Modern antiviral drugs for the treatment of chronic hepatitis C achieve high cure rates; however, treatment of patients with chronic kidney disease (CKD) is still challenging due to the lack of efficacy and safety data in this patient population.1
Sofosbuvir is a pan-genotypic inhibitor of the HCV NS5B RNA-dependent RNA polymerase, which is essential for HCV replication. It is a nucleotide prodrug, whose main (>90%) metabolite is GS-331007.2 Relative to patients with normal renal function, the area under the curve for sofosbuvir and GS-331007, which is eliminated through the kidney, is 171% and 451% higher, respectively in patients with severe CKD.1,2
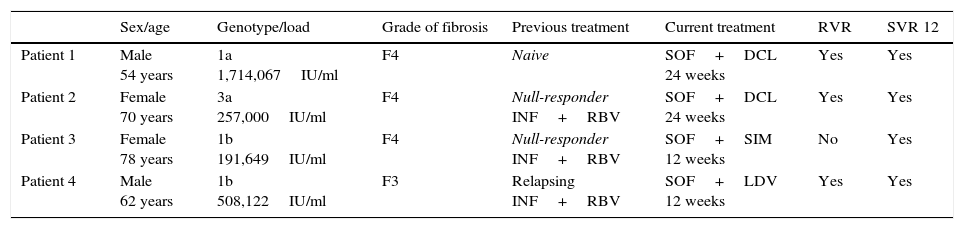
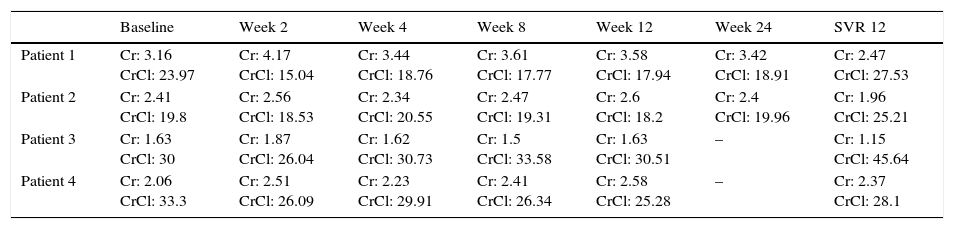
We present data from 4 patients with severe CKD and hepatitis C who were treated in our department with different antiviral regimens containing sofosbuvir (Tables 1 and 2). The first 2 were treated for severe CKD with creatinine clearance (CrCl) of less than 30ml/min. One of them was in a peritoneal haemodialysis program and on a hepatorenal transplant waiting list, the other presented CKD associated with cryoglobulinaemia. These 2 patients had presented hydropic decompensation and were on diuretic therapy. They were treated off label with sofosbuvir+daclatasvir for 24 weeks, with resolution of ascites and clinical improvement. The third patient presented CKD with CrCl 30ml/min and received 12 weeks of treatment with simeprevir+sofosbuvir. Creatinine was elevated during treatment but had improved considerably by the end. The last patient presented CKD due to diabetic nephropathy with CrCl 33ml/min, and received 12 weeks of treatment with sofosbuvir+ledipasvir. Creatinine was elevated during treatment but had almost returned to baseline levels by the end. Treatment was well tolerated by all patients, with no significant adverse effects. None of the patients received ribavirin, and all 4 achieved a sustained viral response at week 12 and 24 post treatment.
Patient characteristics.
| Sex/age | Genotype/load | Grade of fibrosis | Previous treatment | Current treatment | RVR | SVR 12 | |
|---|---|---|---|---|---|---|---|
| Patient 1 | Male 54 years | 1a 1,714,067IU/ml | F4 | Naive | SOF+DCL 24 weeks | Yes | Yes |
| Patient 2 | Female 70 years | 3a 257,000IU/ml | F4 | Null-responder INF+RBV | SOF+DCL 24 weeks | Yes | Yes |
| Patient 3 | Female 78 years | 1b 191,649IU/ml | F4 | Null-responder INF+RBV | SOF+SIM 12 weeks | No | Yes |
| Patient 4 | Male 62 years | 1b 508,122IU/ml | F3 | Relapsing INF+RBV | SOF+LDV 12 weeks | Yes | Yes |
DCL: daclatasvir 60mg/day; INF: interferon; LDV: ledipasvir 90mg/day; RBV: ribavirin; RVR: rapid viral response at week 4 of treatment; SIM: simeprevir 150mg/day; SOF: sofosbuvir 400mg/day; SVR 12: sustained viral response at week 12 after treatment.
Creatinine and creatinine clearance during therapy.
| Baseline | Week 2 | Week 4 | Week 8 | Week 12 | Week 24 | SVR 12 | |
|---|---|---|---|---|---|---|---|
| Patient 1 | Cr: 3.16 CrCl: 23.97 | Cr: 4.17 CrCl: 15.04 | Cr: 3.44 CrCl: 18.76 | Cr: 3.61 CrCl: 17.77 | Cr: 3.58 CrCl: 17.94 | Cr: 3.42 CrCl: 18.91 | Cr: 2.47 CrCl: 27.53 |
| Patient 2 | Cr: 2.41 CrCl: 19.8 | Cr: 2.56 CrCl: 18.53 | Cr: 2.34 CrCl: 20.55 | Cr: 2.47 CrCl: 19.31 | Cr: 2.6 CrCl: 18.2 | Cr: 2.4 CrCl: 19.96 | Cr: 1.96 CrCl: 25.21 |
| Patient 3 | Cr: 1.63 CrCl: 30 | Cr: 1.87 CrCl: 26.04 | Cr: 1.62 CrCl: 30.73 | Cr: 1.5 CrCl: 33.58 | Cr: 1.63 CrCl: 30.51 | – | Cr: 1.15 CrCl: 45.64 |
| Patient 4 | Cr: 2.06 CrCl: 33.3 | Cr: 2.51 CrCl: 26.09 | Cr: 2.23 CrCl: 29.91 | Cr: 2.41 CrCl: 26.34 | Cr: 2.58 CrCl: 25.28 | – | Cr: 2.37 CrCl: 28.1 |
Cr: creatinine (mg/dl); CrCl: creatinine clearance (ml/min).
New oral antiviral drugs against hepatitis C achieve a high cure rate and have few side effects. Nevertheless, the most appropriate treatment has yet to be evaluated in some patient populations, such as those with severe CKD and CrCl <30ml/min. In these patients, current guidelines3,4 still recommend pegylated interferon with low-dose ribavirin in genotype 2, 3, 5 or 6. Triple therapy with dasabuvir+paritaprevir/ombitasvir/ritonavir is recommended in genotype 1 and 4. However, a high interaction rate coupled with the polypharmacy typical of patients with end-stage renal disease often make this impractical. Other therapies recommended in these patients are simeprevir and daclatasvir. These can be administered in combination in select patient groups, although this is not described in current guidelines. In the near future, the combination of grazoprevir/elbasvir may be the regimen of choice in these patients, since it has shown high rates of efficacy in patients with CKD.1,5
Sofosbuvir is a pan-genotypic NS5B polymerase inhibitor in which the primary metabolite GS-331007 is eliminated through the kidneys.1,2 Serum levels of GS-331007 increase significantly in patients with CKD. Although there is so far no evidence of toxicity associated with this metabolite, sofosbuvir is not recommended in patients with CrCl below 30ml/min.1–4
Few case series of patients with severe CKD treated with sofosbuvir have been published. A series of patients with severe CKD treated with 200mg of sofosbuvir plus ribavirin daily was presented at the AASLD-2014 conference. Although the therapy was well tolerated, sustained response was poor. This dosage is considered suboptimal and is not recommended.6 The following year, in the AASLD-2015 conference, small series of CKD patients with CrCl <30ml/min treated with combinations of antiviral drugs, including sofosbuvir 400mg/day were presented. No cases of impaired kidney function were reported, the drug was associated with high cure rates and was well tolerated by all patients.7–9
We also found the therapy to be well tolerated, with no adverse effects and rapid patient-reported improvement in all cases. Although creatinine levels were slightly elevated during treatment, no dosage changes were required, and post-treatment creatinine levels improved over baseline levels in 3 out of 4 patients.
We present efficacy and safety findings in a small series of patients with CKD treated with sofosbuvir. Although prospective studies are no doubt needed, we believe that the benefit of this therapy should be evaluated on a patient-by-patient basis.
Please cite this article as: Rodríguez Gil FJ, Pérez Garrido I. Tratamiento antiviral con sofosbuvir en pacientes con hepatitis C e insuficiencia renal severa. Gastroenterol Hepatol. 2017;40:85–86.