This study aimed to assess the circulating levels of activated nuclear factor kappa B p65 and monocyte chemotactic protein-1 in diabetic retinopathy patients who were taking antihyperglycemic and antihypertensive drugs.
METHODS:In total, 235 healthy controls and 371 Type 2 diabetic patients [171 without retinopathy (DNR) and 200 patients with retinopathy (diabetic retinopathy)] were recruited for this study. Plasma and the nuclear fraction of peripheral blood mononuclear cells were isolated for the quantification of the monocyte chemotactic protein-1 and nuclear factor kappa B p65 levels, respectively.
RESULTS:Non-medicated diabetic retinopathy patients had significantly higher levels of activated nuclear factor kappa B p65 and plasma monocyte chemotactic protein-1 than DNR patients. Diabetic retinopathy patients who were taking antihyperglycemic and antihypertensive drugs showed significant reductions in both the nuclear factor kappa B p65 and monocyte chemotactic protein-1 levels compared with the non-medicated patients.
CONCLUSION:This study demonstrated the significant attenuation of both the nuclear factor kappa B p65 and circulating monocyte chemotactic protein-1 levels in diabetic retinopathy patients taking antihyperglycemic and antihypertensive drugs.
The increasing prevalence of diabetes mellitus (DM) worldwide will inevitably be accompanied by an increased development of irreversible DM complications, including diabetic retinopathy (DR). DR is the most common complication among patients with type 2 DM (1), and it is the leading cause of preventable vision impairment among working-age adults (2). More than 50% of type 2 DM patients are likely to experience DR within 20 years after diagnosis (3). DR is characterized by microvascular lesions such as microaneurysms, basement membrane thickening, loss of pericytes leading to blood barrier dysfunction and pre-retinal neovascularization (4).
The presence of oxidative stress and the generation of reactive oxygen species and advanced glycation end products are known to contribute to the development of DR (4). The role of inflammation in the development of DR is supported by increasing evidence that has shown the involvement of various cytokines and inflammatory cells in the pathogenesis of DR (5). Prolonged hyperglycemia in a person with type 2 DM creates a hypoxic state that triggers abnormal local leukocyte-endothelial interactions, which lead to retinal microvascular damage (6). Nuclear factor kappa B (NF-κB) is a “redox-sensitive” nuclear transcription factor present in many cell types that mainly regulates immune and inflammatory responses as well as apoptosis by controlling the expression of numerous genes coding for pro-inflammatory cytokines, chemokines, inflammatory enzymes and adhesion molecules (7) that are essential for leukocyte migration and adherence to vascular endothelial cells. Increased NF-κB expression had been demonstrated in many inflammatory diseases (7). Monocyte chemotactic protein-1 (MCP-1) is a chemokine regulated by NF-κB that is mainly expressed in smooth muscle cells, macrophages, endothelial cells and adipocytes (8). Its main function is to recruit circulating monocytes into the subendothelial cell layer of the blood vessel wall, and it has been reported to be involved in the pathogenesis of atherosclerosis, cardiovascular disease, obesity and insulin resistance (8).
The 1998 United Kingdom Prospective Diabetes Study provided the first evidence that early intervention with oral glucose-lowering and antihypertensive drugs reduced the incidence of diabetic complications and improved the survival of type 2 DM patients (9-10). However, it is uncertain whether this beneficial effect was derived directly from tight glycemic and blood pressure control or through other means of action. The recent large-scale Diabetic Retinopathy Candesartan control trial reported that antihypertensive drugs (angiotensin-converting enzyme inhibitors and angiotensin II receptor antagonists) delayed the progression of DR (11-12). Antihyperglycemic and antihypertensive drugs are believed to possess anti-inflammatory and endothelial-dysfunction modulating effects independent of their blood glucose- and blood pressure-lowering properties. Thus, this study aimed to assess the circulating levels of activated peripheral blood mononuclear cell (PBMC) NF-κB p65 and plasma MCP-1 in DR patients treated with antihyperglycemic and antihypertensive drugs.
METHODSStudy populationThe study subjects were recruited from the diabetes and ophthalmology clinics at the University Malaya Medical Center, Malaysia, between September 2009 and October 2011. DM had been previously diagnosed according to the World Health Organization criteria. A total of 371 unrelated Type 2 DM patients [171 patients without retinopathy (DNR) and 200 patients with retinopathy (DR)] (210 men, 161 women) aged 58.2±9.7 years (mean±SD; range, 35 to 78 years) were recruited for this study. Detailed medical histories and socio-demographic data for each patient were noted. The patients were carefully selected by excluding any patients with a previous history of inflammatory disease who had received anti-inflammatory drug treatment or antioxidant supplements. Type 1 DM patients and type 2 DM patients with complications other than retinopathy were also excluded from the study. The non-retinopathy controls were recruited from among blood-donor volunteers. They consisted of 235 unrelated healthy subjects (134 men, 101 women) aged 57.1±4.1 years (mean±SD; range, 45 to 65 years). Both the DNR patients and healthy subjects were confirmed to be free from any diabetic complications including retinopathy by attending doctors and ophthalmologists. Written informed consent was obtained from each subject prior to sample collection.
All of the DR patients underwent a complete eye examination that included dilated retinal examination and seven-field stereoscopic Diabetic Retinopathy Study retinal photography (13). The color fundus photographs were graded for DR severity in a blinded fashion by two independent ophthalmologists at the University of Malaya Eye Research Center, Malaysia. The modified Early Treatment of Diabetic Retinopathy Study Airlie House classification of DR was used to grade the retinopathy into non-proliferative diabetic retinopathy (NPDR) and proliferative diabetic retinopathy (PDR) (14). Among the DR patients, 26 had mild NPDR, 85 had moderate NPDR, 14 had severe NPDR and 75 had PDR. The study was performed in adherence to the principles of the 1983 Declaration of Helsinki and approved by the Medical Ethics Review Committee of the University Malaya Medical Center, Malaysia (IRB reference number: 744.12).
Sample collection and preparationSix milliliters of blood was drawn from patients and control subjects. Three ml of the freshly collected blood was sent for routine blood examination at the Clinical Diagnostic Laboratory of the University Malaya Medical Center. The collected whole blood samples in EDTA tubes were centrifuged for 15 minutes at 1000 xg. The plasma was extracted and stored at -80 °C for the MCP-1 enzyme-linked immunosorbent assay. Subsequently, the cell sediments of the EDTA blood tubes were reconstituted with isotonic phosphate-buffered saline solution. The peripheral blood mononuclear cells (PBMCs) were then isolated as previously described (15) using a density gradient centrifugation method. PBMCs (approximately 4 million cells) were subjected to nuclear extraction, and the extracted nuclear fraction was used for the NF-κB p65 transcription factor assay. Analysis of all of the samples was performed within 1 month after collection.
PBMC nuclear extractionThe nuclear fraction of the isolated PBMCs was extracted using a commercially available nuclear extraction kit (Cayman Chemical Company, MI, United States) according to the manufacturer’s protocol. In brief, the extracted PBMCs were collected in ice-cold PBS in the presence of phosphatase inhibitors to prevent the events controlled by dephosphorylation. The pelleted cells were then resuspended in ice-cold hypotonic buffer, causing cell swelling and increased membrane fragility. The addition of detergent (10% Nonidet P-40) ruptured the cell membranes and released the cytoplasmic fraction while maintaining the integrity of the nuclear membranes. After separation of the cytoplasmic fraction from the nuclei by a brief centrifugation, the pelleted nuclei were lysed in ice-cold extraction buffer containing a mixture of protease and phosphatase inhibitors. The nuclear extract was then isolated by microcentrifugation (14,000 xg for 10 minutes at 4 °C) and stored at -80 °C.
Measurement of the NF-κB p65 levels in PBMCsThe levels of activated NF-κB p65 in the nuclei of PBMCs were measured using a transcription factor assay kit (Cayman Chemical Company, MI, United States). The kit utilized a specific double-stranded DNA sequence containing an NF-κB response element to specifically bind the activated NF-κB p65 in the nuclear extract. NF-κB p65 was detected by the addition of a specific primary antibody directed against NF-κB p65. A secondary antibody conjugated to horseradish peroxidase was added to provide a sensitive colorimetric readout at 450 nm. The inter-assay coefficient of variation was 8%. The nuclear protein concentration was determined using a Bradford assay (16), and the activated NF-κB p65 level was expressed as arbitrary units per milligram of protein (AU/mg of nuclear protein).
Measurement of the plasma MCP-1 levelsThe plasma MCP-1 levels were quantitatively measured with a sandwich enzyme-linked immunosorbent assay standard kit (Raybiotech® Inc., GA, United States) according to the manufacturer’s protocol. The plate was coated with a specific monoclonal antibody directed against human MCP-1, and a polyclonal antibody conjugated to horseradish peroxidase was used for sensitive colorimetric detection at 450 nm. The inter-assay coefficient of variation was 7.7%. The mean minimal detectable level of MCP-1 was typically less than 2 pg/ml. The results were expressed as pg/ml.
Statistical analysisThe continuous variables were checked for normality prior to statistical analysis. A chi-squared test with one degree of freedom (for dichotomous variables) and an unpaired t-test (for continuous variables) were used to evaluate the differences between the groups. Comparison of subgroups was performed with one-way analysis of variance (ANOVA) and Tukey’s post-hoc test. Associations between parameters were determined by Pearson’s correlation coefficient (r) with Bonferroni correction. A logistic regression model was used to estimate the odds ratio (OR) and 95% confidence interval (CI) for each risk factor for DR among the type 2 DM patients. Statistical significance was set at p<0.05. All of the data were analyzed using GraphPad Prism® for Windows® version 5.02 (GraphPad® Software Inc., CA, United States).
RESULTSThe general clinical parameters for the healthy controls and the DNR and DR patients are listed in Table 1. Both the DNR and DR patients showed significantly (p<0.05) higher levels of glycated hemoglobin (HbA1c), total cholesterol, high-density lipoprotein (HDL-C) and low-density lipoprotein (LDL-C), higher systolic blood pressures (SBP), a higher prevalence of hypertension, a lower HLD/LDL ratio and lower diastolic blood pressures (DBP) compared to the healthy controls. When the two patient groups were compared, the DR patients had significantly (p<0.05) higher levels of HbA1c and total cholesterol, a longer duration of DM and more subjects who received insulin treatment. No significant differences (p>0.05) in gender, age, body mass index (BMI), triglyceride levels, alanine aminotransferase (ALT) levels or aspartate aminotransferase (AST) levels were observed.
General clinical parameters of healthy controls and DNR and DR patients.
| Demographics | Ctrl (n = 235) | DNR (n = 171) | DR (n = 200) |
|---|---|---|---|
| Age (years) | 57.1±4.1 | 59.2±9.6 | 57.2±9.8 |
| Gender (male/female) | 134/101 | 100/71 | 110/90 |
| Race (Malay/Chinese/Indian) | 106/90/39 | 63/28/80a | 70/47/83a |
| BMI (kg/m2) | 25.6±4.8 (n = 100) | 27.2±4.4 | 26.3±5.0 |
| HbA1c (%) | 5.6±0.4 (n = 100) | 7.9±1.8a | 8.9±2.1a,b |
| SBP (mmHg) | 124.0±8.0 (n = 100) | 136.5±19.5a | 139.3±22.4a |
| DBP (mmHg) | 83.0±7.0 (n = 100) | 79.0±10.5a | 78.4±13.1a |
| Total cholesterol (mmol/l) | 3.8±0.6 (n = 100) | 4.5±1.0a | 4.8±1.5a,b |
| Triglycerides (mmol/l) | 1.8±1.3 (n = 100) | 1.6±0.7 | 1.7±1.0 |
| HDL-C (mmol/l) | 1.0±0.3 (n = 100) | 1.2±0.3a | 1.2±0.3a |
| LDL-C (mmol/l) | 2.1±0.5 (n = 100) | 2.5±0.9a | 2.8±1.2a |
| HDL-C/LDL-C ratio | 0.6±0.2 (n = 100) | 0.5±0.2a | 0.5±0.2a |
| ALT (IU/l) | 30-65 c | 37.8±17.5 | 36.8±24.6 |
| AST (IU/l) | 15-37 c | 22.0±14.0 | 22.8±16.4 |
| Diabetes duration (years) | - | 10.4±7.9 | 15.7±9.1b |
| Retinopathy duration (years) | - | - | 5.0±3.6 |
| Current smoker (yes/no) | 43/192 | 29/142 | 13/187a,b |
| Alcohol intake (yes/no) | 70/165 | 24/147a | 16/184a |
| Hypertension (yes/no) | 0/235 | 104/67a | 119/81a |
| Antihyperglycemic treatment duration (years) | - | 9.5±5.5a (n = 107) | 11.5±7.5a (n = 130) |
| Antihyperglycemic medication (yes/no) | 0/235 | 107/64 a | 130/70 a |
| Insulin (yes/no) | 0/235 | 34/137 a | 98/102 a,b |
| Oral medication (yes/no) | 0/235 | 119/81 a | 81/119 a,b |
| Antihypertensive treatment duration (years) | - | 7.0±3.5 a (n = 104) | 8.5±4.0 a (n = 119) |
| Antihypertensive medication (yes/no) | 0/235 | 104/67 a | 119/81 a |
| ACEI & ARA (yes/no) | 0/235 | 67/104 a | 83/117 a |
| CCB & Diuretics (yes/no) | 0/235 | 37/134 a | 36/164 a |
The data are expressed as the mean ± SD unless otherwise indicated; dichotomous variables are given in absolute numbers. ACEI, angiotensin-converting enzyme inhibitors; ALT, alanine aminotransferase; ARA, angiotensin II receptor antagonists; AST, aspartate aminotransferase; BMI, body mass index; CCB, calcium channel blockers; Ctrl, healthy controls; DBP, diastolic blood pressure; DNR, diabetic non-retinopathy; DR, diabetic retinopathy; HbA1c, glycated hemoglobin; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; and SBP, systolic blood pressure. ap<0.05 versus healthy control; bp<0.05 versus DNR; cnormal value range provided.
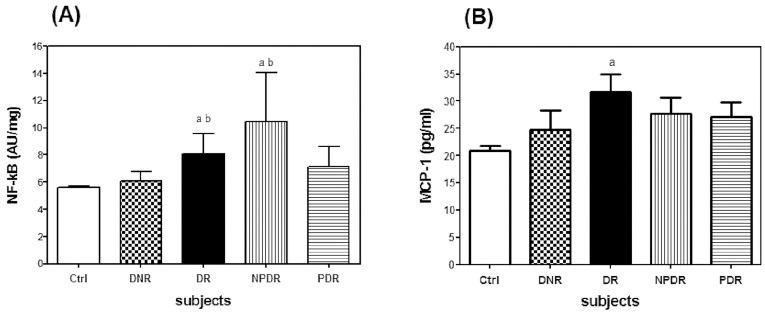
In this study, DR patients who were free from the influence of antihyperglycemic and antihypertensive medications had significantly higher levels of NF-κB p65 and plasma MCP-1 than the healthy controls (Figure 1). In addition, significantly higher levels of NF-κB p65 were found in the DR and NPDR patients compared with the DNR patients. Both the DNR and DR patients who were taking antihyperglycemic and antihypertensive medications showed lower levels of NF-κB p65 and plasma MCP-1 compared with the non-medicated patients (Table 2). Further comparisons between the medicated and non-medicated patients (NPDR and PDR) showed similar trends in the results.
Comparison of the levels of (A) NF-κB p65 and (B) plasma MCP-1 in Ctrl (n = 235), DNR (n = 50), DR (n = 70), NPDR (n = 40) and PDR (n = 30) patients who were not taking antihyperglycemic and antihypertensive medications. The data are expressed as the mean±SD. Ctrl, healthy controls; DNR, diabetic non-retinopathy; DR, diabetic retinopathy; NPDR, non-proliferative DR; PDR, proliferative DR. ap<0.05 versus Ctrl; bp<0.05 versus DNR.
Comparison of the NF-κB p65 and plasma MCP-1 levels in DNR and DR patients with different clinical factors.
| Parameters/groups | Antihyperglycemic medication | Antihypertensive medication | ||
|---|---|---|---|---|
| yes | no | yes | no | |
| NF-kB p65 (AU/mg) | ||||
| DNR | 5.0±1.6a (107) | 6.1±3.2 (50) | 4.9±1.3a (104) | 6.1±3.2 (50) |
| DR | 6.1±2.8b (130) | 8.1±5.8 (70) | 6.2±3.3b (119) | 8.1±5.8 (70) |
| NPDR | 6.0±2.9c (85) | 10.4±8.1 (40) | 5.2±1.6c (74) | 10.4±8.1 (40) |
| PDR | 5.6±1.9d (45) | 7.1±4.5 (30) | 5.2±1.4d (45) | 7.1±4.5 (30) |
| MCP-1 (pg/ml) | ||||
| DNR | 13.4±11.1a (107) | 24.7±19.8 (50) | 12.1±9.0a (104) | 24.7±19.8 (50) |
| DR | 18.7±14.4b (130) | 30.5±18.8 (70) | 16.8±12.1b (119) | 30.5±18.8 (70) |
| NPDR | 20.8±18.5c (85) | 28.3±19.8 (40) | 20.4±15.6c (74) | 28.3±19.8 (40) |
| PDR | 15.6±10.4d (45) | 27.1±17.3 (30) | 18.6±13.1d (45) | 27.1±17.3 (30) |
The data are expressed as the mean±SD; the number in the parentheses indicates the number of subjects. DNR, diabetic non-retinopathy; DR, diabetic retinopathy; MCP-1, monocyte chemoattractant-1; NF-κB p65, nuclear factor kappa B p65; NPDR, non-proliferative diabetic retinopathy; and PDR, proliferative diabetic retinopathy. ap<0.05 versus DNR with no antihyperglycemic and antihypertensive medication; bp<0.05 versus DR with no antihyperglycemic and antihypertensive medication; cp<0.05 versus NPDR with no antihyperglycemic and antihypertensive medication; dp<0.05 versus PDR with no antihyperglycemic and antihypertensive medication.
The correlation results observed in this study (Table 3) were weak (r<0.5) but significant (p<0.05). The NF-κB p65 level was positively correlated with the plasma MCP-1 level in healthy controls and DNR patients as well as with the diabetes duration in both DNR and DR patients. In addition, positive correlations were also observed between the NF-κB p65 and HbA1c levels as well as the triglyceride level in the DNR patients. Both the SBP and DM duration in the DNR patients were positively correlated with the plasma MCP-1 level, but an inverse correlation was found between the plasma MCP-1 and HDL-C levels. In DR patients, the plasma MCP-1 level was positively correlated with the HbA1c level, DM duration and retinopathy duration.
Pearson correlations between NF-kB, MCP-1 and several clinical parameters of different clinical groups.
| Biochemical parameters | Pearson r | p-value |
|---|---|---|
| Ctrl | ||
| NF-κB p65/MCP-1 | 0.19 | <0.01 |
| DNR | ||
| NF-κB p65/MCP-1 | 0.18 | <0.05 |
| NF-κB p65/HbA1c | 0.18 | <0.05 |
| NF-κB p65/diabetes duration | 0.20 | <0.05 |
| NF-κB p65/triglycerides | 0.19 | <0.05 |
| MCP-1/HDL-C | - 0.17 | <0.05 |
| MCP-1/diabetes duration | 0.16 | <0.05 |
| MCP-1/SBP | 0.17 | <0.05 |
| DR | ||
| NF-κB p65/diabetes duration | 0.17 | <0.05 |
| MCP-1/HbA1c | 0.25 | <0.01 |
| MCP-1/diabetes duration | 0.26 | <0.001 |
| MCP-1/retinopathy duration | 0.16 | <0.05 |
Ctrl, healthy controls; DNR, diabetic non-retinopathy; DR, diabetic retinopathy; HbA1c, glycated hemoglobin; HDL-C, high-density lipoprotein cholesterol; MCP-1, monocyte chemoattractant protein-1; NF-κB p65, nuclear factor kappa B p65; and SBP, systolic blood pressure.
The risk factors for DR were investigated among the type 2 DM patients (DNR and DR) using a logistic regression model (Table 4). High levels of HbA1c (OR = 1.20, p<0.05), plasma MCP-1 (OR = 1.04, p<0.05) or activated NF-κB p65 (OR = 1.08, p<0.05) and long diabetes duration (OR = 1.08, p<0.05) were risk factors for DR after adjusting for age, gender and other metabolic factors. Antihyperglycemic (OR = 0.81 for oral and OR = 0.63 for insulin, p<0.05 for both) and antihypertensive (OR = 0.79, p<0.05) medications were protective against the development of DR by type 2 DM patients.
Risk assessment for DR in type 2 DM patients.
| Characteristics | Unadjusted model | Adjusted model a | ||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Age (years) | 0.96 (0.93-1.00) | 0.052 | ||
| Gender Female | 1.00 (reference) | |||
| Male | 0.87 (0.58-1.31) | 0.511 | ||
| BMI (kg/m2) | 0.96 (0.92-1.01) | 0.099 | ||
| SBP (mmHg) | 1.01 (1.00-1.02) | 0.274 | ||
| DBP (mmHg) | 1.00 (0.98-1.02) | 0.736 | ||
| Diabetes duration (years) | 1.08 (1.05-1.11) | 0.000 | 1.08 (1.04-1.13) | 0.000 |
| HbA1c (%) | 1.34 (1.18-1.53) | 0.000 | 1.20 (1.00-1.44) | 0.046 |
| Total cholesterol (mmol/l) | 1.25 (1.04-1.49) | 0.018 | 1.46 (0.76-2.79) | 0.251 |
| Triglycerides (mmol/l) | 1.25 (0.96-1.63) | 0.100 | ||
| HDL-C (mmol/l) | 1.02 (0.53-1.95) | 0.952 | ||
| LDL-C (mmol/l) | 1.25 (1.01-1.54) | 0.045 | 0.68 (0.33-1.42) | 0.308 |
| ALT (IU/l) | 1.00 (0.99-1.01) | 0.998 | ||
| AST (IU/l) | 1.00 (0.99-1.02) | 0.615 | ||
| Current Smokers No | 1.00 (reference) | |||
| Yes | 0.51 (0.30-0.87) | 0.013 | 0.40 (0.15-1.03) | 0.057 |
| Alcohol intake No | 1.00 (reference) | |||
| Yes | 0.58 (0.31-1.07) | 0.081 | ||
| Hypertension No | 1.00 (reference) | |||
| Yes | 0.79 (0.49-1.28) | 0.347 | ||
| Insulin antihyperglycemic medication | ||||
| No | 1.00 (reference) | |||
| Yes | 0.74 (0.37-5.92) | 0.000 | 0.63 (0.16-4.66) | 0.018 |
| Oral antihyperglycemic medication | ||||
| No | 1.00 (reference) | |||
| Yes | 0.67 (0.40-1.12) | 0.003 | 0.81 (0.50-1.78) | 0.033 |
| Antihypertensive medication | ||||
| No | 1.00 (reference) | |||
| Yes | 0.56 (0.37-0.86) | 0.008 | 0.79 (0.40-1.58) | 0.040 |
| NF-κB p65 (AU/mg) | 1.11 (1.01-1.18) | 0.005 | 1.08 (0.98-1.19) | 0.030 |
| MCP-1 (pg/ml) | 1.05 (1.03-1.06) | 0.000 | 1.04 (1.02-1.07) | 0.002 |
Type 2 DM patients (DNR and DR) (n = 371) were included in the analysis model. ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CI, confidence interval; DBP, diastolic blood pressure; DR, diabetic retinopathy; HbA1c, glycated hemoglobin; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; MCP-1, monocyte chemoattractant protein-1; NF-κB p65, nuclear factor kappa B p65; OR, odds ratio; and SBP, systolic blood pressure. a Adjusted for age, gender and metabolic risk factors (BMI, HbA1c and DM duration).
DR is a chronic, low-grade inflammatory disease caused by the presence of microscopic signs of inflammation in the retina such as vasodilatation, fluid exudation, leukocyte migration and altered retinal blood flow (5). Previous reports have shown that the local expression of MCP-1 and NF-κB in the retina as well as their levels in the vitreous fluids are increased in PDR patients (17-18). This study aimed to assess the circulating levels of activated NF-κB p65 in PBMCs and plasma MCP-1 in type 2 DM patients with retinopathy who were taking antihyperglycemic and antihypertensive drugs. The basal levels of plasma MCP-1 and NF-κB p65 in the PBMCs of healthy controls in this study were comparable to previously observed values (19-20). NF-κB and MCP-1 could be involved in the pathogenesis of DR due to the findings of significantly elevated levels of these markers in non-medicated DR patients, and the plasma MCP-1 level was positively correlated with retinopathy duration.
In addition, both DM duration and HbA1c were found to be positively correlated with both of the inflammatory markers in DR patients. This confirms that prolonged hyperglycemia triggers inflammation, possibly by causing abnormal local leukocyte-endothelial interaction in DR patients. Inflammatory chemokines are also potential angiogenic factors (21). Therefore, MCP-1 may act with vascular endothelial growth factor (5) to promote angiogenesis in the retina and cause neovascularization in PDR. The positive correlation between the NF-κB p65 and plasma MCP-1 levels in the healthy controls and DNR patients further supports the regulatory role of NF-κB on MCP-1. Inflammatory chemokines, including MCP-1, also activate NF-κB, leading to the generation of reactive oxygen species and creating a vicious cycle (7). This study also showed that NF-κB p65 was positively correlated with the triglyceride levels and that the plasma MCP-1 levels were inversely associated with the HDL-C levels in DNR patients. The above observations are in agreement with a previous report suggesting the possible involvement of MCP-1 in obesity-related health complications (8).
The 1998 United Kingdom Prospective Diabetes Study, involving 3,867 type 2 DM patients, demonstrated that early intervention with oral glucose-lowering and antihypertensive drugs reduced the incidence of diabetic complications and improved the survival of these patients (9-10). However, there was no evidence to show whether this beneficial effect was derived directly from tight glycemic and blood pressure control or through other means of action. In this study, approximately 60% of the DM patients received antihyperglycemic and antihypertensive medications. The patient groups (DNR, DR, NPDR and PDR) who were taking either antihyperglycemic (insulin, biguanides, sulfonylureas and thiazolidinediones) or antihypertensive drugs (angiotensin-converting enzyme inhibitors, calcium channel blockers and angiotensin II receptor antagonists) had significantly lower levels of NF-κB p65 and plasma MCP-1 compared with the non-medicated group. These findings may explain the beneficial effects of the antihyperglycemic and antihypertensive drugs on diabetic microvascular complications that were observed in the United Kingdom Prospective Diabetes Study. However, this study did not investigate the local effect of these medications on the expression of NF-κB and MCP-1 in the retina of DR patients. A previous report showed that a reduction of local retinal inflammation and the NF-κB and MCP-1 expression levels was found in cultured murine endothelial cells treated with antihypertensive drugs (22). Thus, we speculate that the vitreous levels of NF-κB and MCP-1 in the DR patients are concurrently reduced with the circulating levels of these pro-inflammatory markers.
This transversal study could not identify the actual mechanism by which antihypertensive drugs ameliorate DR. A local renin–angiotensin system exists in retinal glial cells (23), and it is upregulated in DR (24). Angiotensin II is a well-known vasoconstrictor, and it may induce vascular endothelial growth factors that lead to retinal neovascularization (24), a common microscopic sign in PDR. We speculate that the beneficial effect of antihypertensive treatments on DR could partly be due to blocking of the renin–angiotensin system. In addition, a cross-interaction between the advanced glycation end product (AGE)-receptor for AGE (RAGE) and the renin-angiotensin system has been proposed in the pathogenesis of DR (25). Previously, we have shown that RAGE is a pertinent factor leading to DR, and its genetic variants are associated with the development of DR (26). Antihypertensive drugs may reduce the AGE-RAGE interaction by attenuating the NF-κB that controls the cellular expression of RAGE. It should be noted that NF-κB is not the only regulator of diabetes-induced inflammation in DR. The presence of hypoxia in DR could activate another transcription factor, hypoxia inducible factor 1 (27), which was not investigated in this study.
In conclusion, this study showed significant attenuation of both the PBMC NF-κB p65 and circulating MCP-1 levels in DR patients who were taking antihyperglycemic and antihypertensive drugs. Nevertheless, a prospective case-control study is required to substantiate our findings.
This study was supported by a University of Malaya Research Grant (UMRG155-09HTM), a High Impact Research Grant (UM/MOHE-HIR E000042-20001) and the University of Malaya Postgraduate Research Fund, PS239/2010A.
No potential conflict of interest was reported.
Ng ZX, Kuppusamy UR and Chua KH carried out the experiments, data analysis and manuscript preparation. Tajunisah I, Pendek R, Ng ZX, Kuppusamy UR and Chua KH were involved in experimental design, sample collection, and grant and ethics applications.