After pneumonectomy, the development of a new lung cancer or a recurrence in the residual lung is a challenge. Surgery often is considered contraindicated. The goal of our study is to assess the morbidity and mortality of lung resection on a single lung.
MethodsAll patients who underwent lung resection after pneumonectomy from January 1996 through December 2012 were reviewed.
ResultsThere were 12 patients (10 men and 2 women). Mean age was 71 years (range, 54–81 years). Mean preoperative FEV1 was 1470mL (52%) and preoperative FVC 2153mL (61.5%). Subsequent pulmonary resection was performed after a median follow-up of 34.5 months. Wedge resection was performed in all patients. Diagnosis was pulmonary metastatic lung cancer in 2 patients, metachronous lung cancer in 6, metastatic extrathoracic cancer in 3 and benign nodule in 1. Complications occurred in 4 patients (33.4%) while operative mortality was nil.
ConclusionsLung resection on a single lung is a safe procedure associated with acceptable morbidity and mortality. Careful patient selection is very important.
La aparición de una nueva lesión pulmonar, bien un tumour metacrónico o una recidiva, en pacientes neumonectomizados plantea un reto terapéutico, en el que la cirugía en muchas ocasiones se considera contraindicada. El objetivo de nuestro estudio es valorar la morbimortalidad de la cirugía de resección pulmonar sobre pulmón único.
MétodosRevisamos a todos los pacientes a los que se les realizó una neumonectomía y presentaron una nueva lesión en el pulmón remanente entre 1994 y 2012.
ResultadosLa serie consta de 12 pacientes (10 varones y 2 mujeres) con una edad media de 71 años (54–81 años). El FEV1 medio fue de 1.470mL (54,2%) y la CVF de 2.153mL (61,5%). Tras una mediana de 34,5 meses se les intervino de una segunda lesión en el pulmón contralateral, realizándose en todos los casos resecciones pulmonares atípicas. La anatomía patológica mostró metástasis de tumor primario pulmonar en 2 pacientes; tumor metacrónico, en 6 pacientes; metástasis de carcinoma de origen extratorácico, en 3 pacientes y nódulo benigno en un paciente. Se registraron complicaciones en 4 pacientes (33,4%): arritmia cardíaca en 2 pacientes e insuficiencia respiratoria en otros 2 pacientes. No hubo ningún fallecimiento postoperatorio.
ConclusiónLa resección pulmonar sobre pulmón único es un procedimiento seguro con una aceptable morbimortalidad, en la que es de gran importancia una meticulosa selección de los pacientes.
One year after surgery, patients operated on for lung carcinoma (LC) present a risk of relapse from 2% to 5%,1,2 and from 1% to 5% of developing a second primary lung carcinoma, depending on the initial state of the previous tumour.3,4 The challenge in these cases arises when the previous surgery consisted of pneumonectomy. Surgery on a unique lung is rare, given that in the majority of cases the functional status of the patient or the presence of distant metastasis are contraindications for a second procedure.5 Likewise, surgery on a unique lung is often not performed due to lack of knowledge on the part of the doctors, due to the erroneous belief that pneumonectomy contraindicates any subsequent pulmonary resection. This eliminates what would probably have been the sole curative option for these patients. This revision analyses our experience of patients operated for pulmonary lesions after they had received pneumonectomy, in terms of postoperative morbimortality.
MethodsThree hundred and ninety-four pneumonectomies were performed from January 1994 to December 2012. Twelve patients (3%) were operated on for lung lesions due to malignant disease, for either LC or pulmonary metastasis. Inclusion criteria were: patients with resectable lesions, without distant lesions and with sufficient functional status to tolerate pulmonary resection.
All of the patients with a history of LC were monitored postoperatively by CT every 6 months during the first 2 years, and then annually. The follow-up of patients with metastatic lesions was undertaken by the oncology department. The preoperative study of all patients included thoracoabdominal CT, functional respiratory tests, fibrobronchoscopy and, in the last 2 years of the study, PET–CT. Likewise, all of the patients were informed about the procedure and signed their informed consent to be treated using it.
Patient data analysed were: demographic (age and gender), data in connection with their pneumonectomy (histology, TNM), data in connection with the subsequent resection (disease-free interval, preoperative study, surgical aspects, postoperative morbimortality) and series survival. We used the criteria of Martini and Melamed to differentiate those patients with second primary tumours from those with metastasis. Postoperative mortality included those patients who died within the 30 days following surgery, or who died after this time but during the same admission to hospital. To unify the TNM staging we reclassified all of the patients using the new TNM system of 2007.
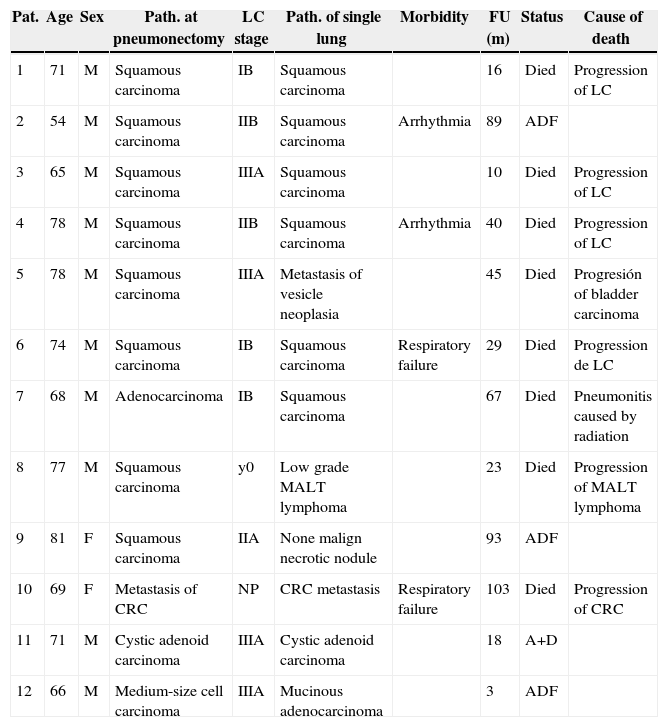
ResultsThe series is composed of 12 patients, 10 men (83.3%) and 2 women (16.7%), with an average age of 71 years old (54–81 years old). Table 1 shows the characteristics of the series. Postoperative histology following pneumonectomy was squamous cell carcinoma in 8 patients (66.6%), lung adenocarcinoma in 1 patient (8.3%), cystic adenoid carcinoma in 1 patient (8.3%), medium-sized cell carcinoma in 1 patient (8.3%) and pulmonary colorectal carcinoma metastasis (CRC) in 1 patient (8.3%). This patient received left upper lobectomy in a first operation, and the pneumonectomy was completed due to another CRC relapse. Pathological study of the patients with a primary lung tumour at the pneumonectomy was: stage yp0 (1 patient, 9%), pIB (3 patients, 27.3%), pIIA (1 patient, 9%), pIIB (2 patients, 18.2%) and pIIIA (4 patients, 36.5%).
Series Characteristics.
| Pat. | Age | Sex | Path. at pneumonectomy | LC stage | Path. of single lung | Morbidity | FU (m) | Status | Cause of death |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 71 | M | Squamous carcinoma | IB | Squamous carcinoma | 16 | Died | Progression of LC | |
| 2 | 54 | M | Squamous carcinoma | IIB | Squamous carcinoma | Arrhythmia | 89 | ADF | |
| 3 | 65 | M | Squamous carcinoma | IIIA | Squamous carcinoma | 10 | Died | Progression of LC | |
| 4 | 78 | M | Squamous carcinoma | IIB | Squamous carcinoma | Arrhythmia | 40 | Died | Progression of LC |
| 5 | 78 | M | Squamous carcinoma | IIIA | Metastasis of vesicle neoplasia | 45 | Died | Progresión of bladder carcinoma | |
| 6 | 74 | M | Squamous carcinoma | IB | Squamous carcinoma | Respiratory failure | 29 | Died | Progression de LC |
| 7 | 68 | M | Adenocarcinoma | IB | Squamous carcinoma | 67 | Died | Pneumonitis caused by radiation | |
| 8 | 77 | M | Squamous carcinoma | y0 | Low grade MALT lymphoma | 23 | Died | Progression of MALT lymphoma | |
| 9 | 81 | F | Squamous carcinoma | IIA | None malign necrotic nodule | 93 | ADF | ||
| 10 | 69 | F | Metastasis of CRC | NP | CRC metastasis | Respiratory failure | 103 | Died | Progression of CRC |
| 11 | 71 | M | Cystic adenoid carcinoma | IIIA | Cystic adenoid carcinoma | 18 | A+D | ||
| 12 | 66 | M | Medium-size cell carcinoma | IIIA | Mucinous adenocarcinoma | 3 | ADF |
A+D, alive with disease; ADF, alive disease-free; CRC, colorectal carcinoma; F, female; FU, follow-up; LC, lung cancer; M, male; Pat., patient; Path., pathology; Respiratory F, respiratory failure.
The average time from pneumonectomy to the subsequent surgery was 34.5 months. The number of lesions observed in CT was 1 in 10 patients (83.3%) and 2 in 2 patients (16.7%), while the average size of lesions was 16.3mm (7–29mm). In preoperative respiratory function tests, the average FEV1 was 1470mL (54.2%) while CVF was 2153mL (61.5%). Only 2 patients had DLCO and KCO as parts of their preoperative functional study. These are more recent patients who were subjected to standardised testing for these values in their preoperative study. The average values for these were 75% and 148%, respectively. In the metastatic study no patient presented extrathoracic involvement. PET–CT was only used for 3 patients (25%) as part of their preoperative metastatic study. This was positive for the lesion in 2 of them (66.6%) and negative in 1 patient (33.4%).
The surgical techniques used were thoracotomy in 11 patients (91.6%) and VATS in 1 patient (8.4%). Atypical resection (AR) was used for all of the patients. A single AR was performed in 10 patients (83.3%), 2 AR were performed in 1 patient (8.35%) and 3 AR were performed in another patient (8.35%). The average duration of the operations was 151min. Only 5 patients (41.6%) were extubated in the operating theatre.
The definitive pathology was squamous carcinoma in 5 patients (41.6%), pulmonary metastasis of a tumour outside the thorax in 3 patients (25%) (CRC, gall-bladder carcinoma and MALT gastric lymphoma), with benign necrotic tumoration in 1 patient (8.3%), cystic adenoid carcinoma in 1 patient (8.3%) and mucinous adenocarcinoma in 1 patient (8.3%). The patient diagnosed with gall-bladder carcinoma metastasis had been operated 13 months previously for this neoplasia. The patient with metastasis of a MALT gastric lymphoma had been operated 20 months beforehand with gastrectomy due to ulcus, and had been diagnosed with lymphoma in the anastomosis 20 months previously. The TNM stage of the patients with a lesion of pulmonary origin was IA in 5 patients (71.4%) and stage IB in 2 patients (28.6%). The resection margins were free of tumour in all of the patients.
With respect to postoperative morbimortality, no complications at all were recorded for 8 patients (66.6%). The only complications which arose in 4 patients (33.4%) were classified as minor (defined as ones which were treated in the ward where patients were hospitalised). These complications were cardiac arrhythmia in 2 patients (50%) and respiratory failure in 2 patients (50%). No patient died during the postoperative period. The average duration of postoperative hospitalisation was 6.5 days (3–14 days).
After an average follow-up of 44.6 months, 8 patients had died (66.6%), 1 patient was alive with disease (8.7%) and 3 patients were alive and disease-free (25%).
DiscussionPatients operated for LC with complete resection present a 5%–10% risk of developing a new PC1 and a 30%–80% risk of relapse. This percentage increases together with patient survival.2,4 The challenge arises when relapse or a new carcinoma occurs in a previously pneumonectomised patient. This is why the most relevant series in connection with surgery in patients with a single lung are infrequent and include, in total, just over 100 cases.5–13 The main reasons why these patients are not sent for surgery are the size of the tumour, the location of the lesion, the state of the new tumour, the poor functional condition of pneumonectomised patients or the false belief held by some doctors that a previous pneumonectomy is a contraindication for another pulmonary resection, as it may lead to a high rate of morbimortality. Our results show that surgery in a unique lung may be undertaken with good results in terms of morbimortality.
The majority of the patients (70%–80%) who presented new pulmonary lesions following pneumonectomy are usually asymptomatic at diagnosis: the majority are detected during clinical and radiological follow-up.5,14–16 Due to this, patients treated surgically for cancer require follow-up over the long term.17 Nevertheless, a meta-analysis and systematic review recently undertaken by Calman et al. showed no clear benefit in the survival of 1669 patients associated with intensive follow-up.18
The preoperative study of patients with pulmonary lesions in a single-lung is the same as it was for the initial cancer. Radiological evaluation not only seeks to assess pulmonary lesions, but also has the purpose of ruling out distant lesions. Thoracoabdominal imaging diagnosis is usually by CT. In certain cases a cranial CT may be recommended to search for metastasis at this level. Likewise, a PET–CT will help us to rule out involvement within the mediastinum and at a distance.5,6 PET–CT was only used for the last 3 patients in our series, as this technique was only included in the preoperative evaluation of patients with a suspicion of lung cancer in the last few years. None of these patients were shown by PET–CT to have involvement of the mediastinum, ganglia hilus or at a distance.
As mentioned above, one of the main reasons why this surgery is contraindicated is the functional status of patients after pneumonectomy. In the series of Donington et al., of 772 patients treated by pneumonectomy,5 only 3% of them could receive a second pulmonary resection. In this series, the average preoperative FEV1 was 1470mL (range from 660 to 2550). In our series, the average preoperative FEV1 was 1470mL, with a range running from 1030 to 1680mL, amounting to 54.2%. Another factor that may lead us to question the possibility of pulmonary resection in patients with a single lung is the need to perform larger pulmonary resection. Thus broad pulmonary resections such as lobectomy, segmentectomy or multiple atypical resections have been associated with worse results in pneumonectomised patients.5,6,8,11 The larger the resection, the poorer will be the respiratory function of the patient, with a greater risk of postoperative mortality.7 Atypical resection was only used in 2 patients in our series, and no segmentectomy or lobectomy was performed. Another important aspect that influences postoperative respiratory lung function is the use of a thoracotomy, which has a restrictive effect on the patient. This is why endoscopic surgery is preferred for these resections. As stated previously in the literature, endoscopic surgery is associated with better postoperative recovery, with less respiratory dysfunction and without compromising long-term oncological results.19–23 In our series endoscopic surgery was only used with 1 patient, coinciding with the period when we started to use this technique, together with the development of cardiothoracic anaesthesia in our hospital. The approach used was 2-port videothoracoscopic surgery, with atypical resection and ventilatory management using apneas.
The success of surgery in single-lung patients depends on the experience of the team who will manage patients of this type. Anaesthesia is one of the key aspects here. Control of patient ventilation using selective lobe blocking and ventilation alternating with apnea are two of the ventilation techniques used in our hospital by a specific cardiothoracic anaesthesia team. Another critical aspect is the extubation of these patients in the operating theatre. In our series only 5 patients were extubated in the operating theatre. These were the most recent patients, and this coincided with improved techniques developed over recent years in thoracic surgery anaesthesia. Likewise, locorregional anaesthesia by epidural or paravertebral catheter has helped to reduce postoperative pain, thereby helping patients to expel secretions, cough and start to walk sooner, reducing the complications deriving from this surgery.
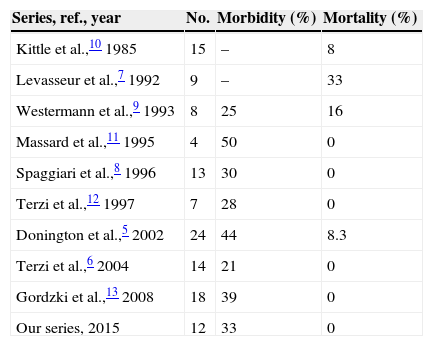
Postoperative morbidity in our series was 33%, which is similar to the rate recorded in the other series.5–13 Four patients presented complications, auricular fibrillation in 2 of them and respiratory failure in the other 2. No other respiratory complications were recorded, such as atelectasis, secretion retention or pneumonia. None of the patients required admission to the Intensive Care Unit. In Donintong's series,5 11 patients (44%) presented some type of complication. As is the case in our series, respiratory complications and atrial fibrillation were the most frequent problems. In Terzi's series6 complications arose in 3 patients (21%): secretion retention, atelectasis and atrial fibrillation. The rates of postoperative mortality published in the bibliography run from 0% to 33%.5–13 No patients died in our series, and this was also the case in the series of Terzi,6 Spaggiari8 and Massard.11 In Donington's series5 2 patients (8.3%) died, both of whom had been subjected to a larger resection. Nevertheless, mortality in those patients who had received a smaller pulmonary resection was 0%, as it was in our series. Table 2 shows the morbimortality in the different series published to date.
Morbimortality of the Different Series Published in the Bibliography.
| Series, ref., year | No. | Morbidity (%) | Mortality (%) |
|---|---|---|---|
| Kittle et al.,10 1985 | 15 | – | 8 |
| Levasseur et al.,7 1992 | 9 | – | 33 |
| Westermann et al.,9 1993 | 8 | 25 | 16 |
| Massard et al.,11 1995 | 4 | 50 | 0 |
| Spaggiari et al.,8 1996 | 13 | 30 | 0 |
| Terzi et al.,12 1997 | 7 | 28 | 0 |
| Donington et al.,5 2002 | 24 | 44 | 8.3 |
| Terzi et al.,6 2004 | 14 | 21 | 0 |
| Gordzki et al.,13 2008 | 18 | 39 | 0 |
| Our series, 2015 | 12 | 33 | 0 |
Eight patients died during follow-up. Of the 4 remaining patients, 3 are disease-free and alive and 1 is alive with disease. Of the three patients who are alive and disease-free, 1 was operated for relapse of his lung carcinoma, 1 for a metachronous tumour and the last for a necrotic nodule. The patient who is alive with disease was operated for cystic adenoid carcinoma. This single-lung patient was operated on twice due to two relapses. He is currently in treatment with radiotherapy.
The chief limitation of our study is due to the retrospective nature of the series. Additionally, the heterogeneous nature of the series and low number of cases do not make it possible to make a study of survival.
To conclude, lung surgery in patients with a previous pneumonectomy is associated with acceptable postoperative morbimortality, while atypical resection is the treatment of choice. It is important to select the patients who are going to be treated using this procedure with care, meticulous study and perioperative management.
Conflict of InterestsThe authors have no conflict of interests to declare.
Please cite this article as: Recuero Díaz JL, Rivas de Andrés JJ, Embún Flor R, Royo Crespo Í, Ramírez Gil E. La cirugía de resección pulmonar sobre pulmón único es un procedimiento seguro. Cir Esp. 2015;93:589–593.