To evaluate the analytical micromethod using liquid chromatography for the quantification of propranolol in children submitted to surgery of tetralogy of Fallot (TLF). Methods: Only 0.2 mL of plasma is required for the assay. Peaks eluted at 8.4 (Propranolol) and 17.5 min (verapamil, internal standard) from a C18 column, with a mobile phase 0.1 M acetate buffer, pH 5.0, and acetonitrile (60:40, v/v) at flow rate 0.7 mL/min, detected at 290 nm (excitation) and 358 nm (emission). Surgery was started 776 min of drug administration (8.7mg, mean); seven blood samples were collected from six patients (4M/2F; 2.1yrs;11.5kg; 0.80m; 18.9kg/m2).
RESULTSConfidence limits of the method showed high selectivity and recovery, sensitivity of 0.02ng/mL, good linearity (0.05-1000ng/mL), precision of 8.6% and accuracy of 3.1%. The mean duration of surgery was 283.2min, with the patients remaining under cardiopulmonary bypass (CPB) for 114min. A declining curve of propranolol plasma concentration was obtained after the last dose in the night that preceded the day of surgery. Plasma concentration also was normalized with hematocrit due to the hemodilution caused by the CPB procedure. On the other hand a decrease on drug plasma concentration was obtained between periods, the beginning of surgery to the postoperative day 2 (7.09 ng/mL and 0.05 ng/mL, p<0.05 respectively) and from the end of CPB to the postoperative day 2 (2.79ng/mL e 0.05ng/mL, p<0.05).
CONCLUSIONPropranolol monitoring of plasma concentrations of children (TLF) normalized after the last preoperative dose revealed a decline from the beginning of surgery to the second postoperative day, suggesting that, once redistribution was restored, propranolol washout was complete.
Avaliar o micrométodo analítico empregando a cromatografia líquida para quantificação de propranolol em crianças operadas de tetralogia de Fallot (TLF).
MÉTODORequereu-se apenas volumes de 0,2mL de plasma para a realização do ensaio. Os picos foram eluídos em 8.4 (Propranolol) e 17.5 min (verapamil, padrão interno) de uma coluna C18, com fase móvel (tampão acetato 0,1 M pH 5,0 e acetonitrila, 60:40, v/v) em fluxo de 0,7 mL/min, sendo detectados em 290 nm (excitação) e em 358 nm (emissão). A cirurgia iniciou-se 776 min depois da dose administrada (8,7mg, média) e sete amostras de sangue foram coletadas de seis pacientes (4M/2F; 2,1 anos;11,5kg; 0,80m;18,9kg/m2).
RESULTADOSOs limites de confiança do método analítico evidenciaram alta seletividade e recuperação, sensibilidade (0,02ng/mL), boa linearidade (0,05-1000ng/mL), precisão de 8,6% e exatidão de 3,1%. A duração média da cirurgia foi de 283,2min, com os pacientes em circulação extracorpórea (CEC) durante 114min. Uma curva de declínio do propranolol no plasma foi obtida após a última dose na noite que precedeu o dia da intervenção. A concentração plasmática foi normalizada com o hematócrito devido à hemodiluição causada pela CEC. Por outro lado obteve-se decréscimo nas concentrações plasmáticas entre os períodos início da cirurgia para o 2o dia de pós-operatório (7,09 ng/mL e0,05 ng/mL, p<0,05 respectivamente) e do final da CEC para o 2o dia de pós-operatório (2,79ng/mL e 0,05ng/mL, p<0,05).
CONCLUSÃOO monitoramento das concentrações plasmáticas normalizadas do propranolol, em crianças com TLF, após a última dose pré-operatória revelou decaimento do início da cirurgia para o segundo pós-operatório, sugerindo que após a correção cirúrgica, uma vez restaurada a distribuição, a eliminação do fármaco foi completa.
Tetralogy of Fallot accounts for 10% of all congenital heart diseases and is the most common cyanotic heart disease during infancy1. Few children are asymptomatic and, by the age of 4 months, most of them develop cyanosis which is normally progressive, a fact leading to delayed growth and development. In addition, fatigue and breathlessness during exercise and episodes of hypoxia are common in these patients. Beta-adrenergic receptor blockers are of great therapeutic value in the care of patients with functional obstruction of ventricular flow. In this respect, propranolol is used successfully in three situations: management of an acute dyspnea attack, prevention of episodes of dyspnea, and improvement of the clinical conditions of children who await for surgical correction. On the other hand, there is a disadvantage in clinical practice because propanolol may cause serious respiratory problems, changes in cardiac output and cardiac insufficiency, all related to the high doses of propranolol required to maintain efficacy 2. Patients with tetralogy of Fallot should be submitted to surgical correction consisting of the closure of the ventricular septal defect with alleviation of right ventricular flow obstruction 3, 4, 5. Administration of propranolol during the preoperative period is advantageous and reduces the incidence of ischemic events, hypertension and secondary tachyarrhythmias 6. Because cardiopulmonary bypass (CPB) and hypothermia cause changes in plasma propranolol concentrations, drug monitoring during and after surgery becomes important.
Different methods have been developed to determine plasma propranolol concentrations, including fluorimetry 7, 8, 9, gas chromatography 10, and high-performance liquid chromatography with fluorescence detection (HPLC-F) 11–16. More recently, an analytical method for the quantification of propranolol in plasma of postoperative adult patients was proposed by Pereira et al (2000). Although this method was found to be selective, its sensitivity was relatively low for our purpose. Therefore, once the washout of propranolol was investigated in pediatric patients undergoing surgical correction of tetralogy of Fallot, the objective of the present study was to optimize a new method with high sensitivity to determine lower propranolol plasma concentrations in the perioperative period.
EXPERIMENTALClinical protocol and blood sample collectionThe study protocol was approved by the Ethics Committees of the Institutions participating of the investigation. Before inclusion in the study, the parents or legal representatives of all children provided a written informed consent to participate of the study. Patients using vasoactive drugs, with episodes of bronchospasm, or without indication for a beta-blocker drug were excluded from the study.
Table 1 summarizes the individual characteristics of six patients with tetralogy of Fallot with surgical indication for the corrective repair of the congenital anomaly. Patients allocated for the study were children of both sexes (4M/2F) 11.5+/-3.9 kg, 2.1+/-1.3 yrs, 0.8+/-0.1 m (height), 18.9+/-6.4 kg/m2 (body mass index). They received a long term treatment dose adjusted (1 to 3 mg/kg of propranolol, three times a day) in the ambulatory as follows: 4.2+/-2.8 mg/kg a day, orally.
Characteristics of the patients studied and daily dose of propranolol administered in the preoperative period.
| Patient | Gender | Age (years) | Weight (kg) | Height (m) | BMI (kg/m2) | Daily dose mg/kg/day |
|---|---|---|---|---|---|---|
| 1 | M | 0.6 | 9.9 | 0.8 | 17.6 | 9.0 |
| 2 | M | 2.7 | 13.2 | 0.9 | 16.3 | 1.5 |
| 3 | F | 2.2 | 12.9 | 0.9 | 16.7 | 4.5 |
| 4 | M | 4.0 | 16.5 | 0.7 | 31.8 | 1.8 |
| 5 | M | 2.6 | 11.3 | 0.9 | 15.2 | 5.4 |
| 6 | F | 0.5 | 5.0 | 0.6 | 15.9 | 3.0 |
| Mean | − | 2.1 | 11.5 | 0.8 | 18.9 | 4.2 |
| SD | − | 1.3 | 3.9 | 0.1 | 6.4 | 2.8 |
| 95%CI | − | 1.0- 3.2 | 8.4- 14.6 | 0.7- 0.9 | 13.8 – 24.0 | 2.0 – 6.4 |
BMI: body mass index; SD: standard deviation; 95% CI: upper and lower limits of the 95% confidence interval.
The last preoperative dose was administered the night before the day of surgery. The interval between the last dose and the beginning of surgery, the duration of surgery and of the CPB, and the hematocrit value are summarized in Table 2 as individual and population data.
Last dose administered before surgery and interval between the administration of the last preoperative propranolol dose and the beginning of surgery.
| Allocation | Last preoperative dose (mg) | Time between last dose and begin of surgery (min) | Duration of surgery (min) | Duration of CPB (min) | Ht% Begin of surgery | Ht% CPB |
|---|---|---|---|---|---|---|
| 1 | 10 | 600 | 360 | 120 | 43 | 23 |
| 2 | 10 | 720 | 240 | 110 | 38 | 21 |
| 3 | 10 | 636 | 230 | 100 | 40 | 22 |
| 4 | 10 | 720 | 241 | 100 | 46 | 27 |
| 5 | 5 | 1362 | 190 | 116 | 34 | 15 |
| 6 | 5 | 618 | 438 | 138 | 50 | 19 |
| Mean | 8.3 | 776.0 | 283.2 | 114.0 | 41.8 | 21.2 |
| SD | 2.6 | 291.6 | 94.9 | 14.3 | 5.7 | 4.0 |
| 95%CI | 6.3-10.4 | 542.7-1009.3 | 207.2-359.1 | 102.6-125.4 | 37.2-46.4 | 17.9-24.4 |
CPB: cardiopulmonary bypass; Ht: hematocrit; SD: standard deviation; 95% CI: upper and lower limits of the 95% confidence interval.
Seven blood samples (2 mL each) were collected at the following times: at the beginning of surgery, at the beginning of CPB, during CPB, at the end of CPB, at the end of surgery, and 24 and 48 h after surgery. Blood was collected into tubes containing EDTA sodium, centrifuged at 3000 g for 30 min, and plasma was stored at -20°C until assay; total volume of blood collected was lower than 20 mL. The samples were analyzed in parallel to the calibration daily curve and internal controls in three different concentrations (low, medium and high). Data obtained from patients were normalized with hematocrit before surgery using the following equation:
where,
Normalized and obtained data were plasma concentrations of the drug
Ht basal: hematocrit before surgery
Ht period: hematocrit obtained in each period
Analytical procedureLiquid–liquid extraction for the clean-up of biological sample
A simple liquid-liquid extraction was employed for the determination of propranolol plasma levels. For this, 0.2 mL aliquots of plasma were added to 0.2 mL internal standard (10 mg/L verapamil hydrochloride, 250ng/assay), 0.2 mL 1.25 M sodium hydroxide and 3 mL dichloromethane. Then, the mixture was vortexed for 1 min and centrifuged at 3000 g for 30 min. The supernatant was aspirated and discarded; the tube containing the organic phase was immersed in a liquid nitrogen bath, and the organic extract was carefully transferred to a conic tube and concentrated to dryness under a nitrogen flow in a water bath at 37°C. Next, the dry extract was dissolved with 0.2 mL of a mixture of acetonitrile and water (8:2,v/v) and 0.02 mL volumes were injected automatically into the liquid chromatograph.
Chromatographic analysisThe samples were analyzed by HPLC-F in a Shimadzu LC-10A liquid chromatograph (Kyoto, Japan) using a Shim-Pack CLC ODS C18 reverse-phase column (Shimadzu; 5 micron, 150 x 6.0 mm length x internal diameter) adapted to an insert NovaPak C18 Waters Assoc. (Milford, USA). The binary mobile phase consisted of 0.1 M acetate buffer, pH 5.0, and acetonitrile (60:40, v/v) at a flow rate of 0.7 mL/min in an isocratic elution system. The peaks were monitored with an RF 10 AXL fluorescence detector (Shimadzu) at excitation and emission wavelengths of 290 and 358 nm, respectively. The peak areas for propranolol and the internal standard were integrated using a Chromatopac CR-6A integrator Shimadzu (Kyoto, Japan).
Validation of the analytical methodThe calibration curve was obtained using aliquots of a stock solution of the drug (1 mg/mL) diluted in drug-free human plasma (blank) and stored at -20°C. The following concentrations of the standard propranolol solution in plasma were prepared: 1000, 500, 100, 50, 25, 10, 5, 2.5, 1, 0.5, and 0.05 ng/mL. The internal standard, verapamil hydrochloride (10 mg/L, working solution) was prepared from a stock solution (1000 mg/L, aqueous) distributed in small aliquots and stored at -20°C until assay.
Each nominal propranolol plasma concentration was plotted against the respective peak area ratio obtained for the drug and its internal standard; in addition the linear correlation coefficient (r2> 0.98) and its respective equation (Y = AX + B) were calculated. At least five of the nine calibration standards were considered for the construction of the daily calibration curve (0.05-100 ng/mL).
The internal controls (80, 40 and 0.5 ng/mL) were prepared in duplicate and analyzed during each analytical run to guarantee the acceptance of daily calibration curve. An analytical run was accepted when at least four of the six controls presented a deviation of less than 15% (for high and medium controls) or less than 20% (for the low control) from the nominal concentration. Then, the calibration curve was applied to quantify propranolol in plasma samples collected from patients included in the clinical protocol.
The linearity of the method was determined using ten propranolol concentrations in triplicate. The detection and quantification limits were evaluated based on the analysis of 10 replicates. The quantification limit was defined as the lowest plasma concentration of the daily calibration curve quantified with acceptable precision and accuracy. The detection limit was defined as one half of the quantification limit. Absolute recovery was determined using three drug concentrations (80, 40 and 0.5 ng/mL) in five replicates and expressed as percentage. The accuracy of the method was evaluated using three concentrations (low, medium and high) in six replicates, and the systematic error of the assay was determined based on the percentage of inaccuracy. Intra-day precision was determined based on the analysis of three concentrations in duplicate, whereas inter-day precision was evaluated by the duplicate analysis of three concentrations (80, 40 and 0.5 ng/mL) on three consecutive days, for a total of 18 assays.
For the determination of the stability of propranolol in plasma, less than 15% (highest concentration) and 20% (lowest concentration) variation was adopted as an acceptable criterion for all concentrations studied. Stability was analyzed after three freezing/thawing cycles of the drug in the biological sample. For the analysis, samples of two propranolol concentrations (250 and 16 ng/mL) were analyzed in triplicate on 3 consecutive days, corresponding to freezing/thawing cycles 1, 2 and 3.
The short-, medium- and long-term stability of the drug was also analyzed. Stability was evaluated as a function of time and storage conditions using extracted samples, in at least three concentrations (16, 62.5 and 250ng/mL) in replicates (n=3) kept at room temperature on the tray of the auto-sampler for the maximum time any sample could remain in this condition. For the determination of short-term stability, two concentrations were submitted to three freezing/thawing cycles in triplicate for each cycle. Long-term stability was determined using two concentrations (8 and 500 ng/mL) in replicates (n=3) stored for different periods of time up to 12 months, thus guaranteeing stability during the study period; the results are reported as percentage.
Robustness of the method was determined using two analytical C18 columns of different batches supplied by the same manufacturer, and using small variations in the proportion of the constituents and in the flow rate of the mobile phase. Six replicates were analyzed and the results are reported as a function of the coefficient of variation. The influence of room temperature variations could be ruled out since the temperature in the laboratory was monitored twice daily and was kept at 21 ± 2°C.
Statistical analysis and calculation of the mean residence timeThe normalized plasma propranolol concentrations were plotted as a function of sampling time. The mean residence time of the drug was determined using the PK Solutions 2.0, Non Compartment Data Analysis software (Ashland OH, USA).
The GraphPad Instat software 3.0 (GraphPad Instat Inc., San Diego CA, USA) was used for statistical analysis, and paired data were compared by ANOVA and Wilcoxon or Friedman tests. The results are reported as median, 95% confidence interval (95% CI) for drug concentration. The characteristics of patients were expressed as mean +/-standard deviation of the mean.
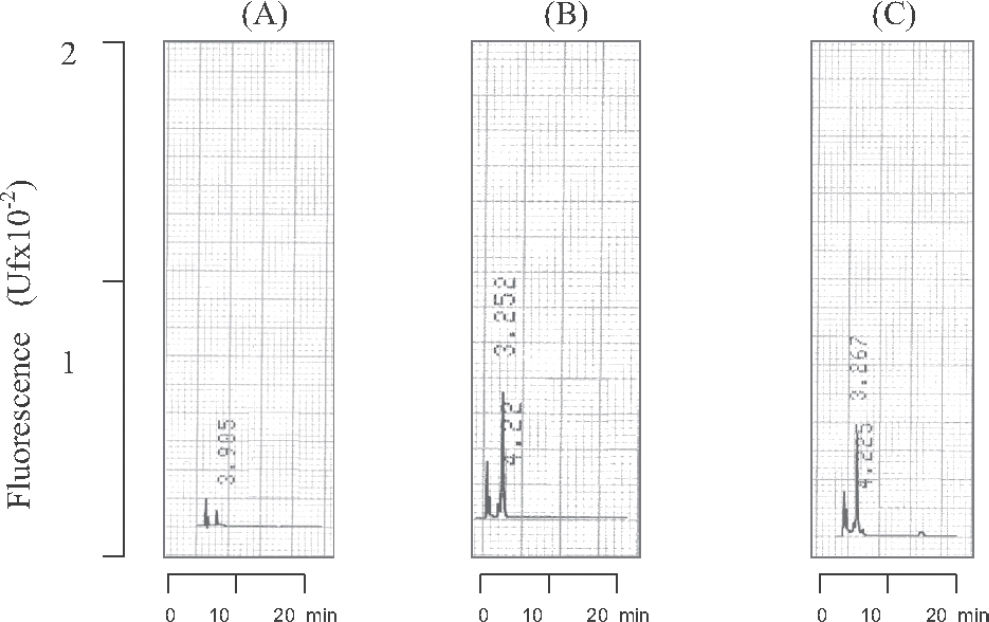
RESULTSValidation of the analytical methodThe specificity of the analytical method for the quantification of propranolol in biological samples was evaluated by injecting extracts of normal (n=2), lipemic (n=2) and hemolysate (n=2) plasma into the chromatographic system. The endogenous components eluted from the chromatographic column did not interfere with the analysis under the conditions described above, with the chromatogram peaks obtained for the endogenous components differing from the retention times obtained for propranolol and the internal standard (verapamil), as can be seen in Figure 1.
The peaks corresponding to propranolol and internal standard were eluted from the chromatographic column at 8.4 and 17.5 min, respectively. The chromatographic profile of the determination of propranolol in plasma is shown in Figure 2, which illustrates the analysis of a plasma blank, blank of plasma sample spiked (propranolol 5ng/mL, plasma standard) with addition of the internal standard and plasma of a patient receiving propranolol. Detection by fluorescence (290 nm and 358 nm for excitation and emission wavelengths, respectively) showed high sensitivity and good selectivity. The total time required for each chromatographic run was 20 min.
Chromatographic profile of plasma propranolol and its internal standard (verapamil). Chromatograms: (A) Blank of plasma samples without internal standard (IS), (B) blank of plasma sample spiked (propranolol 5ng/mL) plus IS. (C) plasma of a patient receiving propranolol. Retention times: 1) Propranolol 8.4 min and, 2) Verapamil (IS) 17.5 min.
The daily calibration curve (Figure 3) showed excellent linearity (r2 : 0.9998), and only 0.2 mL of the biological sample was required. Thus, the new analytical HPLC-F method proposed was selective and sensitive for the quantification of propranolol in plasma using a C18 column, a binary mobile phase and low flow rate.
The method developed presented high sensitivity (quantification limit = 0.05 ng/mL and detection limit = 0.02 ng/mL), linearity in the range of 0.05 to 1000 ng/mL with a linear coefficient of 0.9998, good recovery (97%), and acceptable precision (intra-day 4.7% and inter-day = 8.6%) and accuracy (3.1%). In addition data from studies of stability and robustness were included (Table 3 – 4).
Confidence limits of the analytical method for the determination of propranolol in plasma.
| Parameters | Unit | Propranolol |
|---|---|---|
| Linearity | ng/mL | 0.05-100 |
| Linear correlation coefficient (r2) | r2 : 0.9998 | |
| Detection limit | ng/mL | 0.02 |
| Quantification limit | ng/mL | 0.05 |
| Absolute recovery (80, 40 e 0.5ng/mL) | (%) | 95.0 |
| Precision (80, 40 e 0.5ng/mL) | ||
| Inter-day (mean, SD) | (%) | 8.6+/−3.9 |
| Intra-day | (%) | 4.7+/− 4.3 |
| Systematic error (inaccuracy) | ||
| (80, 40 e 0.5ng/mL) | (%) | 3.1+/−2.9 |
| Robustness (mean) | ||
| C18 columns (2 batches). | (CV%) | 1.5 |
| Flow rate (+/− 0.1mL) | (CV%) | 2.4 |
| Mobile phase | ||
| (+/− 1% in the proportion of acetonitrile) | (CV%) | 6.4 |
SD: standard deviation; CV: coefficient of variation.
Robustness of the method was analyzed based on analysis using different C18 columns (1.5%), variations in the composition or final pH of the mobile phase (6.4%), or in the flow rate (2,4%), as a function of the coefficient of variation.
Analysis of the stability on freezing/thawing cycle showed that propranolol concentration remained stable for at least three cycles for the two concentrations analyzed in triplicate (16 and 250 ng/mL), with a good coefficient of variation, as seen in Table 4.
The maximum time the sample remains on the tray of the automatic injector before analysis showed that propranolol remained stable for a least 18 h. The procedure was conducted for different concentrations (16, 62.5 and 250 ng/mL, replicates n=3) and a coefficient of variation equivalent to 3.8% was obtained, as shown in Table 4.
Regarding long-term stability, propranolol standards in the biological sample were found to be stable in a freezer (-20°C) for a minimum period of 24 months; a coefficient of variation of 0.8% was obtained for the assay performed in triplicate for propranolol high and low concentrations (500 and 8 ng/mL, respectively) in blank plasma spiked (Table 4).
Therapeutic drug monitoringThe validated analytical method was applied for drug monitoring of plasma concentrations in six patients undergoing heart surgery for the correction of a congenital anomaly (tetralogy of Fallot). Analyses were performed using a daily calibration standard curve accepted by the internal controls and propranolol plasma concentrations in patient samples were estimated by the linear regression equation.
The mean duration of surgery was 5.03 h (301.7 ± 99.6 min), with the patient remaining under CPB for 1.94 h (116.3 ± 14.4 min). Surgery was started 12.74 h (794.9 ± 270.8 min) after administration of the last dose of propranolol (8.7 ± 2.7 mg), as shown inTable 2.
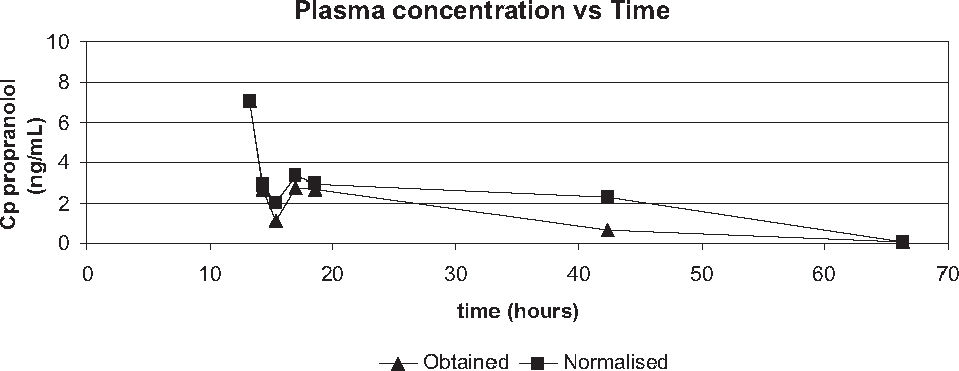
The declining curve of propranolol plasma concentration obtained from patients after the last dose in the night that preceded the day of surgery is illustrated in Figure 4.
The effect of hemodilution that occurs during the surgical intervention on propranolol plasma concentrations (data obtained and data normalized with hematocrit) was investigated by comparison. It showed no significant difference at the beginning of surgery (7.09 and 7.09 ng/mL, p=1.0000) as well as at the end of surgery (2.70 and 2.94 ng/mL, p=0.1250), Table 5. However, significant differences were observed by comparison of drug concentrations (obtained versus normalized) during CPB (1.14 versus 2.06 ng/mL, p=0.0313) and after the CPB (2.79 versus 3.37 ng/mL, p=0.0313), as seen in Table 5.
Comparison of propranolol plasma concentrations during the surgery in patients with tetralogy of Fallot, medians, n=6.
| Period | data obtained ng/mL(IC95%) | normalised data ng/mL(IC95%) | Probability (p)Wilcoxon test a |
|---|---|---|---|
| Beginning of surgery | 7.09(1.76-15.80) | 7.09(1.76-15.80) | p=1.0000 |
| End of surgery | 2.70(1.22-4.68) | 2.94(1.27-5.66) | p=0.1250 |
| Beginning of CPB | 2.69(0.44-8.56) | 2.87(0.58-8.60) | p=0.5000 |
| Intra - CPB | 1.14(0.24-4.29) | 2.06(0.49-8.91) | p=0.0313 |
| End of CPB | 2.79(1.45-8.52) | 3.37(1.31-11.57) | p=0.0313 |
| PO 1st day | 0.63(0.00-3.88) | 2.28(0.51-4.21) | p=0.4375 |
| PO 2nd day | 0.05(0.00-2.31) | 0.05(0.00-1.92) | p=0.9999 |
CPB: cardiopulmonary bypass; PO: postoperative period.
On the other hand, by comparison between periods, normalized data showed a significant decrease on drug concentrations (p<0,05) from the beginning of surgery (7.09 ng/mL) to the postoperative day 2 (0.05 ng/mL) and also, a significant reduction from the end of CPB (2.79ng/mL) to the postoperative day 2 (0.05ng/mL, p<0.05).
Finally, by applying a noncompartmental analysis PK solutions 2.0 software, the mean residence time estimated for propranolol during the period of study was 35.8 h.
DISCUSSIONValidation of the analytical methodBefore the quantification of drug by HPLC-F, with respect to the clean-up of the biological sample, Heeden (1991) described a selective but expensive procedure consisting of solid extraction of propranolol from plasma using Bond Elut columns using two extraction steps. The minimum detectable concentration was 1.0 ng in 1mL plasma. In the present study, simple liquid-liquid extraction with dichloromethane, proposed to quantify propranolol plasma concentrations, presents an absolute recovery comparable to those reported previously with a relative low cost method 11, 13, 18. A detection limit of 20 ng/mL using 0.4 mL plasma was described by Lo and Riegelman (1980), whereas Drummer et al (1981), Harrison et al (1985) and Pereira et al (2000) reported for assays employing higher volumes of plasma, detection limits of 2, 1 and 0.5 ng/mL, respectively. In the present method, 0.02 ng/mL was determined by our procedure, once the detection limit was increased by 20 times compared to Pereira et al, 2000.
As regards chromatographic systems previously described, ion-pair liquid chromatography requires modifying reagents added to the mobile phase, such as alkylamines (triethylamine) and sulfonic acids, in addition to a high flow rate for elution of the analyte. On the other hand, reverse-phase liquid chromatography using an isocratic elution system has been preferred over those previously described. Consequently, in the present study the binary system using reverse-phase chromatography was found to be the best alternative among the most complex systems reported in the literature 14–16, 19. Despite the good selectivity obtained with complex systems, an evident limitation of the reported methods is the high flow rate (1.5-2.0 mL/min) required to elute the drug 11–13, 20, 21.
Some modifications introduced in the present method provided a marked increase in the sensitivity, thus requiring validation of the new procedure compared to the procedure described by Pereira and co-workers (2000) once a highly sensitive procedure able to determine picogram amounts of the drug in the plasma is necessary to investigate the washout of propranolol. The main adaptations responsible for the 20-fold increase in the sensitivity of the method were the use of a mobile phase consisting of a different proportion of acetate buffer, pH 5.0, and acetonitrile (60:40, v/v, instead of 65:35), a reduction in the ionic strength of the medium using 0.1 M instead of 0.4 M acetate buffer, pH 5.0, as proposed by Pereira et al (2000) and, finally, replacement of the analytical Nova Pak C18 column (150 x 3.9 mm, 4 µm; Waters, Milford, USA) with a column of higher capacity and selectivity (Shimpack ODS, 150 x 6 mm, 5 micron; Shimadzu), this modification leading to a marked increase in sensitivity with an additional gain of selectivity and capacity. The method validated was superior to previously reported procedures, because 0.05 ng/mL could be quantified by HPLC-F in 0.2 mL of plasma. Thus, the binary reverse-phase chromatography system described in the present study seems to be the best choice in view of its selectivity, marked sensitivity, and operation at low flow.
Analysis of the robustness of the method demonstrated its reproducibility. In addition, propranolol was found to be stable under the conditions analyzed, considering its short-term stability (freezing/thawing cycle), stability over time and under the storage condition used, and long-term stability. It should be emphasized that no stability or robustness studies were performed earlier.
Quantification of propranolol in plasma of patients submitted to surgical correction of tetralogy of FallotIn outpatients with tetralogy of Fallot chronically treated with propranolol, the effective plasma levels of the drug should range from 40 to 90 ng/mL 22. A reduction in the propranolol dose before heart surgery is necessary to prevent complications during the intervention including a low cardiac output in the postoperative period. On the other hand, administration of propranolol before surgery prevents episodes of hypoxemia during the induction of anesthesia. However, the recommended period for discontinuation of the drug prior to the surgery is still controversial. Barazzone and co-workers (1988) proposed an interval of 6 to 8 h after the last propranolol dose, whereas other investigators recommend a longer period ranging from 12-18 h up to 24 h (maximum) before the beginning of the surgical procedure 6, 17.
For interpretation of the results (Table 5), plasma propranolol concentrations were normalized to measure the effect of hemodilution caused by the CPB during the surgery. As can be seen, the obtained and normalized data declined differently during CPB, p<0.05. Due to re-heating of the patients at the end of CPB, obtained and normalized plasma propranolol concentrations again increased differently, p<0.05. According to Barazzone et al (1988), this increase is mainly due to redistribution of the drug from the lungs to the plasma, a reduction in hepatic metabolism, and a decrease in the volume of distribution as a result of hypothermia. In addition, a reduction in plasma proteins increases the free fraction of the drug by 6.6 to 13.5%, because 90% of propranolol is bound to plasma protein, especially alpha 1-acid glycoprotein. On the other hand, when CPB is installed and hemodilution occurs, the plasma propranolol concentration decreases by 50%. In addition, fatsoluble drugs such as propranolol are easily sequestered by the circulator, a fact that contributes to a reduction of plasma propranolol levels during CPB 6.
In the present study, surgery was started about 15h after administration of the last preoperative propranolol dose. Barazzone and co-workers (1988), investigating patients with tetralogy of Fallot who received propranolol, PO, every 8 h before surgery, reported a median plasma concentration of 18.7 ng/mL (95% CI, 3.4-61.5) at the beginning of CPB, whereas in the present study a median plasma concentration of 2.87 ng/mL (0.49-8.91) was observed. This difference might be explained by the different time intervals between the last preoperative dose administered and the beginning of CPB, which was approximately 15 h in the present study versus 8 h in the investigation of Barazzone et al (1988). In addition, plasma propranolol concentration was 2.28 ng/mL (0.51-4.21) 24 h after the beginning of surgery, i.e., during the late postoperative period, compared to 1.0-5.6 ng/mL reported by Barazzone et al (1988). In fact, concentrations found were lower than the recommended to maintain efficacy (40-90 ng/mL); our data suggests that the washout of propranolol was completed by the second postoperative day as demonstrated by the mean residence time of drug.
CONCLUSIONThe validated analytical method showed good linearity, sensitivity, accuracy and precision for the determination of propranolol in plasma at low concentrations. Robustness and stability studies were acceptable.
The effect of hemodilution on propranolol plasma concentrations during CPB procedure was verified. Drug monitoring after the last preoperative dose revealed a decline of plasma concentrations, data normalized, from the beginning of surgery up to the second postoperative day, suggesting that, once redistribution was restored at the end of surgery, propranolol washout was complete.
To Brazilian Foundation for Research CAPES, CNPq and FAPESP for financial support.