Large vessel occlusion in acute ischemic stroke is associated with low recanalization rates under intravenous thrombolysis. We evaluated the safety and efficacy of the Solitaire AB stent in treating acute ischemic stroke.
METHODS:Patients presenting with acute ischemic stroke were prospectively evaluated. The neurological outcomes were assessed using the National Institutes of Health Stroke Scale and the modified Rankin Scale. Time was recorded from the symptom onset to the recanalization and procedure time. Recanalization was assessed using the thrombolysis in cerebral infarction score.
RESULTS:Twenty-one patients were evaluated. The mean patient age was 65, and the National Institutes of Health Stroke Scale scores ranged from 7 to 28 (average 17±6.36) at presentation. The vessel occlusions occurred in the middle cerebral artery (61.9%), distal internal carotid artery (14.3%), tandem carotid occlusion (14.3%), and basilar artery (9.5%). Primary thrombectomy, rescue treatment and a bridging approach represented 66.6%, 28.6%, and 4.8% of the performed procedures, respectively. The mean time from symptom onset to recanalization was 356.5±107.8 minutes (range, 80-586 minutes). The mean procedure time was 60.4±58.8 minutes (range, 14-240 minutes). The overall recanalization rate (thrombolysis in cerebral infarction scores of 3 or 2b) was 90.4%, and the symptomatic intracranial hemorrhage rate was 14.2%. The National Institutes of Health Stroke Scale scores at discharge ranged from 0 to 25 (average 6.9±7). At three months, 61.9% of the patients had a modified Rankin Scale score of 0 to 2, with an overall mortality rate of 9.5%.
CONCLUSIONS:Intra-arterial thrombectomy with the Solitaire AB device appears to be safe and effective. Large randomized trials are necessary to confirm the benefits of this approach in acute ischemic stroke.
Ischemic stroke is the third leading cause of death and the leading cause of long-term disability worldwide (1); it is the leading cause of mortality in Brazil (2). An increased national incidence of stroke is expected, and preventive and acute medical care is needed (3). Despite the rising occurrence of strokes and stroke-related deaths, the adult Brazilian population has demonstrated an alarming lack of knowledge about stroke prevention, recognition, and treatment (4). Although national initiatives aimed at improving stroke care have been implemented (5), few national studies have examined intra-arterial recanalization therapies for acute ischemic stroke.
Intravenous thrombolysis using a recombinant tissue plasminogen activator (TPA) for arterial recanalization is the standard treatment for acute ischemic stroke (6,7). Recanalization is strongly associated with clinical outcomes (8). Intravenous thrombolysis has not been associated with high recanalization rates in large vessel occlusions (9,10). In this setting, intra-arterial thrombectomy with retrievable stent devices has reportedly achieved higher recanalization rates and shorter recanalization times (11-28). The aim of this study was to assess the safety and efficacy of the Solitaire AB device (ev3, Irvine, CA, USA) in acute ischemic stroke patients who were prospectively evaluated and treated at the Clinics Hospital of the Ribeirão Preto Medical School, São Paulo, Brazil. The literature on intra-arterial recanalization therapies was reviewed and compared to the results of the present study.
METHODSPatient PopulationWe prospectively followed a group of patients who were treated for acute ischemic stroke with the Solitaire AB device (ev3, Irvine, CA, USA) by the same stroke team from June 2011 to January 2012. The study was approved by the ethics committee at our institution. The patients or their legal representatives signed the consent forms that were previously approved by the institutional review board. The patients were selected for intra-arterial recanalization treatment according to our institution's acute stroke protocol for recanalization strategies. Initially, the patients were assessed for eligibility for intravenous thrombolysis using the National Institutes of Neurological Disorders and the European Cooperative Stroke Study 3 trial criteria (1,2). The endovascular recanalization procedures have three modalities: rescue treatment, a bridging approach, and primary thrombectomy. All of the patients who were eligible for standard intravenous thrombolysis were considered for rescue intra-arterial therapy if they exhibited a persistent proximal occlusion at the end of intravenous fibrinolysis. Likewise, the patients were considered for bridging intra-arterial therapy if they presented with a tandem carotid occlusion or basilar occlusion, which are known to have low recanalization rates with intravenous TPA (9,10). The patients who were ineligible for intravenous fibrinolysis were treated with primary thrombectomy. Patients were excluded from intra-arterial therapy if they presented with hypoattenuation in more than one-third of the middle cerebral artery territory on brain computed tomography (CT) or if intra-arterial recanalization could not be performed within eight hours of symptom onset for anterior circulation stroke or within nine hours for posterior circulation stroke.
The arterial occlusion site was assessed with either combined transcranial color-coded duplex (TCCD) and carotid ultrasound (US) or brain computed tomography angiography (CTA). When vessel imaging was unavailable, a score≥10 on the National Institutes of Health Stroke Scale (NIHSS), coma, tetraparesis and locked-in syndrome were used as the proximal occlusion criteria.
Patient assessment and follow-upThe stroke severity was assessed using the NIHSS upon admission. A brain CT scan was quickly performed to determine the brain tissue injury pattern. When available, TCCD and carotid US were also performed in the acute phase to assess the artery occlusion site. If visualizing the intracranial arteries was not possible using TCCD, a CTA scan was performed. A second and third brain CT scan were obtained immediately after thrombolysis and between 24 and 48 hours after treatment, respectively. All of the patients were kept under observation until discharge and were followed up three months after their strokes. The patient outcomes were assessed by certified examiners at discharge (NIHSS) and the three-month follow-up (modified Rankin Scale, mRS). The time from the symptom onset to the recanalization and procedure times were recorded. The procedure time began with the groin puncture and ended at the instant of recanalization. Recanalization was assessed using the thrombolysis in cerebral infarction score (TICI) (29). The number of Solitaire AB device (ev3, Irvine, CA, USA) passes was recorded.
Rescue treatmentStandard intravenous thrombolysis was performed with a 0.9 mg/kg dose of TPA (with a maximum total dose of 90 mg), which was delivered as an initial bolus of 10% with the remaining 90% infused within 60 minutes. The patients were monitored using NIHSS assessment and continuous TCCD. An urgent rescue thrombectomy was performed 60 minutes after the bolus if a persistent proximal occlusion was visible on TCCD or the NIHSS score did not improve by≥4 points (if TCCD was unavailable).
Bridging approachThe patients who presented with carotid tandem occlusion or basilar artery occlusion associated with coma, tetraparesis or locked-in syndrome within 4.5 hours of the symptom onset were placed on intravenous fibrinolysis using TPA at a dose of 0.6 mg/kg (15% as a bolus and the remaining 85% as an infusion after 30 minutes), and the angiography suite was prepared for an urgent thrombectomy.
Primary thrombectomyPrimary thrombectomy was indicated for those patients with acute ischemic stroke who were ineligible for intravenous fibrinolysis and those patients presenting between 4.5 and 8 hours after the onset of an anterior circulation occlusion or between 4.5 and 9 hours after the onset of a posterior circulation occlusion.
Neurointerventional procedureFor the thrombectomy, the patient was transferred to the angiography suite, where the intubation was performed. The patient's blood pressure was carefully monitored throughout the anesthesia induction and the procedure to maintain a minimum pressure of 180 mm Hg (systolic arterial pressure) or 80 mm Hg (mean arterial pressure). If there were no complications, the patient was extubated immediately after the procedure.
All of the procedures were performed using the femoral artery approach. An intravenous bolus of 5,000 IU of standard heparin was administered after the puncture if intravenous TPA was not previously infused. If TPA was infused prior to the endovascular procedure, no heparin was administered after the femoral puncture. A 7-Fr guiding catheter (Guider Softip; Boston Scientific, Natick, MA) or a 6-Fr guiding catheter (Neuron; Penumbra, Alameda, CA) was introduced through a femoral sheath into the internal carotid artery or the most navigable vertebral artery (related to the ischemic vascular territory). The guiding catheter was continuously perfused with a solution of 40 mg of verapamil hydrochloride diluted in 1,000 mL of physiological saline (0.9%). Frontal, oblique, and lateral angiography series were completed to determine the cervical vessel that was related to the ischemic territory of the brain and to define the occluded intracranial vessels. If a proximal carotid or vertebral occlusion was identified, a Wallstent or Express stent, respectively, (both by Boston Scientific Target, Fremont, CA, USA) was used to perform an angioplasty stenting procedure. After stenting, the guiding catheter was placed into the cervical artery, and a manual thrombus aspiration was performed. After this step, or if no proximal cervical artery occlusion was identified, a 0.021-inch Rebar 18 or a 0.027-inch Rebar 27 microcatheter (ev3, Irvine, CA, USA) was navigated distal to the point of occlusion over a 0.014-inch steerable microwire (Transend EX Platinum; Boston Scientific Target). The microwire was exchanged with a 4×20 mm or 6×30 mm Solitaire AB device (ev3, Irvine, CA, USA). The device was left in place for 3 to 5 minutes. Then, the microcatheter was advanced to recoat the first third of the Solitaire AB device (ev3, Irvine, CA, USA). The fully deployed Solitaire AB device and the delivery microcatheter were gently pulled back as a single unit and recovered via manual aspiration of the guiding catheter; this procedure was performed with a 60 mL syringe. Successful recanalization was defined as a TICI score of 3 or 2b in all of the treatable vessels. If the treatable vessel was not opened to a minimum of TICI 2b after a maximum of eight passes of the thrombectomy device, the treatment was considered to be a failure. No intra-arterial fibrinolytics were administered, even if the recanalization attempt was unsuccessful. The groin punctures were closed with an Angio-Seal (St. Jude Medical, St. Paul, MN).
Endpoints, statistical analysis and literature reviewWe collected clinical and radiological data from all of the study subjects. The mean and standard deviation (SD) were calculated for the numeric variables, and Fisher's exact test was used for the categorical data. All of the statistical analyses were performed with SPSS version 20.0 (Chicago, IL). We searched PubMed for studies published through May 1, 2012 that evaluated retrieval devices for acute ischemic stroke and large intra-arterial therapy for acute ischemic stroke trials. The percentage of recanalization, percentage of mRS scores≤2 at 3 months, symptomatic hemorrhage rates and mortality rates in the present study were compared to those in the earlier large trials.
RESULTSTwenty-one patients were evaluated. Vessel imaging (TCCD and US or CTA) was performed on all of the subjects. The mean patient age was 65 years, and the patients' NIHSS scores ranged from 7 to 28 (average 17, SD±6.36) on presentation. Vessel occlusions were located in the middle cerebral artery (61.9%), distal internal carotid artery (14.3%), tandem carotid occlusion (14.3%), and basilar artery (9.5%). Primary thrombectomy, rescue treatment and the bridging approach were performed in 66.6%, 28.6%, and 4.8% of the procedures, respectively. The mean time from symptom onset to recanalization was 356.5±107.8 (SD) minutes (range, 80–586 minutes). The mean procedure times were 60.4±58.8 (SD) minutes (range, 14–240 minutes), and 45.7±35.7 minutes for the successful procedures. We obtained an overall recanalization rate (TICI scores of 2b and 3) of 90.4% and a symptomatic intracranial hemorrhage rate of 14.2%. The number of device passes ranged from 1 to 8 (average 3.1, SD±2.4). There were no device fractures or arterial dissections. The NIHSS scores at discharge ranged from 0 to 25 (average 6.9, SD±7.0). At three months, 61.9% of the patients had an mRS score≤2.
The overall mortality rate was 9.5%. One (4.7%) patient died from pneumonia after being discharged. Symptomatic hemorrhagic complications were observed in three patients (14.2%), one of whom (4.7%) had a subarachnoid hemorrhage and died during hospitalization. The other two patients (9.4%) presented with hemorrhagic transformation and were completely dependent on caregivers at the three-month follow-up. Contrast medium enhancement was observed in 12 cases (57.1%). The patients' clinical and technical data are summarized in Table 1. Figures 1, 2 and 3 illustrate procedures with patients 12, 14 and 20, respectively.
Clinical and technical data of patients.
| Patient | Age/Gender | NIHSS on presentation | NIHSS on discharge | Symptomatic intracranial hemorrhage | CT scan constrast enhance | mRS - 3 months | Artery site occluded | Recanalization time (minutes) | Procedure time (minutes) | Treatment modality | No of device passes | TICI post-treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 79/F | 7 | 1 | N | Y | 0 | Proximal LMCA | (c) | 40 | R | 1 | 3 |
| 2 | 69/M | 20 | 3 | N | Y | 2 | Proximal LMCA | (c) | 106 | M | 6 | 3 |
| 3 | 79/M | 21 | 8 | N | Y | 5 | Proximal LMCA | 412 | 57 | R | 2 | 3 |
| 4 | 58/F | 12 | 0 | N | N | 1 | Proximal LMCA | 365 | 20 | M | 3 | 3 |
| 5 | 68/M | 12 | 4 | N | N | 2 | Proximal LMCA | (c) | 16 | M | 1 | 3 |
| 6 | 75/M | 19 | 15 | N | Y | 6 | Proximal RMCA | 329 | 29 | R | 1 | 3 |
| 7 | 59/M | 18 | 7 | N | Y | 4 | Right tandem | 336 | 51 | B | 1 | 3 |
| 8 | 86/F | 14 | NA | Y | (a) | 6 | Proximal RMCA | (c) | 46 | M | 5 | 2b |
| 9 | 71/M | 26 | 3 | N | Y | 2 | Proximal LMCA | 360 | 20 | M | 2 | 3 |
| 10 | 61/M | 24 | 1 | N | N | 2 | Right tandem | 157 | 97 | M | 4 | 3 |
| 11 | 67/M | 20 | 25 | Y | (b) | 5 | Right tandem | 447 | 240 | M | 8 | 0 |
| 12 | 42/M | 20 | 0 | N | N | 0 | Distal RICA | 367 | 43 | R | 2 | 3 |
| 13 | 80/M | 18 | 17 | N | Y | 5 | Proximal RMCA | 320 | 160 | M | 8 | 0 |
| 14 | 49/M | 26 | 6 | N | Y | 2 | Proximal LMCA | 162 | 16 | M | 1 | 3 |
| 15 | 57/F | 16 | 4 | N | N | 2 | Distal RICA | 586 | 140 | M | 5 | 3 |
| 16 | 81/F | 28 | 20 | N | Y | 4 | Distal LICA | 415 | 81 | R | 7 | 2b |
| 17 | 85/F | 19 | 10 | Y | (b) | 5 | Proximal RMCA | 268 | 16 | M | 2 | 3 |
| 18 | 81/M | 8 | 4 | N | Y | 2 | BA | 452 | 22 | M | 4 | 3 |
| 19 | 48/M | 8 | 2 | N | N | 1 | Proximal RMCA | 481 | 27 | M | 1 | 3 |
| 20 | 33/F | 7 | 4 | N | Y | 2 | BA | 300 | 14 | M | 2 | 3 |
| 21 | 37/F | 15 | 4 | N | Y | 2 | Proximal LMCA | 304 | 29 | R | 1 | 3 |
(NA): not available; (a): subarachnoid hemorrhage; (b): symptomatic intra-parenchimal hemorrhage; (c): cases that represented wake-up stroke, thus without accurate onset time; (Y) yes, (N) no; (M) primary mechanical thrombectomy; (R) rescue treatment; (BA) bridging approach.
(a) Digital subtraction angiography (DSA) of the right common carotid artery ([RCCA] arterial phase, oblique view) shows an occlusion of the distal right internal carotid artery (RICA) (black arrow); (b) a road map of the RICA (oblique view) shows a guiding catheter in the RICA (arrowhead); the distal tip of the microcatheter (white arrow) and the microwire have crossed the occluded portion of the RICA and moved through the thrombi and into the right middle cerebral artery (RMCA) (black arrow); (c) a road map of the RICA (oblique view) shows the terminal tip of microcatheter (white arrow) distal to the occlusion site; (d) a road map of the RICA (oblique view) shows the terminal tip of the microcatheter (white arrow) and the terminal radiopaque marks of a 6x30 mm Solitaire AB device (ev3, Irvine, CA, USA), which indicate the start of deployment (white arrowhead); (e) a road map of the RICA (oblique view) in which the proximal stent radiopaque mark is seen ahead of the distal tip of microcatheter (white arrowhead), indicating the full deployment of the stent into the RMCA and the distal RICA; (f) a control DSA of the RCCA (arterial phase, oblique view) that was performed after the stent retrieval shows that the distal RICA, right anterior cerebral artery (RACA) and RMCA branches have been completely opened; (g) a picture shows the Solitaire AB (ev3, Irvine, CA, USA) and the removed clot.
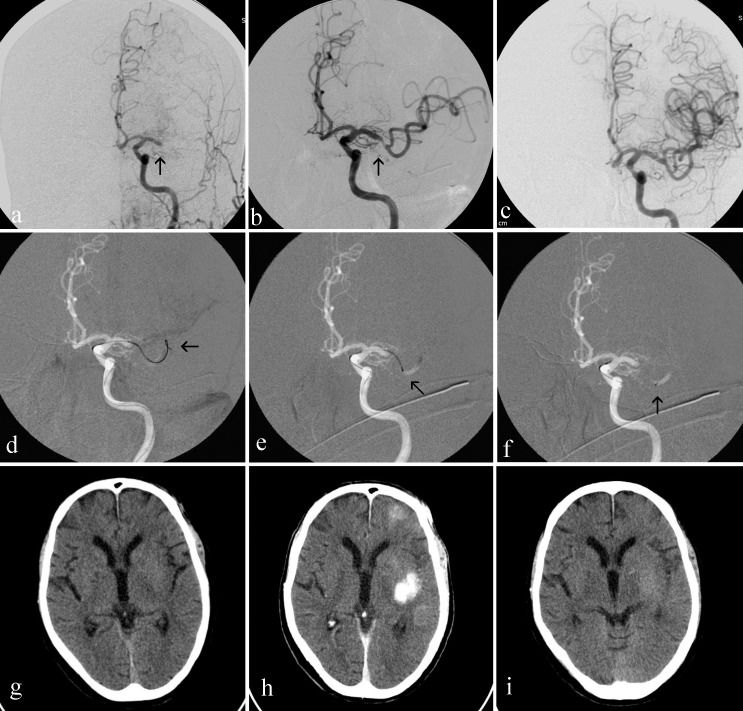
(a) DSA of the left common carotid artery (LCCA), arterial phase, frontal view, shows a proximal occlusion of M1 segment of left middle cerebral artery (LMCA) (black arrow); (b) DSA of left interna carotid artery (LICA), arterial phase, frontal view, shows a Solitaire AB (ev3, Irvine, CA, USA) deployed into the M1 segment and promptly flow restoration of LMCA (black arrow); (c) Final control DSA of the LCCA, arterial phase, frontal view, shows the complete recanalization of the LMCA; (d, e, f) road maps of LICA, frontal views, show (d) the microwire crossed the occluded LMCA (black arrow), (e) the terminal tip of the microcatheter (black arrow) placed beyond the thrombi into the M1 segment of the LMCA and (f) terminal radiopaque marks of a 4x20 mm Solitaire AB device (ev3) deployed into the occluded site of LMCA; (g, h, i) Brain CT scans, (g) performed on presentation revealing no signs of hemorrhage or ischemic injury, (h) performed immediately after intra-arterial thrombectomy showing contrast medium enhance in the left middle cerebral artery territory and (i) performed 48 hours after intra-arterial thrombectomy showing contrast medium cleared and slight parenchymal hypoattenuation signs.
(a, b) a DSA of (a) the right vertebral artery (RVA) and (b) the left vertebral artery (LVA) (white arrows), arterial phase, frontal views, shows an occlusion of the proximal basilar artery (BA) (white arrowheads); (c) a DSA of the LCCA (frontal view) shows the BA (white arrow) filled by the left posterior communicating artery; (d, e) a Road map of the RVA (frontal view) shows (d) the microwire (white arrow) crossing the occluded section of the BA and (e) the distal tip of the microcatheter (white arrow) advanced by the microwire through the thrombi; (f) a DSA of the BA (frontal view) shows the contrast medium being injected through the microcatheter distal to the thrombi in the BA (white arrow); (g) the distal (white arrow) and proximal (white arrowhead) radiopaque marks of the stent (ev3, Irvine, CA, USA) reveal its full deployment into the distal RVA and BA; (h, i) A final control DSA of the RVA (arterial phase, [h] frontal and [i] lateral views) shows the complete recanalization of the BA.
We reviewed the literature and compared our results with the results from 17 studies that investigated retrieval devices for treating acute stroke (11-27). We investigated the recanalization rate, recanalization time, procedure time, symptomatic intracranial hemorrhage rate, 90-day mortality rate, average patient NIHSS upon presentation, and study outcomes. Overall, the results were similar (Table 2).
The main studies assessing the use of retrieval devices for treating acute ischemic stroke (searched in PubMed through May 1, 2012).
| Author - Year | Sample | Mean NIHSS baseline | Symptomatic intracranial hemorrhage n (%) | 3 month mRS≤2 n (%) | 90-day mortality n (%) | Mean time from onset to recanalization (minutes) | Mean procedure time (minutes) | Mean number of device passes | TICI 2b, 3 n(%) |
|---|---|---|---|---|---|---|---|---|---|
| Castanho et al. 2010 | 20 | 19 | 2 (10) | 9 (45) | 4 (20) | 422 | 50.0 | 1.4 | 18 (90) |
| Roth et al. 2010 | 22 | 19.4 | 2 (9) | 11 (50) | 4 (18.1) | 277 | NR | 1.7 | 20 (90.9) |
| Naiak et al. 2010 | 7 | 18.8 | 1 (14.2) | 4 (57) | NR | 244 | 84.0 | 1.8 | 4 (57) |
| Brenkfeld et al. 2011 | 17 | 19 | 0 (0) | 7 (43) | NR | NR | 52.5 | 1.3 | 16 (94.1) |
| Costalat et al. 2011 | 50 | 14.7 | 5 (10) | 27 (54) | 6 (12) | 377 | 54.0 | 2.0 | 44 (88) |
| Mendes Pereira et al. 2011 | 141 | 18 | NR | 77 (54.6) | 29 (20.5) | NR | 45 | NR | 121 (86) |
| Rohde et al. 2011 | 10 | 19 | 2 (20) | NR | 3 (30) | 260.7 | 88.7 | 3.0 | 10 (100) |
| Fesl et al. 2011 | 14 | 16.5 | 4 (29) | 6 (45) | 1 (8.3) | 267.0 | 29.0 | NR | 13 (93) |
| Miteff et al. 2011 | 26 | 21.4 | NR | 11 (42.3) | 5 (19.2) | 476.4 | 95.6 | NR | 11 (42) |
| Mohlenbruch et al. 2011 | 25 | 14 | 3 (12) | 15 (60) | 2 (8) | 237 | 54 | 2.0 | 22 (88) |
| Park et al. 2011 | 8 | 18.3 | 0 (0) | 4 (50) | 0 (0) | 221.7 | 41.5 | 1.5 | 8 (100) |
| Stampfl et al. 2011 | 18 | 21 | 3 (16.6) | 6 (33.3) | 5 (27.7) | 289.2 | 48.3 | NR | 16 (88.8) |
| Cohen et al. 2012 | 17 | 20.5 | 2 (11.7) | 15 (88.2) | 1 (5.8) | 299.1 | 45 | 2.3 | 16 (94.1) |
| Mpotsaris et al. 2012 | 26 | 16 | NR | 10 (38.4) | 2 (7.6) | 327 | NR | 2.1 | 18 (69.2) |
| Parrilla et al. 2012 | 48 | 17.4 | NR | NR | NR | 409.3 | 121.3 | NR | 48 (100) |
| San Román et al. 2012 | 60 | 18 | 7 (12) | 27 (45) | 17 (28) | 302 | 80 | NR | 44 (73.3) |
| Menom et al. 2012 | 14 | 13.6 | 1 (7.1) | 8 (57.1) | 2 (14.2) | NR | 84 | NR | 12 (85.7) |
| Total | 523 | 17.9 | 38 (13.4) | 465 (50.1) | 81 (17.9) | 316.3 | 64.8 | 1.9 | 441 (84.3) |
| Castro-Afonso et al. 2012 | 21 | 17 | 3 (14.2) | 13 (61.9) | 2 (9.5) | 356.5 | 60.1 | 3.1 | 19 (90.4) |
Comparing the outcomes of the present study with the results of six large trials assessing intra-arterial treatment for stroke, (28), our study obtained a higher recanalization rate than the PROACT-II, MERCI, IMSII and Multi-MERCI trials. However, our recanalization results were similar to those of the Penumbra pivotal stroke trial and the primary results of the SWIFT trial. The outcome (mRS scores≤2 at 3 months) and three-month mortality rate results in our study were significantly improved over the results found in the MERCI, Multi-MERCI, and Penumbra pivotal stroke trials. No differences were observed in the symptomatic intracranial hemorrhage. The outcomes of the different trials are presented in Table 3.
The outcomes of the present study compared to the results from 6 large trials assessing intra-arterial treatment for acute ischemic stroke.
| PROACT-II | MERCI | IMSII | Multi-MERCI | Penumbra pivotal stroke trial | SWIFT trial (SolitaireTM arm) | Present study | |
|---|---|---|---|---|---|---|---|
| Sample (∗), n | 121 | 141 | 55 | 164 | 125 | 58 | 21 |
| Recanalization, n (%) | 80 (66.1) p = 0.037 | 68 (48.2) p<0.001 | 33 (60.0) p = 0.013 | 112 (68.2) p = 0.041 | 102 (81.6) p = 0.530 | 48 (88.9) p = 0.499 | 19 (90.4) |
| Symptomatic intracranial hemorrhage, n (%) | 11 (9.0) p = 0.436 | 11 (7.8) p = 0.396 | 8 (14.5) p = 0.999 | 16 (9.7) p = 0.458 | 14 (11.2) p = 0.713 | 1 (1.7) p = 0.055 | 3 (14.2) |
| 3-month mortality, n (%) | 30 (24.7) p = 0.161 | 61 (43.2) p = 0.003 | 13 (23.6) p = 0.212 | 56 (34.1) p = 0.024 | 41 (32.8) p = 0.037 | 10 (17.2) p = 0.499 | 2 (9.5) |
| 3-month mRS≤2, n (%) | 48 (39.6) p = 0.093 | 39 (27.6) p = 0.005 | 37 (67.2) p = 0.788 | 59 (35.9) p = 0.031 | 52 (41.6) p = 0.010 | 32 (58.2) p = 0.619 | 13 (61.9) |
A high recanalization rate (90.4%) without an accompanying increase in the number hemorrhagic complications was observed in this prospective series of patients with acute stroke caused by a proximal artery occlusion and treated with intra-arterial thrombectomy with the Solitaire AB stent (ev3, Irvine, CA, USA). In addition, a significant proportion of patients exhibited a slight or no long-term disability. Our overall results in the Brazilian population are in accordance with previous studies using retrievable stents (11-27).
The new retrievable intracranial stents have shown superior results in large vessel occlusions when compared with other recanalization devices and techniques. The higher retrievable stent recanalization rates observed in the context of acute ischemic stroke could be attributed to a device design that is technically simpler, promotes rapid recanalization, removes the clot, and avoids the deployment of a definitive stent, which may lead to rethrombosis and necessitate using a dual antiplatelet regime (11-27).
Although the preliminary trials assessing retrievable stents have shown promising technical and clinical results, more data regarding the use of retrievable stents in acute ischemic stroke will be available in the near future. The results of four large studies assessing the Solitaire device (ev3, Irvine, CA, USA) are expected soon: the STAR Trial (Solitaire FR Thrombectomy for Acute Revascularization - NCT01327989), the IMSIII Trial (Interventional Management of Stroke III - NCT00359424), the EXTEND-IA (Extending the Time for Thrombolysis in Emergency Neurological Deficits - NCT00887328), and the THRACE trial (Trial and Cost Effectiveness Evaluation of Intra-arterial Thrombectomy in Acute Ischemic Stroke - NCT01062698).
The main limitations of the present study are the lack of a control group and the small sample size. Therefore, our conclusions concerning the safety and efficacy of the procedure are limited. The one-year study duration also precluded an analysis of the local learning curve. Nevertheless, the initial reports regarding the safety and effectiveness of new devices and technologies are usually the first critical step in the rapidly evolving field of endovascular intervention.
Despite the small sample size, the use of the Solitaire AB device (ev3, Irvine, CA, USA) in a Brazilian population provided good technical and clinical results. Our results underscore the importance of a combined stroke team and the enrollment of patients under a strict stroke protocol. Considering the recent efforts to organize an acute stroke network in Brazil (5), we believe that this study could serve as a preliminary ischemic stroke treatment model and thus motivate the government, hospitals and public health administrators to widely support and promote acute stroke care for the Brazilian people.
Intra-arterial thrombectomy with the Solitaire AB device (ev3, Irvine, CA, USA) appears to be safe and effective and provided a high recanalization rate when used with a strict stroke protocol. Large randomized trials are necessary to confirm the benefits of this treatment approach to acute ischemic stroke.
No potential conflict of interest was reported.
Castro-Afonso LH guaranteed the integrity of the entire study, contributed to the study concepts and design, definition of intellectual content, literature research, clinical studies, data acquisition and analysis, and statistical analysis. Abud DG guaranteed the integrity of the entire study, contributed to the study concepts and design, definition of intellectual content, clinical studies, and data acquisition and analysis. Pontes-Neto OM and Cougo-Pinto PT contributed to the study concepts and design, data acquisition and analysis, and statistical analysis. Abud TG contributed to the definition of intellectual content, data acquisition and analysis, and statistical analysis. Nakiri GS contributed to the study concepts, data acquisition and analysis, and statistical analysis. Oliveira L, Monsignore LM, Santos D, Dias FA, Fábio SR, Coletto FA contributed to the data acquisition and analysis.







![(a) Digital subtraction angiography (DSA) of the right common carotid artery ([RCCA] arterial phase, oblique view) shows an occlusion of the distal right internal carotid artery (RICA) (black arrow); (b) a road map of the RICA (oblique view) shows a guiding catheter in the RICA (arrowhead); the distal tip of the microcatheter (white arrow) and the microwire have crossed the occluded portion of the RICA and moved through the thrombi and into the right middle cerebral artery (RMCA) (black arrow); (c) a road map of the RICA (oblique view) shows the terminal tip of microcatheter (white arrow) distal to the occlusion site; (d) a road map of the RICA (oblique view) shows the terminal tip of the microcatheter (white arrow) and the terminal radiopaque marks of a 6x30 mm Solitaire AB device (ev3, Irvine, CA, USA), which indicate the start of deployment (white arrowhead); (e) a road map of the RICA (oblique view) in which the proximal stent radiopaque mark is seen ahead of the distal tip of microcatheter (white arrowhead), indicating the full deployment of the stent into the RMCA and the distal RICA; (f) a control DSA of the RCCA (arterial phase, oblique view) that was performed after the stent retrieval shows that the distal RICA, right anterior cerebral artery (RACA) and RMCA branches have been completely opened; (g) a picture shows the Solitaire AB (ev3, Irvine, CA, USA) and the removed clot. (a) Digital subtraction angiography (DSA) of the right common carotid artery ([RCCA] arterial phase, oblique view) shows an occlusion of the distal right internal carotid artery (RICA) (black arrow); (b) a road map of the RICA (oblique view) shows a guiding catheter in the RICA (arrowhead); the distal tip of the microcatheter (white arrow) and the microwire have crossed the occluded portion of the RICA and moved through the thrombi and into the right middle cerebral artery (RMCA) (black arrow); (c) a road map of the RICA (oblique view) shows the terminal tip of microcatheter (white arrow) distal to the occlusion site; (d) a road map of the RICA (oblique view) shows the terminal tip of the microcatheter (white arrow) and the terminal radiopaque marks of a 6x30 mm Solitaire AB device (ev3, Irvine, CA, USA), which indicate the start of deployment (white arrowhead); (e) a road map of the RICA (oblique view) in which the proximal stent radiopaque mark is seen ahead of the distal tip of microcatheter (white arrowhead), indicating the full deployment of the stent into the RMCA and the distal RICA; (f) a control DSA of the RCCA (arterial phase, oblique view) that was performed after the stent retrieval shows that the distal RICA, right anterior cerebral artery (RACA) and RMCA branches have been completely opened; (g) a picture shows the Solitaire AB (ev3, Irvine, CA, USA) and the removed clot.](https://static.elsevier.es/multimedia/18075932/0000006700000012/v1_202212011629/S1807593222021615/v1_202212011629/en/main.assets/thumbnail/cln-67-12-1379-g001.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)

![(a, b) a DSA of (a) the right vertebral artery (RVA) and (b) the left vertebral artery (LVA) (white arrows), arterial phase, frontal views, shows an occlusion of the proximal basilar artery (BA) (white arrowheads); (c) a DSA of the LCCA (frontal view) shows the BA (white arrow) filled by the left posterior communicating artery; (d, e) a Road map of the RVA (frontal view) shows (d) the microwire (white arrow) crossing the occluded section of the BA and (e) the distal tip of the microcatheter (white arrow) advanced by the microwire through the thrombi; (f) a DSA of the BA (frontal view) shows the contrast medium being injected through the microcatheter distal to the thrombi in the BA (white arrow); (g) the distal (white arrow) and proximal (white arrowhead) radiopaque marks of the stent (ev3, Irvine, CA, USA) reveal its full deployment into the distal RVA and BA; (h, i) A final control DSA of the RVA (arterial phase, [h] frontal and [i] lateral views) shows the complete recanalization of the BA. (a, b) a DSA of (a) the right vertebral artery (RVA) and (b) the left vertebral artery (LVA) (white arrows), arterial phase, frontal views, shows an occlusion of the proximal basilar artery (BA) (white arrowheads); (c) a DSA of the LCCA (frontal view) shows the BA (white arrow) filled by the left posterior communicating artery; (d, e) a Road map of the RVA (frontal view) shows (d) the microwire (white arrow) crossing the occluded section of the BA and (e) the distal tip of the microcatheter (white arrow) advanced by the microwire through the thrombi; (f) a DSA of the BA (frontal view) shows the contrast medium being injected through the microcatheter distal to the thrombi in the BA (white arrow); (g) the distal (white arrow) and proximal (white arrowhead) radiopaque marks of the stent (ev3, Irvine, CA, USA) reveal its full deployment into the distal RVA and BA; (h, i) A final control DSA of the RVA (arterial phase, [h] frontal and [i] lateral views) shows the complete recanalization of the BA.](https://static.elsevier.es/multimedia/18075932/0000006700000012/v1_202212011629/S1807593222021615/v1_202212011629/en/main.assets/thumbnail/cln-67-12-1379-g003.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)