The use of nutritional supplements to treat hypercholesterolemia is gradually increasing, however further studies on their efficacy and safety are required.
Patients and methodsThe present clinical trial included patients with moderate hypercholesterolemia and cardiovascular risk who were treated either with a nutraceutical preparation containing 3.75mg of monacolin K, 515mg of berberine and 50mg of coenzyme Q10 per tablet (Lipok®) or with a placebo. The clinical and laboratory variables were analyzed at baseline and at three and six months. None of the patients was diabetic, and none was being treated with lipid-lowering drugs or with any other nutritional supplements affecting lipid metabolism.
ResultsIn patients of the intervention group and of the placebo group, baseline LDL-C was 134.7mg/dL (14.4) and 138.7mg/dL (15.2), respectively. At three months after treatment start, LDL-C had decreased by 26.1mg/dL (−32.4 to 19.7) and increased by 4.5mg/dL (−1.5 to 10.5) in the respective groups. In the intervention group, a similar decrease in non-HDL-C and total cholesterol was observed, while no significant changes were observed in either group for HDL-C, triglycerides and lipoprotein(a). A good tolerance and safety profile was observed.
ConclusionIn conclusion, this study demonstrates that the combination of monacolin K, berberine and coenzyme Q10 is effective and safe for treating hypercholesterolemia in patients with a moderate degree of excess LDL-C and cardiovascular risk.
El uso de suplementos nutricionales para tratar la hipercolesterolemia está aumentando de forma progresiva; sin embargo son necesarios más estudios sobre su eficacia y seguridad.
Pacientes y métodosEn el presente ensayo clínico fueron incluidos pacientes con hipercolesterolemia y riesgo cardiovascular moderados que fueron tratados con un preparado nutracéutico que contenía 3,75mg de monacolina K, 515mg de berberina y 50mg de coenzima Q10 por comprimido (Lipok®) o con placebo. Se analizaron las variables clínicas y de laboratorio en situación basal y a los 3 y 6 meses. Ningún paciente era diabético y ninguno seguía tratamiento con fármacos hipolipidemiantes u otros suplementos nutricionales con efectos sobre el metabolismo lipídico.
ResultadosEn los pacientes del grupo de intervención y del grupo placebo, el c-LDL basal era de 134,7mg/dL (14,4) y 138,7mg/dL (15,2), respectivamente. A los 3 meses de tratamiento el c-LDL había disminuido 26,1mg/dL (de –32,4 a 19,7) y aumentado 4,5mg/dL (de –1,5 a 10,5) en ambos grupos, respectivamente. En el grupo de intervención se observó un descenso similar del c-no HDL y del colesterol total, mientras que no ocurrieron cambios significativos en ninguno de los 2 grupos en el c-HDL, los triglicéridos y la lipoproteína (a). Se observó un buen perfil de tolerancia y seguridad.
ConclusiónEste estudio demuestra que la combinación de monacolina K, berberina y coenzima Q10 es eficaz y segura para tratar la hipercolesterolemia en los pacientes con un grado de exceso de c-LDL y de riesgo cardiovascular moderados.
Hypercholesterolemia is a major cause of atherothrombotic cardiovascular disease (ACVD)1 and its detection and treatment in the general population is a fundamental preventive measure for controlling the growing epidemic of this disease.2 However, the control of hypercholesterolemia in the various degrees of cardiovascular risk (CVR) is still very poor.3 This is largely due to the inadequate use of lipid-lowering drugs, which is due to various factors among which therapeutic inertia by health professionals and lack of patient treatment adherence are notable.4,5 Intolerance and concern about potential side effects are common causes of non-adherence to lipid-lowering drugs.6 Therefore, in recent years there has been a progressive increase in the use of dietary supplements that affect lipid metabolism, or nutraceuticals,7,8 to treat hypercholesterolemia, particularly in subjects who are intolerant to statins and who, despite maintaining a healthy lifestyle, have a moderate CVR or a moderate degree of hypercholesterolemia. In fact, these products have been included in guidelines for the treatment of dyslipidaemia published by various scientific societies (European guidelines on dyslipidaemias)2,9 in which red yeast rice (RYR) figures prominently. RYR is a nutraceutical obtained by fermenting rice (Oryza sativa) by the action of a yeast, Monascus purpureus,10 during which monacolins are produced, particularly monacolin K, whose structure is very similar to that of lovastatin and, like the latter, its main mechanism of action is decreasing intracellular synthesis of cholesterol by partial and reversible inhibition of the HMG-CoA reductase enzyme. To enhance its hypocholesterolemic action, RYR has been associated to other bioactive compounds, including products to inhibit cholesterol absorption, such as plant sterols, or enhancers for the hepatic uptake of cholesterol such as berberine, through increased LDL receptor activity.11 Berberine is an alkaloid contained in various species of plants of the Berberis genus (Berberis vulgaris, Berberis aquifolium, and Berberis aristata).12 It decreases LDL cholesterol (LDL-C) by increasing the activity of the LDL receptor by various mechanisms, among them the reduction of the protein PCSK9.13 Despite the progressive increase in the use of nutraceuticals to lower plasma cholesterol, the clinical evidence to justify their use is still limited. The present study analyzed the lipid effects of a combination of red yeast rice and berberine with coenzyme Q10 (ubiquinone), a molecule that has been used to prevent muscular symptoms related to statin use14 in a population with moderate hypercholesterolemia and CVR treated at lipids units.
Patients and methodsThis was a prospective, randomized, double-blind placebo-controlled study in patients attending medical consultations at two hospitals to treat hypercholesterolemia. The main objective was to analyze the efficacy in lowering cardiovascular risk through lipid reduction of one tablet per day of a nutraceutical product containing 3.75mg of monacolin K, 515mg of berberine, and 50mg of coenzyme Q10 per tablet (Lipok®) compared with placebo. The primary endpoint was the difference in the change in the concentration of LDL-C between the intervention group and the placebo group, from the baseline visit to the first follow-up visit at three months. The secondary endpoints were the differences in the changes in non-HDL cholesterol concentration (non-HDL-C) and triglycerides between the two groups.
The study was conducted in accordance with the Declaration of Helsinki, and the study protocol was approved by the Ethics Committee of Bellvitge University Hospital. All patients signed an informed consent form at their first screening visit. The trial was registered in clinicaltrials.gov with the identifier: NCT03828006.
PatientsPatients were recruited consecutively by the physician who provided them with medical care as they attended the lipids unit.
Patients included in the study were aged 18 years and over, with a BMI of between 18.5 and 34.9kg/m2, LDL-C plasma concentrations of 100–160mg/dL (according to SEA guidelines 2022),15 and a ten-year CVR of <20% according to the ACVD risk score.16 Patients intolerant to any of the active treatment components were excluded, as were those with a history of previous ACVD, myopathy or hyperlipidaemia secondary to diabetes mellitus, liver, kidney or thyroid disease, pregnant women or women with childbearing potential. Also excluded were subjects following treatment with nutritional supplements with potential lipid-lowering effects, such as plant sterols and lipid-lowering drugs, antifungals, macrolides or menopausal hormone replacement therapy.
Patients were visited on four occasions; at an initial screening visit, a baseline visit and two follow-up visits at three and at six months. All patients were instructed to maintain their usual lifestyle throughout follow-up.
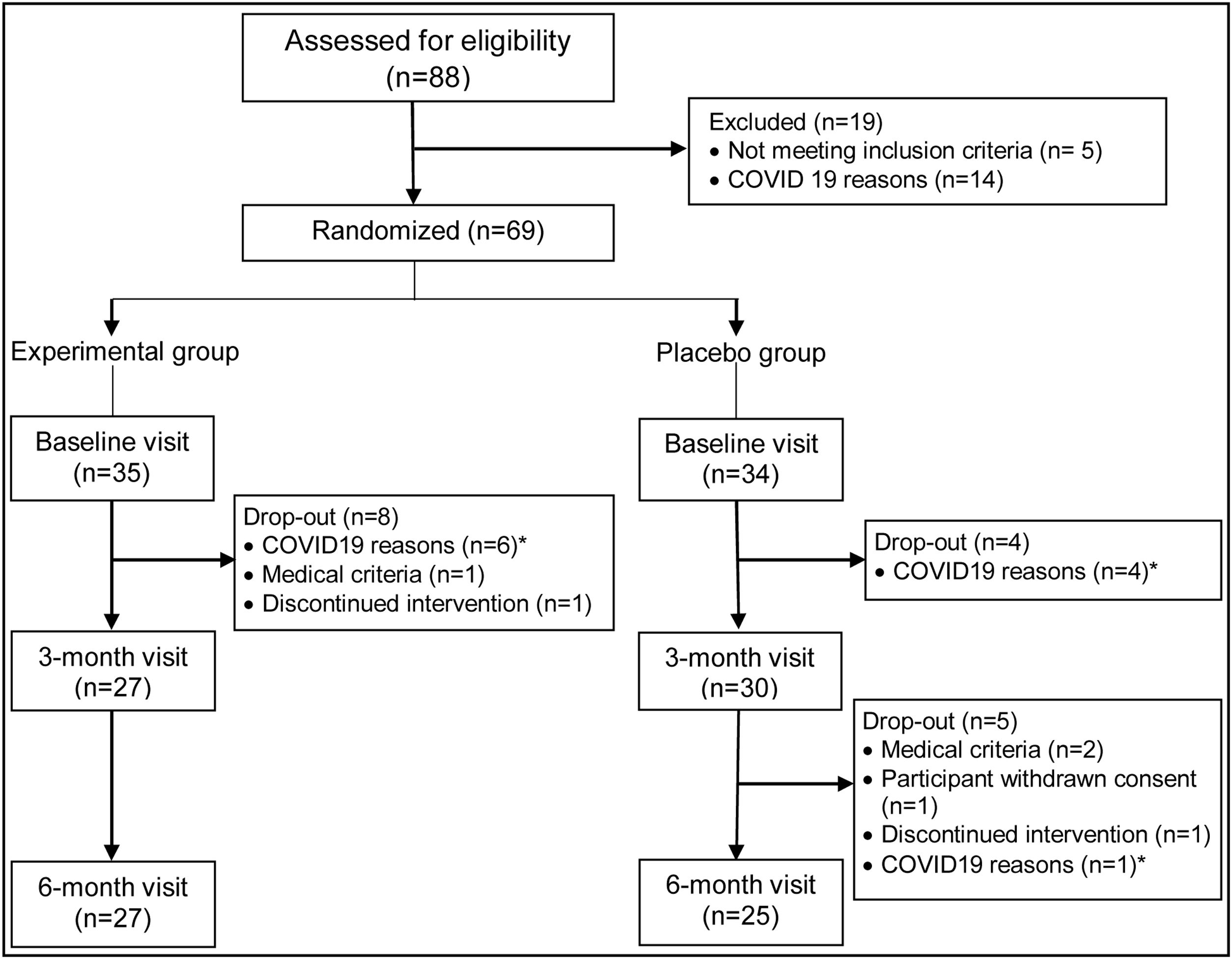
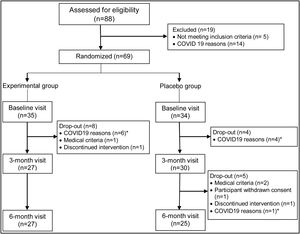
Eighty-eight patients were initially screened, of whom 83 met the inclusion criteria. Sixty-nine patients finally agreed to participate in the trial. Fifty-seven patients attended the three-month visit, and 52 patients attended the final visit at six months. Figure 1 sets out the causes of patient dropouts.
ProceduresAt the screening visit, patients’ clinical history was recorded, blood samples were taken and a physical examination was carried out. After the screening visit, the investigator scheduled a date for the baseline visit within a period not exceeding four weeks. At the baseline visit, the investigator checked if patients met the inclusion/exclusion criteria and verified the results of blood tests. The study treatment was prescribed randomly in accordance with a computer-generated randomization table, independently for each center, with which each patient was assigned to a treatment group as they were recruited for the study. At the follow-up visits, held at three and six months, a physical examination was performed and blood samples taken, and a tablet count was made to assess treatment compliance. Adherence to treatment was considered adequate when patients returned less than 20% of the tablets they should have taken during the follow-up period. Adherence to the Mediterranean diet was assessed by means of the PREDIMED diet adherence questionnaire (available at http://www.predimed.es/uploads/8/0/5/1/8051451/p14_medas.pdf.17 Patients’ physical activity was recorded by means of a questionnaire. The degree of vascular risk was assessed by means of the American College of Cardiology's “ASCVD Risk Estimator Plus” (http://tools.acc.org/ASCVD-Risk-Estimator-Plus/#!/calculate/estimate/).16 Adverse events were monitored throughout the study.
Statistical analysisQualitative variables were analyzed using the chi-square or Fisher's exact tests and are presented as absolute frequencies and percentages. For quantitative variables it was checked whether they fitted a normal distribution using normal Q–Q plot graphs. Variables that did not fit a normal distribution were analyzed using the Mann–Whitney U test and are expressed by the median value and interquartile range. The comparison between treatment groups at the baseline visit were analyzed by analysis of variance (ANOVA) and the data are expressed as mean (SD); changes at three months were analyzed by analysis of covariance (ANCOVA), adjusting by basal values as covariates; data are presented as estimated mean (95% confidence interval). A further analysis of the results was done at six months. All tests were performed using the SPSS statistical package for Windows, version 22.0 (IBM-SPSS Inc., Armonk, New York).
ResultsTable 1 shows the baseline characteristics of the intervention and placebo groups. No significant differences were observed between the two. There was a predominance of women, the average BMI was approaching normal weight, and there was a low percentage of patients with high blood pressure or tobacco consumption. The degree of CVR was low-moderate in both groups. As Table 1 shows, the mean total cholesterol and LDL-C values at baseline were moderately high. Patients’ adherence to Mediterranean diet was intermediate and their average weekly hours of physical activity was greater than five hours per week.
Study patients’ baseline characteristics.
| Characteristic | Experimental groupn=27 | Placebo groupn=30 | p value |
|---|---|---|---|
| Women | 21 (77.8%) | 25 (80%) | 0.837 |
| Age (years) | 57.6±10.3 | 53.1±10.6 | 0.105 |
| Weight (kg) | 69.6±10.8 | 66.5±8.9 | 0.235 |
| BMI (kg/m2) | 25.8±3.1 | 25.5±2.4 | 0.681 |
| Waist circumference (cm) | 86.0±10.1 | 86.9±9.1 | 0.745 |
| Treated arterial hypertension | 4 (14.8%) | 6 (20.0%) | 0.734 |
| Current smoking | 4 (14.8%) | 2 (6.7%) | 0.408 |
| Total cholesterol (mg/dL) | 221.3±21.5 | 224.3±22.4 | 0.613 |
| LDL-C (mg/dL) | 134.7±14.4 | 138.8±15.2 | 0.311 |
| 10-Year risk of incident hard ASCVD | 4.7 [1.4–8.3] | 2.0 [1.3–3.8] | 0.059 |
| PREDIMED diet questionnaire | 9.4±2.1 | 8.4±2.1 | 0.076 |
| Physical activity (h/week) | 5.6±4.8 | 5.5±4.1 | 0.936 |
Normal distribution quantitative endpoint variables are expressed as means and standard deviations and qualitative variables as number and percentage n (%). BMI: body mass index. ACVD: atherothrombotic cardiovascular disease.
Table 2 shows changes in lipid profile, blood pressure and lifestyle habits that occurred between the baseline visit and the three-month follow-up visit. Total cholesterol decreased by an average of 26.7mg/dL in the intervention group and increased by 5.8mg/dL in the placebo group. These differences were statistically significant. LDL-C and non-HDL-C, i.e., cholesterol content in atherogenic lipoproteins, underwent a decrease of a similar magnitude to that observed for LDL-C in both groups, again with significant differences. By contrast, changes in concentrations of HDL-C, triglycerides and lipoprotein(a) were very scarce and, like the variations in systolic and diastolic blood pressure and glycosylated hemoglobin, were not significant. Table 3 shows that the changes in the lipid profile after three months were maintained after six months. No significant changes were observed in the evolution of the remaining clinical and laboratory variables analyzed with regard to observations made at three months of follow-up.
Evolution at three months of follow up.
| Experimental groupn=27 | Placebo groupn=30 | p value | ||
|---|---|---|---|---|
| Total cholesterol (mg/dL) | Baseline | 221.3±21.5 | 224.3±22.4 | 0.613 |
| Change at 3 months | −26.7 (−33.9 to −19.6) | +5.8 (−1.1 to +12.6) | <0.001 | |
| LDL-C (mg/dL) | Baseline | 134.7±14.4 | 138.7±15.2 | 0.311 |
| Change at 3 months | −26.1 (−32.4 to −19.7) | +4.5 (−1.5 to +10.5) | <0.001 | |
| HDL-C (mg/dL) | Baseline | 65.6±14.8 | 66.2±16.9 | 0.885 |
| Change at 3 months | +0.5 (−3.0 to +3.9) | +0.04 (−3.2 to +3.3) | 0.857 | |
| Non-HDL-C (mg/dL) | Baseline | 155.8±18.4 | 158.1±19.4 | 0.641 |
| Change at 3 months | −27.2 (−34.0 to −20.4) | +5.7 (−0.7 to +12.2) | <0.001 | |
| Triglycerides (mg/dL)a | Baseline | 111 [66 to 125] | 84 [67 to 119] | 0.533 |
| Change at 3 months | −7 [−23 to +10] | −2 [−15 to +18] | 0.277 | |
| Lipoprotein (a) (mg/dL)a | Baseline | 19 [10 to 57] | 15 [8 to 51] | 0.472 |
| Change at 3 months | +1 [0 to +19] | 0 [−3 to +3.3] | 0.082 | |
| HbA1c (%) | Baseline | 5.4±0.3 | 5.4±0.4 | 0.865 |
| Change at 3 months | −0.07 (−0.18 to +0.04) | −0.02 (−0.12 to +0.08) | 0.518 | |
| Systolic blood pressure (mmHg) | Baseline | 124.5±15.2 | 123.7±13.0 | 0.834 |
| Change at 3 months | −1.1 (−5.6 to+3.4) | −5.8 (−10.0 to −1.5) | 0.134 | |
| Diastolic blood pressure (mmHg) | Baseline | 75.7±8.3 | 75.2±8.5 | 0.798 |
| Change at 3 months | −0.9 (−4.0 to +2.2) | −0.5 (−3.4 to +2.4) | 0.855 |
Baseline visit analyzed by ANOVA. Data are expressed as means and standard deviations x¯±SD. Changes at three months analyzed by ANCOVA adjusted by basal values; data are expressed as estimated means (95% confidence interval). Bold p values indicate significant values (p<0.05).
Evolution at six months of follow up.
| Experimental groupn=27 | Placebo groupn=25 | p value | ||
|---|---|---|---|---|
| Total cholesterol (mg/dL) | Baseline | 221.3±21.5 | 221.7±23.1 | 0.950 |
| Change at 6 months | −25.7 (−34.2 to −17.2) | −0.4 (−9.3 to +8.4) | <0.001 | |
| LDL-C (mg/dL) | Baseline | 134.7±14.4 | 136.3±15.4 | 0.711 |
| Change at 6 months | −26.8 (−34.9 to −18.7) | −2.1 (−10.5 to +6.2) | <0.001 | |
| HDL-C (mg/dL) | Baseline | 65.6±14.8 | 65.9±17.5 | 0.935 |
| Change at 6 months | +0.2 (−3.8 to+4.1) | +0.2 (−4 to +4.3) | 0.998 | |
| Non-HDL-C (mg/dL) | Baseline | 155.8±18.4 | 155.8±19.9 | 0.997 |
| Change at 6 months | −25.8 (−34.4 to −17.2) | −0.6 (−9.6 to +8.4) | <0.001 | |
| Triglycerides (mg/dL)a | Baseline | 111 [66 to 125] | 85 [65.5 to 121] | 0.589 |
| Change at 6 months | −4 [−22 to+19] | +13 [−4.55 to +29.5] | 0.122 | |
| Lipoprotein (a) (mg/dL)a | Baseline | 19 [10–57] | 16 [9–50] | 0.601 |
| Change at 6 months | 0 [−2 to +2] | 0 [−3 to +2] | 0.587 | |
| HbA1c (%) | Baseline | 5.4±0.3 | 5.4±0.4 | 0.933 |
| Change at 6 months | +0.16(−0.17 to +0.49) | −0.01 (−0.34 to +0.33) | 0.484 | |
| Systolic blood pressure (mmHg) | Baseline | 124.5±15.2 | 125.0±13.2 | 0.904 |
| Change at 6 months | −2.4 (−6.8 to +2.1) | −3.7 (−8.4 to+0.9) | 0.671 | |
| Diastolic blood pressure (mmHg) | Baseline | 75.7±8.3 | 75.3±8.4 | 0.856 |
| Change at 6 months | +0.5 (−1.9 to +2.8) | +1.0 (−1.4 to +3.5) | 0.736 |
Baseline visit analyzed by ANOVA. Data are expressed as means and standard deviations x¯±SD. Changes at six months analyzed by ANCOVA adjusted by basal values; data are expressed as estimated means (95% confidence interval). Bold p values indicate significant values (p<0.05).
Throughout the study, no significant changes were observed in patients’ degree of adherence to the Mediterranean diet nor weekly hours of physical activity. Good adherence to the study treatment was observed for 92.6% of patients in the intervention group and 90% of patients in the placebo group. During the six months of follow-up, good tolerance to the treatment was observed without any serious adverse events being recorded for any patient. Adverse events were observed in five patients (18.5%) of the intervention group and in five (16.7%) of the placebo group, which were mild in four patients in each group, or moderate in two patients, one in the intervention group had myalgia in the pelvic girdle, possibly related to the treatment, which remitted without the need for additional measures, and another in the placebo group presented an anxiety-depressive disorder which was attributed to family stress and was not solved despite anxiolytic treatment with paroxetine 20mg once a day. Only one patient in the intervention group and two patients in the placebo group had an adverse effect that might be related to the treatment. Notably, no increases were observed in alanine aminotransferase values or muscular symptoms throughout the follow up Fig. 1.
DiscussionIn the present study conducted in patients with a moderate degree of hypercholesterolemia and CVR, treatment with a nutraceutical preparation containing monacolin K, berberine and coenzyme Q10 was well tolerated and caused a decrease in LDL-C of 26mg/dL. Likewise, a similar decrease was observed in non-HDL-C, i.e., in the cholesterol transported by all atherogenic lipoproteins taken together,18 and in total cholesterol. Such a degree of decrease may be expected given the effect of monacolin K described in a recent expert paper,19 which outlines that a dose of 3–10mg/day of monacolin K was associated with a 15–25% decrease in LDL-C. Furthermore, our results are consistent with those of other studies conducted which combined monacolin K and berberine at doses similar to20 and lower than those observed in a clinical trial in which the patients included had a higher degree of hypercholesterolemia21 and, therefore, had a higher response to cholesterol-lowering treatment. In that study, 50 hypercholesterolemic patients were randomized to receive treatment with RYR, berberine and policosanols or placebo. Patients in the intervention and placebo groups had basal LDL-C of 174mg/dL (SD 25.13) and 170mg/dL (SD 22.04), respectively. LDL-C decreased by 41mg/dL (SD 29) in the intervention group and by 15mg/dL (SD 19) in the placebo group. It is unlikely that policosanols may have played a part in this decrease, since after a detailed analysis of the clinical trials conducted with these compounds, it has been established that they are ineffective at lowering plasma cholesterol.22
Furthermore, in the present study we have not observed any significant changes in HDL-C concentrations, which can be explained by the lower effect of drugs inhibiting cholesterol synthesis on c-HDL.23 Neither were any changes observed in the concentrations of lipoprotein(a), which is consistent with the practical absence of effect of current lipid-lowering drugs with the exception of PCSK9 protein inhibitors, on this lipoprotein, whose metabolism is not well known.24
The results of this study demonstrate that the association of monacolin K and berberine may be an effective therapeutic measure for treating patients with a moderate degree of hypercholesterolemia and CVR for whom no treatment of a higher intensity is indicated to reduce cholesterol concentration. More studies with a larger number of patients and longer-term follow-up are necessary to confirm the effects on lipids and the safety of the combination of nutraceuticals studied in the present study. Also, it is of great clinical importance to ascertain whether the decrease in atherogenic cholesterol achieved by means of nutraceutical products is effective in preventing cardiovascular disease. There is evidence from a prospective study conducted with yeast rice in a Chinese population concerning its effects on the incidence of cardiovascular disease events.25 The study included 4870 patients with previous myocardial infarction who did not follow lipid-lowering treatment and had total cholesterol levels ranging between 170 and 250mg/dL and mean LDL-C of 134mg/dL who were randomized to treatment with a capsule containing 300mg of a rice yeast extract equivalent to a dose of monacolin K of 2.5–3.2mg/day, or a placebo. LDL-C decreased by 17.6% in the active treatment group compared to the placebo group and the incidence of non-fatal myocardial infarction or coronary death decreased by 45% and 4.7% in relative and absolute terms, respectively. A good safety profile and a significant decrease in cardiovascular mortality and for all causes of 30% and 33%, respectively, were observed. These results were more favorable than expected given the degree of the decrease in cholesterol. In a systematic review and meta-analysis of clinical trials of cardiovascular prevention with statins or other lipid-lowering drugs, it was observed that for every 40mg/dL decrease in LDL-C, the relative risk of major cardiovascular disease events was 0.77 (95% IC, 0.75–0.79, P<0.001).26 The authors argued that the greater-than-expected decrease in CVR might have been influenced by other substances present in the yeast rice used in the trial, such as vegetable sterols or isoflavones, although they provided no data on the analysis of these substances.
Noteworthy are the good tolerance and high degree of adherence to treatment, without observing any serious adverse events or alterations of liver enzymes or muscle symptoms. This is consistent with the concept that RYR or its main component, monacolin K, has a similar or superior safety profile to that of low doses of statins.27 There is also a need to consider that RYR requires proper processing to extract its main active components, monacolins, and discard other compounds with potential toxic effects on the kidney, such as citrinin,28 hence products meeting the legal regulations for marketing should be selected. Moreover, berberine has been seen to have a good safety profile with no adverse effects on the liver or kidney.29 The potential protective effect of coenzyme Q10 against muscular symptoms resulting from the inhibition of HMG-CoA reductase, which as mentioned, may lead to a decrease of said enzyme,30 may also have been a reason behind the absence of muscle effects observed in this study.
This study's main limitation is its sample size, with a predominance of women, and the relatively short follow-up period. However, it was conducted in a patient population selected under strict criteria who have been followed up at lipid units served by experienced professionals in these disorders.
“This study shows that the combination of monacolin K, berberine and coenzyme Q10 improves total cholesterol, LDL-c and non-HDL-C levels in patients with hypercholesterolemia and at moderate risk.”
This study was funded by a grant from Bioksan. Bioksan has not participated in the data analysis, nor in drafting the article. We are also grateful for the assistance of the company Analysis & Research Network S.L. in the design and creation of the database and in monitoring the study.