important role as a second messenger and signaling ion, and affects redox metabolism. The cell-damaging oxidative stress that can be the result of a zinc deficiency is a fundamental principle.7 Although the Zn++ ion is redox-inert, it has important antioxidant properties.8 The protection through antagonizing redox-active transition metals like copper and iron, and the protection of protein sulfhydryl groups from oxidative damage are among the most important antioxidant properties of zinc.9 Moreover, zinc is important in the metabolism of neurotransmitters and growth, sexual and thyroid hormones, as well as storage of insulin (zinc insulin complex) in the Langerhans cells of the pancreas (Figure 1).10–14
Subject of present investigations are the multiple functions of Zn++ ions. Zn++ ions, apparently not protein bound, are found in many cells, predominantly in intracellular compartements.15 They are messengers in cellular control, and in intra- and intercellular communication.16
Zinc is the most abundant intracellular trace element and can be found in all tissues. Muscle and bone contain the largest quantities of zinc, accounting for 85% of the body’s stores of this trace element, followed by skin and liver, which together make up an additional 11%. The remainder is distributed across other tissues. The body of an adult weighing 70 kg contains a total of 2–3 g of zinc.17 The zinc content in diets typically consumed in industrial nations is generally adequate to supply this requirement. A diet including meat is considered rich in zinc. More importantly, meat contains zinc in an easily absorbed form.18
Zinc is an important regulator of different caspases, a family of cysteine proteases that drives apoptosis, the programmed demise of cells.19 Zinc impacts the activation and inhibition of various molecular processes. As a component of intracellular signal molecules, zinc is able to mimic the effects of hormones, growth factors and cytokines.20 In addition, zinc serves as an important intracellular second messenger, similar to calcium. The stability of free intracellular Zn++ ions exerts a decisive effect on the development and progression of many diseases.21 Intracellular zinc homeostasis is subject to the control of numerous proteins. The existence of such controls underscores the crucial importance of zinc.22 Metallothioneins and zinc transporters are the main components in this process. Metallothioneins are important for the absorption and storage of zinc.22 A distinct group of zinc transporters facilitate the influx and efflux of zinc through the cell membrane.23
To date, a total of 10 zinc transporters (ZnT, SLC30 family) and 14 Zip transporters (Zip, SLC39 family) have been identified in human cells. ZnT reduce the intracellular availability of zinc by accelerating the zinc efflux from the cell or into intracellular vesicles, while Zip increase the intracellular zinc availability by accelerating the influx of extracellular zinc and possibly by triggering the outflow of zinc from intracellular vesicles into the cytoplasm.24 Both transporter types have a specific tissue expression with different responsibilities in the regulation of nutritionally-induced zinc deficiency or oversupply and in the regulation of physiological stimuli, such as hormones or cytokines. Moreover, zinc transporters coordinate the subcellular, cellular and organelle-based translocation of zinc ions.25
Zinc ions are released from proteins in a zinc-thiolate coordinated milieu or from stores in cell organelles through specific reactions involving zinc transporters.26 Targets for regulatory zinc ions are proteins with docking sites for transient zinc binding such as membrane receptors, enzymes, protein-protein interactions and sensor proteins that control gene expression.26
The initiation, transmission, targeting and termination of zinc signals depend on specific proteins that control association and dissociation of metal ions. These recent findings confirm the important functions of zinc and zinc metalloproteins in cellular control.26
According to studies by Foster, et at.27 the expression of zinc transporter genes (mRNA of ZnT1 and Zip1) is highly regulated but independent of dietary zinc intake. This study included the evaluation of zinc transporters expression in combination with an analysis of zinc intake and plasma zinc concentrations. The expression of zinc transporter mRNA varied considerably between individual transporters. ZnT1, ZnT1 and Zip1 were most frequently expressed. Ultimately, this also raised the question whether zinc transporter analyses can be used as a surrogate indicating intracellular zinc deficiency, analogous to the use of ferritin in iron deficiency. Further research will be necessary to definitively answer this question. Abnormalities of zinc metabolism also affect the function of mitochondria. Thus may result in DNA damage, birth defects and developmental disorders. An oxidative environment heightens the cellular availability of zinc ions while a reductive milieu reduces this availability.24 It has been suggested that a disturbance of zinc’s antioxidant function in persons with zinc deficiencies may increase their sensitivity to factors that damage the DNA and, in turn, reduce the protective mechanisms of DNA repair mechanisms.28
Zinc metabolism in the liverAs the main organ involved in zinc metabolism, the liver plays an important role in maintaining systemic zinc homeostasis. Subsequently, liver diseases can alter zinc levels and, in turn may be influenced by zinc deficiency.29 Zinc release from hepatocytes is differentially regulated. Turnover studies using 65Zn++ showed that complete exchange of zinc in hepatocytes requires less than two days.30
The regulatory processes depend almost completely on hormonal control by insulin, glucagon and the glucocorticoids.31 Depending on the metabolic situation, these substances trigger a transient dysregulation of zinc metabolism with subsequent plasma zinc deficiency. Stress or mediator substances, like proinflammatory cytokines or lipopolysaccharides, can have similar effects. Changes in zinc status directly affect gene expression. For example, mRNA levels for metallothionein, cholecystokinin, uroguanilin, endothelin, retinol-binding protein etc. rise or fall in response to changes in zinc concentration.41,48 Zinc deficiency affects different hepatic functions and, because of the liver’s central role in metabolism, this impacts metabolic processes in other organs (32, for details see reference 6).
There are tight interactions between metallothionein, an acute-phase protein, and zinc. Metallothionein is important for the absorption, distribution and intracellular accumulation of zinc; in addition, the increased intake of zinc triggers an increase in metallothionein synthesis. Close interactions have also been identified between zinc and interleukin-6 (IL-6), one of the most important proinflammatory cytokine regulating the acute-phase genes. The effects of zinc in the hepatic synthesis of acute-phase proteins, in the regulation of gluconeogenesis and in the control of reactive substrates (e.g. nitrogen monoxide) or hydrophilic radicals, as well as the control of microbial growth, are all functions of IL-6. It regulates the zinc transporter Zip14 in the liver, thus contributing hypozincemia during the acute-phase reaction.33
Zinc transporters also appear to play a key role in the hepatocytes, though considerable gaps in our understanding remain. To date, the regulatory effects of IL-6 have been identified for ZnT5 (e.g. bone growth) and with robust data also for Zip5 and Zip6 (mammary gland, prostate, steroid hormones). Zip14 is involved in IL-6’s induction of hypozincemia during inflammatory processes. Interacting with IL-6, Zip14 also influences serum iron (hypoferritinemia) in the context of inflammatory processes by modulating the synthesis of hepcidin in the liver.34 Zip14 triggers the uptake of both zinc and non-transferrin-bound iron (NTBI).35 Patients with hereditary hemochromatosis have significantly elevated NTBI concentrations that may ultimately lead to iron overload of the liver, pancreas, heart, bones and other organs with resulting severe damage to these tissues (hepatocellular carcinoma (HCC), cardiomyopathy, diabetes mellitus, etc.). There is also evidence for a direct effect of the HFE gene, which underlies hereditary hemochromatosis, on Zip14-mediated iron transport.35 According to our current understanding, the transporter Zip14 mediates hepatic uptake of zinc during liver regeneration.36 These findings indicate that zinc transporter activity regulates liver tissue growth by providing zinc. Regulating Zip14 activity might therefore represent a potential therapeutic target to promote liver regeneration in patients with chronic liver disease. However, Zip14 also plays a critical role in the development of tumors, such as HCC. Franklin, et at.37 observed downregulation of Zip14 together with zinc depletion in tumor cells in patients with HCC that accelerates in relation to tumor progression. The authors conclude that Zip14 is involved in the reduced ability of tumor cells to take in zinc (as outlined below in more detail).
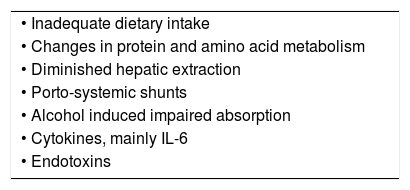
Zinc deficiency and liver diseasesZinc deficiency or an altered zinc metabolism in patients with liver disease is caused by a variety of factors, such as inadequate intake changes in the protein and amino acid metabolism, diminished hepatic extraction, portosystemic shunts, alcohol induced impaired absorption, and the effects of cytokines, mainly IL-6 and endotoxins (Table 1. For details see reference 6).
Severe muscle catabolism can lead to a substantial loss of zinc in the urine.38 Cirrhotic patients with ascites are catabolic and show massive reduction of muscle. Diuretic therapy in patients with cirrhosis and ascites results not only in an increased renal zinc excretion but also to reduced serum albumin and reduced capacity of albumin to bind zinc.39
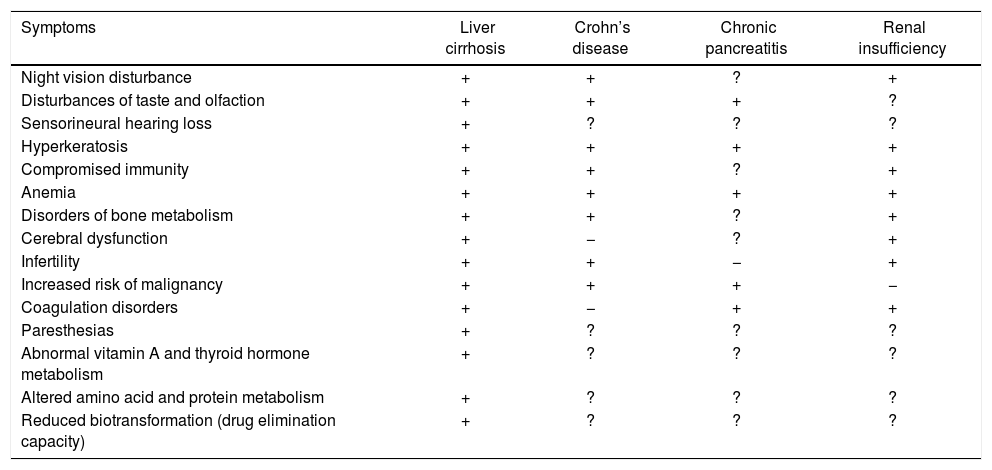
Many of the clinical features of liver cirrhosis have been linked to zinc deficiency, including loss of body hair, testicular atrophy, cerebral dysfunction, poor appetite, immune dysfunction, altered taste and smell, reduced vitamin A and thyroid hormone metabolism, altered protein metabolism, delayed wound healing and diminished drug elimination capacity (Table 2. For details see reference 6). There is a wide range of possible pathomechanisms of zinc deficiency in liver cirrhosis. Zinc deficiency can lead to oxidative tissue damage and/or the modulation of selected signaling cascades in the liver.40 Zinc deficiency may also induce oxidative stress9 and subsequent conditions such as vulnerability to hepatitis, loss of acute-phase response protection against hepatitis and lipid oxidation. By altering the redox state, zinc deficiency compromises the functioning of oxidative-sensitive transcription factors that can affect cell function, proliferation and survival.41–43
Symptoms of zinc deficiency in several chronic diseases.
| Symptoms | Liver cirrhosis | Crohn’s disease | Chronic pancreatitis | Renal insufficiency |
|---|---|---|---|---|
| Night vision disturbance | + | + | ? | + |
| Disturbances of taste and olfaction | + | + | + | ? |
| Sensorineural hearing loss | + | ? | ? | ? |
| Hyperkeratosis | + | + | + | + |
| Compromised immunity | + | + | ? | + |
| Anemia | + | + | + | + |
| Disorders of bone metabolism | + | + | ? | + |
| Cerebral dysfunction | + | − | ? | + |
| Infertility | + | + | − | + |
| Increased risk of malignancy | + | + | + | − |
| Coagulation disorders | + | − | + | + |
| Paresthesias | + | ? | ? | ? |
| Abnormal vitamin A and thyroid hormone metabolism | + | ? | ? | ? |
| Altered amino acid and protein metabolism | + | ? | ? | ? |
| Reduced biotransformation (drug elimination capacity) | + | ? | ? | ? |
Zinc deficiency can impact the many functions of the liver and, in particular the liver’s capacity for regeneration. By inducing oxidative stress, zinc deficiency can result in cell and tissue damage by modulating specific signal cascades with resulting damage to enzymes, mitochondria and ribosomal structures.44
The oxidative stress induced by zinc deficits contributes to inflammation of the hepatic parenchyma (hepatitis) and also to failure of eliciting the acute-phase response as a shield against viruses and toxic substances. A change in redox status limits the oxidative-reductive transcription factors, which impacts a variety of cell functions.41,42 In liver disease, this stress may occur through increased gut permeability with endotoxemia, infections such as spontaneous peritonitis, or release of stress hormones.43–45
Liver cirrhosis is associated with a profound immune dysfunction. A damage of the reticulo-endothelial system following a restricted immune surveillance function and a reduced hepatic synthesis of proteins involved in innate immunity and patterns recognition, which hinders the bacterial capacity of phagocytic cells.46–49
Zinc deficiency and its role in complications of liver cirrhosisAscites is the most common and the most life-limiting complication of liver cirrhosis. The liver plays a central role in the regulation of nutrition by controlling metabolism of macro- and micronutrients, their distribution and appropriate use. Consequently, protein-energy-malnutrition is common in patients with advanced liver disease and represents a significant prognostic factor affecting survival, success of liver transplantation and quality of life.50–52 The catabolic state of cirrhosis is characterized by an imbalance of plasma amino acids: a decrease of branched-chain amino acids (BCAAs: Valine, Leucine, Isoleucine) and an increase of aromatic amino acids (AAAs: Tyrosine, Phenylalanine, Tryptophane).53 In addition to the rearrangement of amino acids and protein metabolism accompanied by accelerated skeletal muscle breakdown and impaired ammonia removal in the liver and muscles, patients with cirrhosis suffer from glucose and fat metabolism disturbances with increased energy expenditure, and often develop a hypercatabolic state.51,52,54
Recently, several authors reported that the supplementation of BCAAs alone, or in combination with zinc may contribute to an improvement of hypoalbuminemia and ascites through an increased supply of substrate for proteins and the stimulation of protein synthesis.55,56,57 Albumin has been shown to be a multifunctional protein with antioxidant, immunomodulatory and detoxification functions.58 It plays a fundamental role in the distribution of many metabolites, hormones, drugs, and essential transition metal ions such as zinc and copper.59 Albumin is the major zinc carrier in the plasma, and typically binds approximately 80% of the plasma zinc.60,61 As described in detail above, liver cirrhosis is associated with a high incidence of zinc deficiency which contributed to nitrogen metabolism disorder.62,63 BCAAs are involved in various biological processes such as stimulation of albumin and glycogen synthesis, improvement of insulin resistance, induction of mitochondrial biogenesis, inhibition of ROS production and hepatocyte apoptosis, and promotion of liver regeneration.64 Randomized clinical trials showed a possible effect of BCAAs in the management of chronic liver disease.64,65 The ability of BCAA-rich supplements to reduce hypoalbuminemia is based on two mechanisms:56,65
- •
The increased supply of substrate for protein synthesis and
- •
The enhanced protein synthesis by BCAAs via stimulation of albumin mRNA translation.
Furthermore, Kuwahata, et al.66 reported that continuous supplementation with BCAAs induced phosphorylation of ribosomal protein S6 in livers of rats with chronic liver disease. Thus, following increased albumin synthesis BCAAs supplementation may increase osmotic pressure, which results in a decrease of extracellular fluid and ascites.56 Zinc supplementation might result in a decrease of muscular BCAAs consumption and consequently, the administered BCAAs might be used for albumin synthesis, which leads to an increase in serum albumin levels and a decrease in the amount of ascites.67 Zinc stores in liver, bone and muscle can be replenished independently of the severity of liver cirrhosis and complaints such as diabetes. This process requires a time period of more than six months.6
Recently, Padula, et al.68 found in children with severe malnutrition that cytotoxic effects and chromosomal damage can be repaired in vitro with zinc sulfate supplementation. However, it must be noted that further studies using larger patient groups are necessary to determine the detailed mechanism of action and the long-term clinical efficiency of this treatment in liver cirrhosis.
Hepatic encephalopathy (HE) is a potentially reversible neuropsychiatric syndrome that may occur in association with severe acute and chronic liver diseases. Up to 70% of patients with cirrhosis of the liver experience episodes of HE. Although the pathogenesis of HE is multifactorial, high ammonia levels play a key role. Ammonia induces swelling of the astrocytes and associated changes that disrupt neuronal transmission and compromise energy production in the brain. This edema of the astrocytes impacts the functions of crucial proteins in the brain and triggers oxidative and nitrosative stress.69,70 Other factors beyond ammonia that are involved in the pathogenesis of HE include infections, the effects of pro-inflammatory cytokines and dysfunction of neutrophils.69,71
Poor zinc status impairs nitrogen metabolism by reducing the activity of urea cycle enzymes in the liver72,73 and of glutamine synthetase in the muscle.74,75 Zinc deficiency is, therefore, associated with altered nitrogen metabolism, both in experimental model of cirrhosis in the rat73 and in patients with advanced liver disease.76 It has been also postulated that zinc deficiency may play a role in the pathogenesis of hepatic encephalopathy as serum zinc concentrations are reduced in patients with this condition and correlate inversely with blood ammonia concentrations.53,77 This postulate is supported by the observation made by Van der Rijt, et al.78 of a patient with severe recurrent hepatic encephalopathy and zinc deficiency. Long-term zinc supplementation improved the patient,s encephalopathy and quality of life by lowering of blood ammonia levels (urea synthesis, glutamine synthesis in the liver, glutamine synthesis in the muscle. A small number of controlled studies have been undertaken to examine the efficiency of zinc in the treatment of hepatic encephalopathy in patients with cirrhosis.6,43,79
Overall, however, the number of controlled studies of zinc substitution in HE remains small and their findings are contradictory. One interesting report suggests that zinc substitution has a beneficial effect on the muscle cramps frequently suffered by patients with liver cirrhosis.80
More recent findings by Katayama, et at.81 do, however, support the benefit of zinc substitution in patients with HE, reporting an additive effect of both zinc plus BCAAs and of zinc plus lactulose. A combination of two agents with different pharmacological properties appears to enhance efficacy in a way not achieved by the individual agents given as monotherapy.16
Hepatocellular carcinoma (HCC) is the fifth most common form of cancer worldwide and represents the third most common cause of cancer-related death.82 Cirrhosis of the liver, even an advanced fibrosis, however, is the decisive risk factor leading to development of HCC.
The pathogenetic mechanisms underlying hepatocarcinogenesis have yet to be fully elucidated, though recent years have seen the identification of a number of signal transduction pathways that are involved in this process and may represent target structures for systemic therapy. Still, given the enormous heterogeneity of HCC, comprehensive efforts are required to clearly define the molecular genesis of HCC.
A close association between chronic inflammatory processes and the development of cancer is well established. The majority of HCC cases develop as a result of genetic instability coupled with long-term chronic inflammation.83 The latter may be due to a variety of causes, including viral infections or toxic causes (alcohol, aflatoxin). In addition, it appears that persisting metabolic disturbances, such as are found in the metabolic syndrome and especially in diabetes mellitus, may induce and perpetuate chronic inflammatory processes, thus promoting the development of cancer. A large number of mediators and signal pathways are implicated in the tumorigenesis triggered by inflammation. Chronic inflammation results in an accelerated aging process of the cells, to a shortening of the telomeres and to increasing genetic instability. In the interplay between chronic inflammation and virally induced immunological changes can be clearly appreciated in the example of hepatitis C.86 Infection with HCV induces immunological changes that lead to destruction of the hepatocytes while at the same time triggering regenerative processes, which perpetuate the inflammation, cause oxidative stress and may damage DNA. Here, HCC appears to be a direct consequence of persistent inflammation with consequent fibrosis formation, ultimately leading to the development of cirrhosis of the liver.84,85
What role could zinc play in this process? Zinc and copper are two essential trace elements with a multiplicity of physiological functions, some of which overlap. Zinc and copper are subject to strict competition for binding sites on metallothionein. The plasma concentrations of both elements are affected by stress, infections, trauma and malignant processes. Here, zinc and copper levels may correlate inversely. Elevated serum copper concentrations are as a rule associated with reduced zinc concentrations.86-88
Ebara, et at.86 found a correlation between elevated serum copper concentrations and the development of HCC. By contrast, the zinc concentration in HCC tissue was lower than that measured in the surrounding healthy hepatic parenchyma. Jie, et at.89 reported on an imbalance between copper concentrations in HCC tissue and that of surrounding healthy liver tissue. In a retrospective analysis of 399 patients with liver cirrhosis 13 (18.5%) developed HCC. Of these 13 patients, 56 (16.1%) exhibited zinc deficiency (< 11.0 μmol/l). While only 15 of 21 patients (55.5%) with alcohol-induced cirrhosis exhibited reduced zinc concentrations, a zinc deficiency was identified in 19 of 20 patients (95.0%) with chronic hepatitis C.18
It remains unclear whether these changes in serum and tissue zinc and copper concentrations contribute to tumor development or are, instead, effects of the malignant transformation itself.
Based on observations of intracellular zinc concentrations in different carcinomas, it has been postulated that these changes may contribute to tumor development due to their impact on the signal function of zinc resulting from inhibitory or activating effects on a wide variety of molecular structures, such as receptors, kinases, caspases, phosphatases and transcription factors.90,91 A decisive role very probably accrues to changes in the expression of different zinc transporters (Zip, ZnT), as has been suggested in preliminary research on certain forms of mammary92 and pancreatic carcinoma.93 Franklin, et at.31 observed a decrease in the gene expression of Zip14, coupled with a reduction in zinc concentrations during the development and progression of HCC.
Further investigations will be needed to clarify whether the inhibition or activation of specific zinc transporters in hepatocytes can influence the zinc-dependent signal cascades that are important in hepatocarcinogenesis. Although, because of the great heterogeneity of HCC, this will certainly impact only a small group of patients, this still represents a very interesting and valuable task and will go a long way toward realizing the concept of individualized, that is, personalized medicine.
Wilson’s diseaseWilson’s disease is an autosomal recessive disorder of copper metabolism that leads to accumulation of copper in various tissues and organs, including liver and brain. The objective of treatment is a reduction in the amount of accumulated copper by inducing a negative copper balance. Currently, four drugs are employed in patients with Wilson,s disease to eliminate copper, namely the copper chelating agent, penicillamine; trientine; tetrathiomolybdate, which forms a three-fold complex with copper and protein; and zinc.6 Treatment with zinc was introduced 1961 by Schouwink.94 The administration of zinc based on research that showed that zinc caused a negative copper balance, controlled urine and plasma copper levels, removed stored copper, and protected the liver, at least in part, by inducing the expression of intestinal and hepatic metallothionein.43,95,96 Zinc induces copper binding metallothioneins both in enterocytes, reducing metal intestinal absorption into portal circulation, and in hepatocytes, reducing damaging effects of free liver copper.96,97 The dose that frequently used for adults with Wilson’s disease is 50 mg elemental zinc 3 times daily. Zinc is suitable for maintenance treatment as well treatment of pre-symptomatic patients, pregnant patients and children.98 Zinc is also indicated as maintenance therapy for patients with Wilson’s disease initially treated with chelators, and as first- line therapy for patients with neurological onset.97,99 Patient’s serum or plasma zinc concentrations and urinary copper excretion should be monitored every six to eight weeks. Urinary copper excretion should exceed 125 per 24 h. Excessive zinc doses may result in copper deficiency. Zinc therapy is an attractive therapeutic agent because it is inexpensive and relatively nontoxix compared to chelation therapy.43 The developement of acute hepatitis following initiation of zinc adminstration has been described by Castilla-Higuerto, et al.100 as a rare side effect of zinc therapy in Wilson’s disease. Elevated liver enzymes normalized promptly following discontinuation of zinc. Similar observations were published by Roberts,101 who pointed to elevated liver transaminases during long-term zinc therapy in Wilson’s disease as evidence of this phenomenon. In such cases, patients must be switched to a chelator.
Nevertheless, recent studies have confirmed safety of zinc therapy in Wilson’s disease.97,102
ConclusionZinc deficiency occurs in many types of liver diseases, especially in more advanced/decompensated disease, and its complications, ascites, hepatic encephalopathy and hepatocellular carcinoma. Zinc supplementation may be useful as adjunctive treatment in these complications. Zinc supplementation could be highly inexpensive and, within well-described daily dosage limits, quite safer, improve patients with liver cirrhosis. The restauration of several functions require additional factors because other micronutrient deficiencies often accompany a zinc deficiency. More research is needed to further elucidate the different roles of zinc in both normal and disease states.
Conflict of InterestNone of the authors have any conflict of interest to report.