To analyze the nutritional status of pediatric patients after orthotopic liver transplantation and the relationship with short-term clinical outcome.
METHOD:Anthropometric evaluations of 60 children and adolescents after orthotopic liver transplantation, during the first 24 hours in a tertiary pediatric intensive care unit. Nutritional status was determined from the Z score for the following indices: weight/age, height/age or length/age, weight/height or weight/length, body mass index/age, arm circumference/age and triceps skinfold/age. The severity of liver disease was evaluated using one of the two models which was adequated to the patients’ age: 1. Pediatric End-stage Liver Disease, 2. Model for End-Stage Liver Disease.
RESULTS:We found 50.0% undernutrition by height/age; 27.3% by weight/age; 11.1% by weight/height or weight/length; 10.0% by body mass index/age; 61.6% by arm circumference/age and 51.0% by triceps skinfold/age. There was no correlation between nutritional status and Pediatric End-stage Liver Disease or mortality. We found a negative correlation between arm circumference/age and length of hospitalization.
CONCLUSION:Children with chronic liver diseases experience a significant degree of undernutrition, which makes nutritional support an important aspect of therapy. Despite the difficulties in assessment, anthropometric evaluation of the upper limbs is useful to evaluate nutritional status of children before or after liver transplantation.
The liver is the largest and most important metabolic organ, playing a pivotal role in integrating several biochemical pathways of carbohydrate, fat, protein, and vitamin metabolism (1,2). Thus, children with chronic liver disease are at high risk for developing undernutrition, with important prognostic implications.
The pathogenesis of undernutrition in chronic liver diseases is multifactorial and includes a reduction in nutrient and caloric intake, anorexia and dietary restrictions, impaired intestinal absorption, abnormalities in nutrient metabolism (carbohydrate, lipid, and protein), and increased proinflammatory cytokine levels, resulting in a hypermetabolic state (1,3). Due to several metabolic alterations that characterize end-stage liver disease, most adult and pediatric liver transplantation candidates present with undernutrition in the hospital. This condition is especially prevalent in developing countries.
Nutritional therapy is one of the most important procedures in the management of liver disease and should be considered an essential adjunct to clinical therapies, especially when the patient is a candidate for liver transplantation (4).
Orthotopic liver transplantation (OLT) revolutionized the management of liver disease from the results achieved with the technique, as well as improved understanding of the indications, contraindications, and problems associated with the technique (5). The indications for and clinical outcomes of OLT depend on the patient’s nutritional status. As undernutrition is known to be associated with a greater risk of post-operative complications and higher mortality rates in patients with liver disease (6,7), this correlation is also believed to be present in patients undergoing liver transplantation (8). Recipients with undernutrition were found to be at greater risk for increased operative blood loss, have longer stays in the intensive care unit, and have higher mortality rates and higher total hospital charges after liver transplantation (8,9). In the immediate phase after liver transplantation, protein catabolism is markedly increased, as demonstrated by the excretion of large amounts of urinary nitrogen (10).
Multiple techniques have been proposed to detect undernutrition in patients with liver disease (11-14). Nevertheless, it is well known that some common nutritional parameters can be misleading in advanced liver disease because of water retention and ascites (11), compromised protein synthesis and coexisting alterations in renal function (12). Although there is no gold standard for the assessment of nutritional status in patients with liver disease, anthropometric measurements, such as arm muscle circumference and triceps skinfold, have been utilized in large groups of patients, mainly in those awaiting OLT, and proved to be useful in identifying muscle and fat depletion.
In the current study, the nutritional status of a group of pediatric patients was prospectively analyzed immediately after OLT, and the relationship between nutritional status and short-term clinical outcomes (mortality and length of stay in the pediatric intensive care unit) were analyzed.
MATERIALS AND METHODSFor this descriptive and prospective study, the participants were selected from infants, pre-school children, school children, and adolescents sequentially admitted to the pediatric intensive care unit (PICU) of Instituto da Criança, Hospital das Clínicas da Faculdade de Medicina da Universidade de Sao Paulo, Brazil, immediately after OLT between January 2006 and December 2009. This tertiary PICU has 20 beds for clinical and surgical patients. The third author (UT) performed or supervised all operations. The Ethical Committee of the institution approved the study protocol.
Children with liver disease were followed in the outpatient Pediatric Surgery Unit, where the OLT was indicated. The unit serves patients from various regions of the country with different clinical conditions. There is no prescribed nutritional program for these patients; only obvious nutritional deficiencies are treated.
We performed anthropometric nutritional evaluations for 60 patients after deceased donor or living-relative donor OLT, during the first 24 hours in the PICU. The study was approved by the Research and Ethics Committee of the institution.
Anthropometric nutritional assessment was carried out during the first 24 hours after admission following OLT. To minimize the possibility of errors, all measurements were performed by the first author of the study (PZ) using the same method described in a previous study (15). The assessment included weight (W), height (H) or length (L), arm circumference (AC), and triceps skinfold thickness (TST).
Weight was measured with a scale that was calibrated for accuracy before each use. Children over 16 kg were weighed standing up, and infants were weighed using a scale accurate to the nearest 5 g. Children and adolescents (six cases) who could not be weighed independently were held by an adult (a parent or evaluator) while being weighed. The child’s weight was obtained by subtracting the weight of the adult from the total weight of the child and adult. In general, children and adolescents were extubated in the first 24 hours allowing them to be weighed more easily.
Length was measured in children up to three years of age using a pediatric anthropometer accurate to the nearest 0.1 cm. Children were placed in the supine position for the measurement.
In children older than three years, height was obtained with a wooden stadiometer, which was accurate to the nearest 0.1 cm. In children whose condition prevented the use of conventional measuring techniques (i.e., mechanical ventilation, vasoactive drugs), height was predicted from measurements of the distance between the knee and the ankle, with the child in the supine position. The length between the heel and the anterior surface of the leg at the knee (femoral condyle) was measured using a pediatric anthropometer. The following equations proposed by Chumlea et al. (16) were used:
a) Caucasian girls = 43.21 + (2.15 × distance knee/ankle);
b) African-American girls = 46.59 + (2.02 × distance knee/ankle);
c) Caucasian boys = 40.54 + (2.22 × distance knee/ankle); and
African-American boys = 39.60 + (2.18 × distance knee/ankle).
Using the data for W and H or L, we obtained the following indices: W/age (A), H/A or L/A, W/H or W/L. We calculated body mass index for age (BMI/A) using the following equation: BMI = W (kg)/H2 (m).
AC was measured with metric tape marked at increments of 0.5 cm. Measurements were taken at the midpoint of the distance between the acromion and the olecranon, with the arm extended along the body.
TST was obtained using a Lange Skinfold Caliper (Cambridge Scientific Industries, Cambridge, MD) with a constant pressure of 10 g/mm2 on the contact surface. The measurement was taken on the back of the arm, parallel to the longitudinal axis, at the midpoint between the acromion and olecranon. We used the average of three consecutive measurements.
Nutritional status was determined from the Z score (Z) for the following indices: W/A, H/A or L/A, W/H or W/L, BMI/A, AC/A, and TST/A. We used reference values from the World Health Organization (WHO) (17). WHO Anthro (version 3.1)/2010 and WHO Anthro Plus/2007 were used for the calculations. Anthropometric evaluation is important for classifying nutritional status and is correlated with the nutritional condition prior to transplantation. Although the post-operative state may influence the nutritional condition, we believe that this influence is not sufficient to change the nutritional classification (undernutrition or not).
The severity of liver disease was evaluated by the extent of Pediatric End-stage Liver Disease (PELD), which indicates the need for a liver transplantation. The extent of PELD is a numerical value applied to children under 12 years old and considers the outcomes of laboratory tests such as bilirubin, albumin, international normalized ratio (INR) and stunting based on gender, weight and height. For patients over 12 years old, the Model for End-stage Liver Disease (MELD) was used to quantify the urgency of OLT.
The population was analyzed for age at admission, sex, type of donor (cadaveric or living-relative donor), mortality, discharge and cause of liver disease. The results were tabulated in an Excel® spreadsheet.
The mean, median and standard deviation (SD) Z were obtained for all anthropometric indicators (W/A, H/A or L/A, W/H, BMI/A, AC/A and TST/A) as well as PELD and length of stay in the PICU.
The distribution of anthropometric indicators in the study population was considered normal by the Kolmogorov-Smirnov test.
Anthropometric indicators of nutritional status and their relationship with disease severity (PELD/MELD), mortality and PICU stay were analyzed using correlation and linear regression analyses with Pearson coefficient calculations.
Data were considered statistically significant at p<0.05, and a 95% confidence interval was used for measures of central tendency. We used SPSS 12.01 to perform the calculations.
RESULTSThe study population consisted of 60 patients evaluated in the PICU after OLT, with a median age of 26 months (2.8 years).
The predominant age group was comprised of children in pre-school and children who were less than two years old; most of these children were female. The median length of PICU stay was seven days, and the average PELD/MELD value was 24. Table 1 shows the main characteristics of this population. The mean, median and standard deviation of Z anthropometric indicators are shown in Table 2, and the frequency of malnutrition in children is shown in Table 3.
Main characteristics for study subjects.
| Population | n | % |
|---|---|---|
| Age | ||
| <2 years | 29 | 48.4 |
| 2 a <5 years | 17 | 28.3 |
| 5 a <10 years | 8 | 13.3 |
| 10 a 20 years | 6 | 10.0 |
| Sex | ||
| Male | 22 | 36.6 |
| Female | 38 | 63.4 |
| Kind of donor | ||
| Deceased donation | 36 | 60 |
| Living donor | 24 | 40 |
| Diagnoses | ||
| Biliary atresia (AVB) | 37 | 61.7 |
| Cirrhosis | 5.0 | 8.3 |
| Deficiency of ornithine transcarbomilase | 1.0 | 1.7 |
| Cholesterol ester storage disease | 1.0 | 1.7 |
| Autoimmune hepatitis | 1.0 | 1.7 |
| Chronic hepatitis | 2.0 | 3.3 |
| Fulminant hepatitis | 6.0 | 10.0 |
| Hepatoblastoma | 1.0 | 1.7 |
| Alagille syndrome | 4.0 | 6.6 |
| Tyrosinemia | 2.0 | 3.3 |
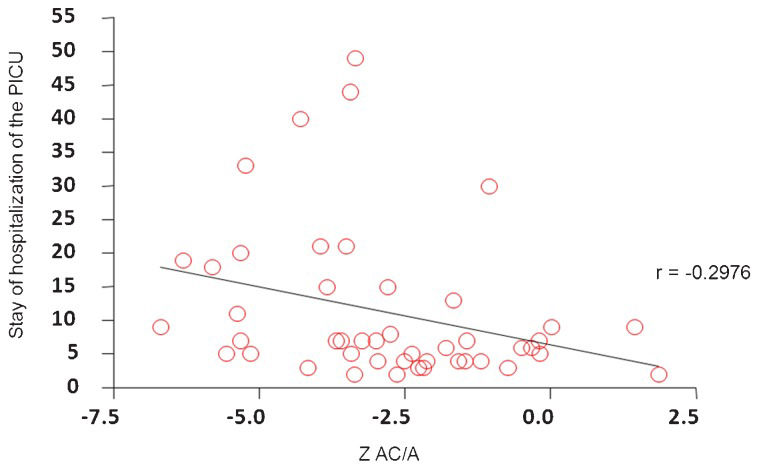
There was no correlation between nutritional status assessed by BMI/A and PELD/MELD (r = 0.05792; p = 0.66) and mortality (p = 0.92), despite the high mortality rate, which was 18% (11 children) overall and 16% to 20% among malnourished children (Table 4). We did not find a correlation between nutritional status assessed by BMI/A (r = -0.03242), H/A (r = -0.2005) and W/A (r = -0.1975) and the length of stay in the PICU. This correlation was found only with AC/A (r = - 0.2976), as shown in Figure 1.
Hospital protein-energy undernutrition was first described in 1980. Current studies report variable percentages depending on the methods used and countries involved (18,19). Children who are admitted to the hospital, mainly in the ICU, are at a high risk of developing undernutrition, particularly children with an underlying disease and severe clinical condition (higher Pediatric Risk of Mortality Score) (20-(22). Undernutrition is associated with increased morbidity and mortality in children, including a higher risk of infections due to poor immune defense, wound-healing problems, and reduced gut function, and longer dependency on mechanical ventilation and longer hospital stays (19).
Roggero et al. (23) found that the predominant factors that influence undernutrition in children with chronic liver disease are age for infants and the degree of hepatic failure for older children. Frequently, undernutrition is associated with higher incidence of infection, surgical complications, and a lower survival rate after liver transplantation.
With the exception of patients with fulminant hepatic failure, most candidates for human OLT present with significant undernutrition, especially in developing countries. Nutritional deficiencies usually occur prior to clinical signs of hepatic insufficiency. Assessing the nutritional status of patients with chronic liver disease is difficult and controversial because the parameters utilized to perform the evaluation are frequently altered by organomegaly, ascites, and edema. Furthermore, patients with end-stage liver disease (ESLD) exhibit significant impairment in hepatocyte synthetic function, which can result in depressed albumin, prealbumin, and transferrin serum levels, thus impairing an adequate nutritional diagnosis (2,9,24).
There is no diagnostic “gold standard” for assessing nutritional status in patients with liver disease. No single criterion should be neglected when assessing nutritional status in these patients. The assessment must be as complete as possible and should include the following components: medical and dietary history, subjective global assessment, anthropometric measurements, biochemical parameters and indices, and a more complex body composition analysis (24,25).
No controversy currently exists regarding the importance of nutritional status as an important predictor of post-transplant outcomes and the benefits of therapeutic nutritional improvement (25). The main objective of the present study was to evaluate the nutritional status of children and adolescents immediately after liver transplantation and to determine the relationship with short-term clinical outcomes (mortality and length of stay in the PICU). Nutritional status was measured immediately after transplant; this parameter primarily reflects the pre-operative condition in these patients, who usually have major fluid overload and frequently have positive balance during surgery as a consequence of the provision of fluids and blood products. However, in this study, even with fluid overload, there was a high percentage of undernutrition demonstrating that this condition has a high prevalence among patients awaiting or undergoing OLT (deceased donation and living donor).
Easily applicable techniques include anthropometric measurements in liver transplant candidates, and current guidelines for enteral nutrition recommend the use of anthropometry parameters to assess the nutritional status of these patients, despite the limitations of this technique. In our study, we used anthropometry to assess children and adolescents after OLT.
We found 50.0% undernutrition by H/A; 27.3% by W/A; 11.1% by W/H or W/L; 10.0% by BMI/A; 61.6% by AC/A, and 51.0% by TST/A. These results confirm that undernutrition is common in children with liver diseases waiting for or undergoing OLT, as demonstrated by previous studies (6,23,26,27), and that prevalence of undernutrition varies according to the index used. Israels et al. (28) evaluated 128 pediatric patients with cancer using anthropometric indexes and found 59.3% malnourished children by muscular arm circumference (MAC) and TST compared to 17.2% diagnosed by W/H. Body weight is not a sensitive parameter because edema, ascites and hepatosplenomegaly overestimate nutritional status (1,23). Nonetheless, we found that 27.3% of children and adolescents were malnourished, as evaluated by W/A.
The degree of height deficits for age is generally considered to be indicative of chronic undernutrition (24). Half of the patients studied had z-scores of -2.02 SD for H/A or L/A, demonstrating chronic impairment of nutritional status, although 48% of children were less than 2 years old. Roggero et al. (23) also found deficits in H/A in children under 1 year waiting for liver transplants, showing that growth can be impaired in children with liver disease who are candidates for OLT even at early ages. H/A can be a useful index in assessing these children.
W/H and BMI, which is generally used to detect acute undernutrition, as well as W/A, may underestimate the degree of undernutrition in children with chronic liver disease (29). This statement is supported by our findings: W/H identified only 11% of children as malnourished and BMI/A identified 10% of children as malnourished. A similar overall result was also found in critically ill patients, in whom edema and fluid retention are usually present. Body weight is increased by edema, resulting in underestimation of under nutrition. In these studies, the minority of patients was considered malnourished by W/H or BMI/A (30-32).
Skinfold measurements and MAC are considered useful parameters with which to assess subcutaneous fat and muscle mass (25) and can help to identify children with chronic under nutrition. In these cases, skinfolds and circumferences are considered to be more accurate measurements than height because variations in these parameters appear earlier than changes in height. However, peripheral edema may falsely increase such measurements (24). To minimize factors contributing to error in anthropometric assessment, such as edema, measurements of skinfold thickness in the upper body are indicated because edema is more readily accumulated in the lower body (24). Furthermore, it is recommended that measurements be performed by the same individual.
The results of our study showed that body muscle mass and fat mass values were reduced more markedly. Despite the possible bias, we found undernutrition in almost 70% of patients by AC/A and more than half of patients by TST/A. We found similar results in a previous study, which utilized measurements in the arm and showed higher accuracy in identifying malnutrition among 90 children and adolescents in a tertiary pediatric intensive care unit, 90% of whom had underlying disease (15). Anthropometric evaluation was useful for classifying nutritional status and followed these patients even with the physiological changes common after OLT.
It is assumed that undernutrition increases mortality after liver transplantation (2,6). Among the patients of the current study, 18.4% died, and although we have not found any association between nutritional status and mortality, the high incidence of undernutrition may have caused a negative impact on mortality (Table 4). Figueiredo et al. (29) investigated the impact of nutritional status on outcomes after liver transplantation. They prospectively studied the predictive value of preoperative nutritional status on the adverse outcomes in 53 patients that received OLT and concluded that none of the nutritional parameters (body cell mass, anthropometry, subjective global assessment) was associated with increased risk of mortality or global resource utilization.
Although no study has demonstrated that preoperative undernutrition is an independent predictor of perioperative mortality, an increased need for blood transfusions, prolonged ICU stays, longer duration of hospitalization and, consequently, higher hospital costs for liver transplantation have been reported (32). We found an association between nutritional status, as determined by AC/A and length of stay in the PICU, showing that malnutrition negatively influences the immediate postoperative OLT and that it indirectly increases hospital costs.
Our findings show that patients with chronic liver diseases exhibit substantial undernutrition. This condition is known as a basic indicator of surgical risk, making nutritional support an important aspect of therapy for children with chronic diseases (33), such as end-stage liver disease, and/or waiting for an OLT. Despite the difficulties in assessing and technical limitations, we recommend that a simple, inexpensive, easily reproducible, and noninvasive technique, such as anthropometric evaluation of the upper limbs, is useful for assessment of nutritional status. Serial anthropometric measurements are recommended to evaluate the effects and adequacy of nutritional interventions.
No potential conflict of interest was reported.
Zamberlan P carried out dates and helped to draft the manuscript. Leone C performed data analyses and statistical analysis. Tannuri U and Carvalho WB participated in the coordination of the study and critically revised it. Delgado AF participated in design, coordination of the study and helped to draft the manuscript.