Mass spectrometry Matrix-Assisted Laser Desorption-Ionisation Time-of-Flight (MALDI-TOF) helps in the rapid identification of microorganisms causing blood stream infection. Rapid and reliable methods are required to decrease the turnaround time for reporting antimicrobial susceptibility results from blood culture isolates.
MethodsAn evaluation was performed on the reliability of a method for antimicrobial susceptibility testing of positive blood culture isolates from briefly incubated solid medium cultures.
ResultsThe agreement between the evaluated and standard methods was 99.3%. The major and minor error rates were 0.4% and 0.3%, respectively, and no very major errors were observed.
ConclusionThe inoculation of briefly incubated solid medium cultures into antimicrobial susceptibility testing panels is an easy and reliable technique, and helps to decrease the turnaround time for reporting antimicrobial susceptibility results of positive blood cultures.
La espectrometría de masas Matrix-Assisted Laser Desorption-Ionisation Time-of-Flight (MALDI-TOF) permite la identificación rápida de los microorganismos causantes de bacteriemia. Se requieren métodos fiables y rápidos que permitan acortar el tiempo necesario hasta disponer de los resultados de sensibilidad a antibióticos de los aislados de hemocultivos.
MétodosSe evalúa la fiabilidad de un método que combina la identificación con MALDI-TOF y el estudio de sensibilidad en paneles de microdilución inoculados a partir de un subcultivo incubado durante solo 4h.
ResultadosLa concordancia de los resultados de sensibilidad a antibióticos de la técnica evaluada frente a la técnica de referencia fue del 99,3%, sin que se observaran errores máximos.
ConclusiónLa inoculación de paneles de microdilución a partir de un subcultivo de solo 4h de incubación es un método fiable y fácil de realizar que permite acortar el tiempo de informe de hemocultivos positivos.
Bloodstream infections are a major cause of morbidity and mortality.1 Haemoculture continues to be the leading technique for aetiological diagnosis of bacteraemia, also allowing for susceptibility tests on isolates.2 The rapid information provided by these results allows for early initiation of appropriate antibiotic treatment that improves the prognosis of septic patients.3,4
Matrix-Assisted Laser Desorption-Ionisation Time-of-Flight (MALDI-TOF) mass spectrometry is a fast and reliable technique for bacterial identification.5,6 Performing MALDI-TOF directly on positive haemocultures considerably reduces the turnaround time for results.7,8 Strategies have been used combining MALDI-TOF identification and inoculation of antibiogram panels directly from the positive haemoculture vial; nevertheless, published protocols include different centrifugation and washing steps that are laborious for implementation in laboratory routines.8–11 Good results have been reported when combining MALDI-TOF identification with inoculation of microdilution panels from subcultures incubated for short periods of time.12,13
With the objective of decreasing reporting times for positive haemocultures, this article evaluates the reliability of the susceptibility results obtained from WIDER system panels (Francisco Soria Melguizo, Madrid, Spain) inoculated from a subculture after 4h incubation.
MethodsFor a period of 3 months (January–March 2015) 138 consecutive positive haemocultures were studied corresponding to 138 different patients. The haemocultures were processed with the BD BACTEC™ FX system (Becton Dickinson, MD, USA). A Gram stain was performed on the positive vials and they were subcultured in chocolate agar (37°C, 5% CO2).
After 4h of incubation, a swab of growth in chocolate agar was carried out with a wooden spatula and the identification was performed with the MALDI-TOF system (Bruker, MALDI Biotyper 3.0) using the direct transfer technique with on-plate formic acid.6,8,12 A score >1.7 was considered acceptable.
When the identification obtained an acceptable score, the minimum inhibitory concentration (MIC) was determined with the microdilution technique on WIDER MIC/IDGP and MIC/IDGN panels for gram-positive and gram-negative bacteria, respectively. To inoculate the panels, a bacterial suspension was prepared from the 4-h subculture. The Prompt™ Inoculation System Wands were used, following the manufacturer's instructions. To check the purity of the inoculum, 10μl of the bacterial suspension was seeded onto blood agar. The panels were incubated at 37°C, with the reading being carried out after 14–18h of incubation.
Meanwhile, after 24h of incubation, based on the colonies isolated in the chocolate agar medium, the standardised inoculation of the isolates was performed on the WIDER panels. The results obtained with this technique were considered as a reference to evaluate those obtained from the 4-h subculture.
To compare the results, the MICs obtained have been transformed into clinical categories (susceptible, intermediate or resistant) in accordance with the expert WIDER system which applies the criteria of the Clinical and Laboratory Standard Institute (CLSI).14 The results obtained with the technique evaluated compared to the standard technique have been classified as consistent (identical interpretation with both methods), maximum errors (false susceptibility), major errors (false resistance), and minor errors (susceptible/resistant vs intermediate).
The WIDER panels used allow for identification of the isolate; nevertheless, the reliability of MALDI-TOF as an identification technique has been sufficiently evaluated and our objective was solely to evaluate the reliability of the susceptibility results obtained from the subculture which had been incubated for 4h. To this end, discrepancies in the identification between the MALDI-TOF and WIDER systems have not been evaluated. When a discrepancy was found in the identification, the identification obtained with MALDI-TOF was manually entered into the system.
ResultsOf the 138 haemocultures studied, 19 were excluded. In 9 isolates (2 Bacteroides spp., one Streptococcus pneumoniae, 3 coagulase-negative staphylococci, one Escherichia coli and 2 polymicrobial cultures), identification was not achieved with MALDI-TOF (score<1.7) after 4h of incubation. Another 6 isolates (3 S. pneumoniae, one Streptococcus agalactiae, one Streptococcus pyogenes and one Listeria monocytogenes), although correctly identified, were excluded because susceptibility studies using the microdilution technique are not conducted for these pathogens in the laboratory routine. Another 4 cases in which yeasts were observed in the Gram stain were also excluded.
In 119 haemocultures, an acceptable identification was obtained (score>1.7) with MALDI-TOF and a MIC was conducted based on the subculture incubated for 4h: 98 (83%) were gram-negative bacilli (GNB) and 21 (17%) gram-positive cocci (GPC) (Table 1).
Bacteraemia-causing agents identified with MALDI-TOF (score>1.7).
| Aetiology | Microorganisms, n (%) |
|---|---|
| Gram-positive cocci | |
| Staphylococcus aureus | 9 (42.8) |
| Staphylococcus epidermidis | 5 (23.8) |
| Enterococcus faecalis | 5 (23.8) |
| Enterococcus faecium | 2 (9.5) |
| Total | 21 (100) |
| Gram-negative bacilli | |
| Escherichia coli | 51 (52.0) |
| Klebsiella pneumoniae | 14 (14.3) |
| Pseudomonas aeruginosa | 8 (8.1) |
| Enterobacter aerogenes | 6 (6.1) |
| Enterobacter cloacae | 5 (5.1) |
| Klebsiella oxytoca | 4 (4.0) |
| Proteus mirabilis | 3 (3.0) |
| Acinetobacter baumannii | 1 (1.0) |
| Aeromonas veronii | 1 (1.0) |
| Citrobacter freundii | 1 (1.0) |
| Citrobacter koseri | 1 (1.0) |
| Proteus vulgaris | 1 (1.0) |
| Kluyvera ascorbata | 1 (1.0) |
| Serratia marcescens | 1 (1.0) |
| Total | 98 (100) |
Only in 3 cases (one Citrobacter koseri, one Enterobacter cloacae and one Enterobacter sakazakii) were discrepancies in the identification of the species observed between the 2 systems used.
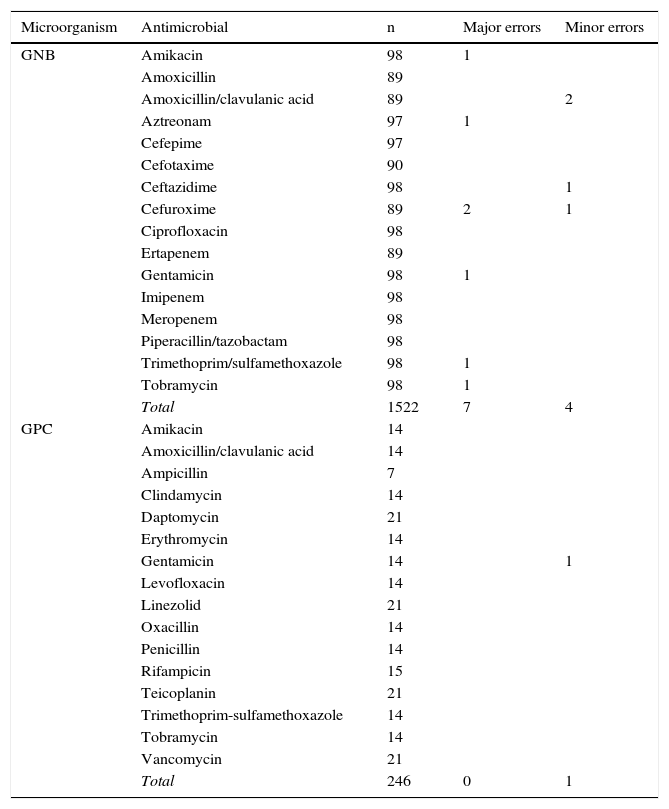
For GNBs, a total of 1522 combinations of antibiotic/microorganism were tested. For GPCs, the total number of studied combinations was 246. The agreement between the susceptibility results obtained with the standard technique and the evaluated technique was 99.3%; the major and minor error rate was 0.4% and 0.3%, respectively, with no maximum errors observed (Table 2). No differences were observed in the error rates between the different included bacterial species. No carbapenemase-producing strains were detected, and between 3 strains of extended-spectrum beta-lactamase-producing E. coli and one strain of methicillin-resistant S. aureus, there was complete agreement with the reference method.
Correlation between the results of the susceptibility study obtained with the standard technique and the evaluated technique.
| Microorganism | Antimicrobial | n | Major errors | Minor errors |
|---|---|---|---|---|
| GNB | Amikacin | 98 | 1 | |
| Amoxicillin | 89 | |||
| Amoxicillin/clavulanic acid | 89 | 2 | ||
| Aztreonam | 97 | 1 | ||
| Cefepime | 97 | |||
| Cefotaxime | 90 | |||
| Ceftazidime | 98 | 1 | ||
| Cefuroxime | 89 | 2 | 1 | |
| Ciprofloxacin | 98 | |||
| Ertapenem | 89 | |||
| Gentamicin | 98 | 1 | ||
| Imipenem | 98 | |||
| Meropenem | 98 | |||
| Piperacillin/tazobactam | 98 | |||
| Trimethoprim/sulfamethoxazole | 98 | 1 | ||
| Tobramycin | 98 | 1 | ||
| Total | 1522 | 7 | 4 | |
| GPC | Amikacin | 14 | ||
| Amoxicillin/clavulanic acid | 14 | |||
| Ampicillin | 7 | |||
| Clindamycin | 14 | |||
| Daptomycin | 21 | |||
| Erythromycin | 14 | |||
| Gentamicin | 14 | 1 | ||
| Levofloxacin | 14 | |||
| Linezolid | 21 | |||
| Oxacillin | 14 | |||
| Penicillin | 14 | |||
| Rifampicin | 15 | |||
| Teicoplanin | 21 | |||
| Trimethoprim-sulfamethoxazole | 14 | |||
| Tobramycin | 14 | |||
| Vancomycin | 21 | |||
| Total | 246 | 0 | 1 |
GNB: gram-negative bacilli; GPC: gram-positive cocci; n: number of combinations of antibiotic/microorganism tested.
The identification and susceptibility report was issued for the 119 cases studied in less than 24h from the detection of the positive haemoculture.
DiscussionThe direct identification technique with MALDI-TOF based on positive haemocultures has been shown to be effective and reliable; however, the pre-processing of the sample is quite labour intensive. Even to the detriment of speed, in order to better organise work flow, some authors recommend grouping positive haemocultures and performing the identification in blocks twice a day.15 Thus, identification from a 4-h subculture could be obtained in a similar amount of time with the advantage of not needing to pre-process the sample. In our series, an acceptable identification was not obtained in just 9 (6%) of the 138 haemocultures initially included in the study. In the series by Hoyos-Mallecot et al.8 conducting direct identification from the haemoculture, with a score of 1.7, 4% of GNB and 26% of gram-positive bacteria were not identified. It is possible that the sample obtained from a subculture contains a larger number of proteins that enables more reliable profiles to be obtained, increasing the sensitivity of the technique.
Strategies that combine MALDI-TOF identification and MIC panel inoculation directly from the haemoculture vial can accelerate the times to issuance of reports, and in general have shown good results; nevertheless, most published methods require samples to be pre-treated. Machen et al.,10 conducting a lysis filtration pre-processing, obtained a global error rate of 5.5% with 1.3% of maximum errors with the Vitek-2 system. After first centrifuging the sample, Wimmer et al.11 found an error rate close to 2% with 0.26% of maximum errors using the Phoenix system.
Previous studies have shown that the identification obtained with MALDI-TOF from a swab of a subculture, which has been incubated for very few hours, is consistent with that obtained using the recommended identification technique based on 18–24h subcultures.11,12 In a series of 104 haemoculture isolates, Idelevich et al.12 evaluated inoculation of MIC panels using the Vitek system based on growth in 2.5–7-h subcultures, finding that the mean incubation time for GPC was 3.8h and for GNB was 2.4h. Based on these results, we set the incubation time at 4h, and, using WIDER system MIC panels for the first time, we succeeded in identifying 93.5% of the isolates studied. For the 119 isolates that we subjected to MIC after 4h of incubation, we obtained 99.3% consistency with the reference technique, with no observed maximum errors. Also, the method that we evaluated has the advantage of not requiring any sample pre-processing. Therefore, it entails no additional costs and can be easily adapted to the laboratory work flow.
The limitations of the technique include anaerobic bacteraemias in which insufficient growth is achieved after 4h of incubation, and polymicrobial bacteraemias. Another limitation of the study would be the low number of gram-positives included in this series.
In conclusion, inoculation of MIC panels with a subculture which has been incubated for 4h, after identification via MALDI-TOF, is a reliable method compared to the conventional inoculation system after 24h of incubation, and can shorten the reporting time for positive haemocultures.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Ballestero-Téllez M, Recacha E, de Cueto M, Pascual Á. Identificación y determinación de sensibilidad a antibióticos de aislados de hemocultivos a partir de subcultivos de corta incubación. Enferm Infecc Microbiol Clin. 2017;35:582–585.