Technology Enhanced Medical Education International Conference (THEME 2019)
Más datosThis study aims to determine the relationship between the severity of peripheral diabetic neuropathy and sleep quality in patients with type 2 diabetes.
MethodsThis study is an observational analytic cross-sectional design. Data collected consisted of age, gender, HbA1c level, duration of DM, Diabetic Neuropathy Symptom (DNS) score, the severity of diabetic neuropathy, and sleep quality. Diabetic Neuropathy Symptom (DNS) score is a diabetic neuropathy screening consisting of scores from 1 to 4, and a score of ≥1 is considered significant. The severity of diabetic neuropathy was measured using an England score consisting of mild, moderate, and severe. While sleep quality is measured by the Pittsburgh Sleep Quality Index (PSQI), which consists of 7 components, namely subjective sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbance, use of sleeping drugs and dysfunction of daily activities. Each component has a value of 0–3. The score of the seven components added to the value between 0 and 21. The number of samples that meet the research criteria is 18 samples. Sampling by consecutive sampling method for three months at the neurologic clinic of Wahidin Sudirohusodo Hospital Makassar. The data obtained were recorded and grouped according to the purpose and type of data, then the statistical method was chosen following the significance limit p<0.05, namely by using the Spearman correlation test.
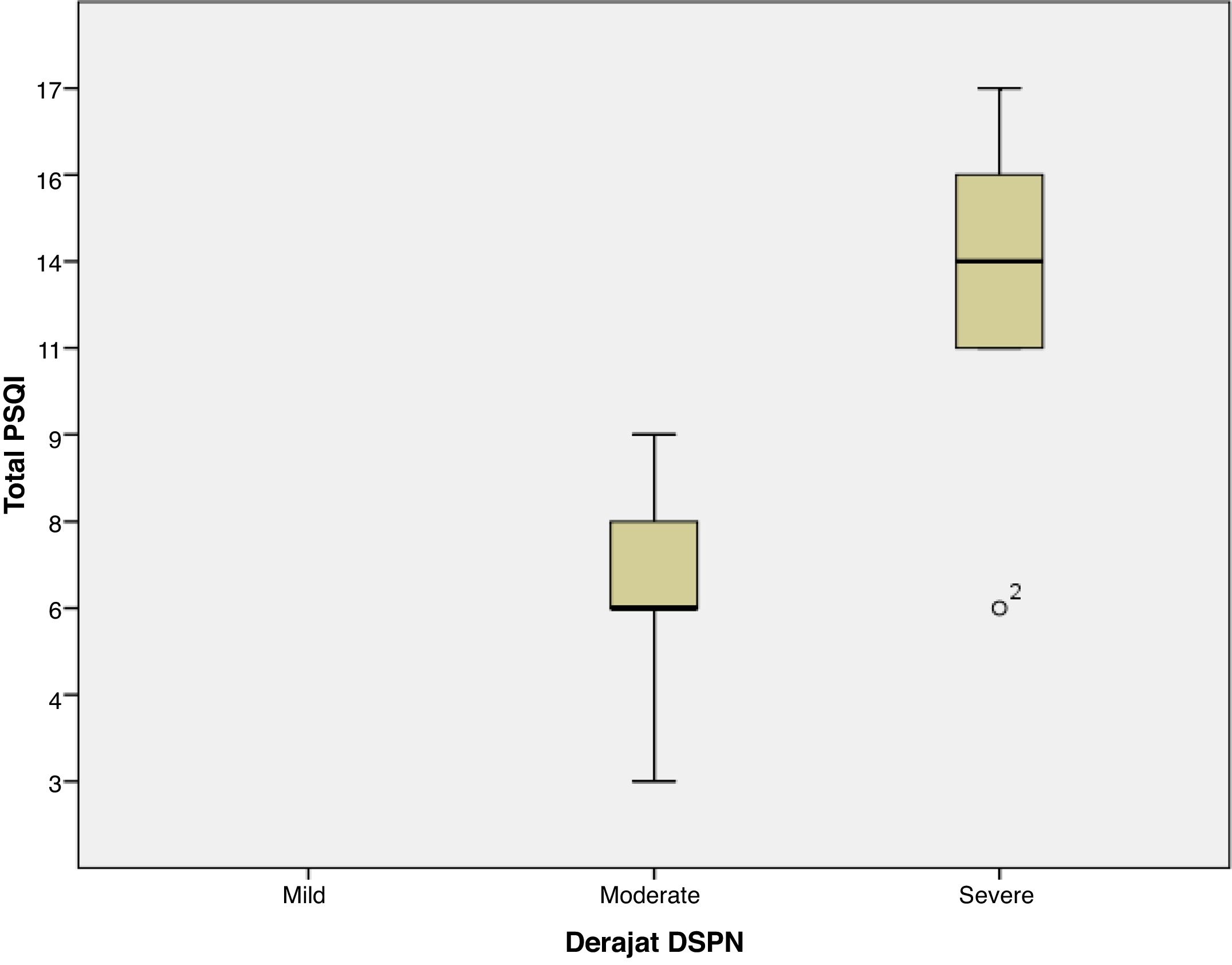
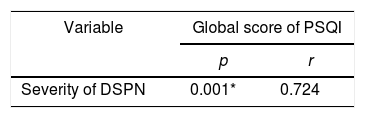
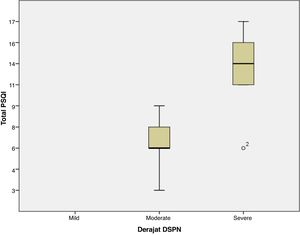
ResultThe majority of respondents had moderate diabetic neuropathy, 11 patients, and seven patients with severe neuropathy. The lowest PSQI score is three, and the highest is 17, with the global average PSQI score obtained 8.72±4.22. The results showed that there was a significant relationship between the severity of peripheral diabetic neuropathy with sleep quality (global PSQI score) with a value of p=0.001. The relationship between the two variables is unidirectional and has a strong correlation with the positive coefficient correlation, r=0.724.
ConclusionThere is a significant relationship between the severity of peripheral diabetic neuropathy and sleep quality (global PSQI score). The more severe the degree of peripheral diabetic neuropathy, the worse the quality of sleep.
Diabetes Mellitus (DM) is one of the most common causes of peripheral neuropathy in the world. More than half of diabetic patients experience neuropathy, and half of those who have neuropathy are diabetic patients.1,2 Diabetes mellitus is a group of metabolic diseases characterized by hyperglycemia that occurs due to abnormal insulin secretion, insulin action, or both. Diabetes mellitus has become one of the biggest global health problems in the 21st century.3 The World Health Organization (WHO) predicts an increase in the number of people with DM in Indonesia from 8.4 million in 2000 to around 21.3 million in 2030.4
Diabetic peripheral neuropathy (DPN) is one of the most common DM complications in 20–60% of patients with type 2 DM.5 The most common diabetic neuropathy is Distal Symmetrical Polyneuropathy (DSPN), which accounts for around 75% of diabetic neuropathy. The clinical definition of DSPN is the presence of symptoms and or signs of peripheral nerve dysfunction in people with diabetes after other causes have been excluded. Estimates of the incidence and prevalence of DSPN vary widely, but evidence from cohort studies and The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) shows that DSPN occurs at least 20% in type 1 DM patients after a disease duration of 20 years and about 10–15% of newly diagnosed type 2 DM patients, with a prevalence that rises to 50% after the duration of the disease ten years.6
The most common symptoms of DSPN include burning pain, a sensation of being pricked and electrocuted, paresthesias, hyperesthesia, and deep pain. These symptoms are generally substantial at night and disturb sleep.7 Type 2 DM patients with complications of peripheral neuropathy increase the risk of sleep disorders, especially obstructive sleep apnea, decreased sleep efficiency, sleep fragmentation, and are most often nocturnal hypoxia. This sleep abnormality significantly interferes with the patient's diurnal activity, which causes excessive daytime sleepiness, decreased productivity, cognitive impairment, mood disorders, high accident rates, increased insulin resistance, and poor glycemic control. Thus, early diagnosis and management of sleep disorders in DPN patients are crucial for patient management holistically, increasing productivity, reducing rates of diabetes complications, and improving quality of life.5
The diagnosis of DSPN is generally made based on neurological signs and symptoms and electrophysiological measurements. Many screening instruments can be used to evaluate neuropathy. The instruments most frequently used in the literature are the Michigan Neuropathy Screening Instrument questionnaire (MNSIq) and physical assessment and the Diabetic Neuropathy Symptom (DNS) score. The gold standard for detecting DSPN is a nerve conduction velocity test.8 Sleep disturbance can occur due to reduced quality, a quantity of sleep, or disruption of the circadian rhythm. The Pittsburgh Sleep Quality Index (PSQI) is a useful instrument used to measure sleep quality and patterns in adults. PSQI can distinguish “good” and “bad” sleep quality by measuring seven components: subjective sleep quality, sleep latency, sleep duration, the efficiency of sleep habits, sleep disturbance, use of sleeping pills, and daytime dysfunction during the past month.9 Therefore, the research problem is to analyze a relationship between the severity of peripheral diabetic neuropathy and the quality of sleep in patients with type-2 diabetes.
MethodResearch locationThis research was conducted at the Brain Center clinic of Wahidin Sudirohusodo Hospital (RSWS) Makassar during the period of April 2019 to June 2019. The study population was all patients with type II DM who seek treatment at the Brain Center clinic at RSWS Makassar. This study uses DNS (Diabetic Neuropathy Symptoms) scores as screening before the Nerve Conduction Study. The research sample is all affordable populations that meet the research criteria (inclusion and exclusion criteria). Sampling is done by consecutive sampling method where all samples that come in sequence and meet the criteria are included in the study until the required number of samples is fulfilled, namely 18 samples.
Type and sources of dataData measured from the sample are data related to demographics, patient age, sex, HbA1C levels, duration of DM, DNS scores, and assessment of the severity of neuropathy based on England scores determined from the results of nerve conduction studies which classify the severity of mild, moderate and severe diabetic neuropathy. Sleep quality assessment based on PSQI can assess good and bad sleep quality with a global PSQI score between 0 and 21, where the score <5 sleep quality is reduced, and the score ≥5 is good sleep quality.
Data collection techniquesThe data was collected from anamnesis and the medical record. Assessment of HbA1C levels was measured from laboratory results in the last three months. Diabetic neuropathy screening was done by using DNS scores through data collection sheets. After that, the nerve conduction study was examined in the Clinical Neurophysiology Laboratory of Wahidin Sudirohusodo Hospital Makassar, and then an assessment of the severity of neuropathy was performed using an England score. Assessment of sleep quality of samples using the Pittsburgh Sleep Quality Index (PSQI) questionnaire.
ResultsThis study analyzes the relationship between the severity of peripheral diabetic neuropathy and the quality of sleep in patients with type 2 diabetes at Wahidin Sudirohusodo hospital Makassar. The number of samples that met the inclusion criteria as many as 18 samples where the primary data characteristics of the study sample showed the distribution of the sample age with the age group ≤50 years had a total of 7 people, the age group of 51–60 years amounted to 5 people, the age group of 61–70 years amounted to 4 people and those aged >70 years are two people. Female sex is ten samples (55.6%) more than males, which totaled eight samples (44.4%). DM duration ≤10 years and >10 years are the same, every 9 samples (50%). HbA1C level ≥7 was 18 samples (100%). Based on PSQI, which has good sleep quality of 4 people (22.2%) and poor sleep quality of 14 people (77.8%). Research sample with moderate DSPN 11 samples (61.1%) and severe seven samples (38.9%). Global PSQI scores ranged from 3 to 17 samples, with an average value of 8.72±4.226. In this study, it was found that the severity of peripheral diabetic neuropathy had a statistically significant correlation with sleep quality (global PSQI score) with a p-value of 0.001. The correlation between the two variables has a robust and positive linear relationship, which means that the more severe the severity of peripheral diabetic neuropathy, the worse the quality of sleep (correlation coefficient, r=0.724). When the degree of peripheral diabetic neuropathy was associated with the PSQI component, a significant relationship was found between the degree of peripheral diabetic neuropathy with subjective sleep quality (p=0.006), sleep latency (p=0.010), sleep duration (p=0.036) and sleep efficiency (p=0.003) (Table 1 and Fig. 1).
Analysis of the relationship between severity of diabetic neuropathy with sleep quality.
| Variable | Global score of PSQI | |
|---|---|---|
| p | r | |
| Severity of DSPN | 0.001* | 0.724 |
Patients with type 2 diabetes mellitus with complications of peripheral neuropathy are at high risk of experiencing sleep disorders, especially obstructive sleep apnea, decreased sleep efficiency, sleep fragmentation, and frequent nocturnal hypoxia.5 Peripheral diabetic neuropathy develops slowly, progressively and symmetrically, mainly presents sensory and autonomic symptoms with the involvement of small nerve fibers, then involves large sensory nerve fibers and finally involves motor nerve fibers at a later stage.10
Most patients have positive sensory symptoms (excessive response to stimulation or spontaneous), such as paresthesias and pain. Symptoms can be numbness, tingling, imbalance and falling, pricking, and burning sensation. Symptoms that occur usually get worse at night. In general, these symptoms are mild but can be severe. Negative sensory symptoms (decreased response to certain stimuli) are symptoms of loss of sensitivity in the segment involved. The presence of severe neuropathic pain, in the form of hyperesthesia (excessive response to tactile stimuli), hyperalgesia (excessive sensitivity to painful stimuli), hyperpathia (persistent pain even after the pain stimulus is removed) or even allodynia (painful sensations caused by painless stimuli). It can develop into hypo/deep anesthetic sensitivity such as tactile, vibration, and proprioceptive. Besides, when there is sensory impairment of large nerve fibers, deep hypo or no reflexes are found, especially in the Achilles reflex, and may be generalized no reflexes in very severe cases.10
In this study, it was found that the severity of peripheral diabetic neuropathy had a statistically significant relationship with sleep quality (global PSQI score) with a p-value of 0.001. The degree of peripheral diabetic neuropathy also had a significant relationship with subjective sleep quality (p=0.006), sleep latency (p=0.010), sleep duration (p=0.036) and sleep efficiency (p=0.003). Symptoms of diabetic neuropathy that are worse at night cause sleep disturbances that can worsen the sleep quality of sufferers. This finding is different from studies conducted by Meng et al. who did not find an association between diabetic neuropathy with sleep complaints. It is because DPN patients may not realize the existence of sleep breathing disorder, which can cause microarousal so that it is not enough to wake the patient. This microarousal may not affect total sleep time, but it disrupts sleep quality with diurnal consequences in the form of EDS, cognitive disorders, and mood disorders. However, other studies conducted by Ceron et al. (2015) and Reutrakul and Mokhlesi (2017) found that there is a two-way relationship between DPN and sleep architecture. Decreased sleep efficiency and increased sleep fragmentation in addition to sleep apnea syndrome, it causes activation of the hypothalamic-pituitary-adrenal axis, which leads to an increase in excessive sympathetic activity, oxidative stress, and an increase in systemic inflammation that can result in metabolic dysfunction and increased insulin resistance.5
ConclusionThere is a relationship between the severity of peripheral diabetic neuropathy with sleep quality (global PSQI score), which is statistically significant. The more severe the degree of peripheral diabetic neuropathy, the worse the quality of sleep.
Conflict of interestThe authors declare no conflict of interest.
The researcher would like to thank all those who have helped in this research process. Our grateful, especially to all the patients who have agreed to participate as respondents in this study.
Peer-review under responsibility of the scientific committee of the Technology Enhanced Medical Education International Conference (THEME 2019). Full-text and the content of it is under responsibility of authors of the article.