During the last decade, increased attention has been paid to the development of new treatment approaches in assisted reproduction. It is crucial to establish a safe, effective and comfortable stimulation regimen to increase acceptability and reduce patients’ treatment stress and the high dropout rate that occurs after a failed treatment.
The present study aimed to describe the acceptance and results of a novel treatment regimen, consisting of Corifollitropin alfa (CFT) for ovarian stimulation (OS) and oral Desogestrel (DSG) for control of endogenous LH rise in oocyte donors (OD) who had a previous OS cycle with CFT and GnRH-antagonist (ANT) for LH suppression. This retrospective, cohort study, was performed in a private infertility clinic including 42 oocyte donors between April 1st 2016 and April 1st 2017. To evaluate the degree of acceptability of the DSG treatment cycle, a satisfaction questionnaire was used (EFESO questionnaire). When analyzing results, the degree of satisfaction observed with the novel stimulation protocol was high, without finding significant differences between both groups of treatment related to clinical variables.
Durante la última década se ha prestado más y más atención al desarrollo de nuevos esquemas terapéuticos en reproducción asistida. Es crucial establecer un régimen de estimulación seguro, efectivo y cómodo para la paciente, incrementando así la aceptación y reduciendo el estrés secundario al tratamiento y la alta tasa de abandono que ocurre tras un tratamiento fallido.
El objetivo del presente estudio es analizar la aceptación y los resultados de un novedoso régimen de tratamiento, basado en corifolitropina alfa para la estimulación ovárica y desogestrel oral para el control del aumento endógeno de LH en donantes de ovocitos que ya realizaron un ciclo previo con corifolitropina y antagonista de la GnRH (ANT) para la supresión de la LH. Se trata de un estudio retrospectivo de cohortes, realizado en una clínica privada de infertilidad en el que se incluyeron 42 donantes de ovocitos entre el 1 de abril de 2016 y el 1 de abril de 2017. Para evaluar el grado de aceptabilidad del tratamiento con desogestrel, se utilizó un cuestionario de satisfacción (cuestionario EFESO). Al analizar los resultados, el grado de satisfacción observado con el nuevo protocolo de estimulación fue alto, sin encontrar diferencias significativas entre ambos grupos al analizar diferentes variables clínicas.
In recent years, there has been an increasing trend toward using assisted reproduction techniques (ART) including oocyte donation programs.
Infertility is a difficult condition for patients, and although ART could be an option for these couples, it is also a major cause of stress (Requena et al., 2013). Ovarian stimulation (OS) can be an additional source of stress, therefore the importance of the design of optimal OS protocols for all patients undergoing ART aiming to decrease their physical, psychological and emotional burden (Cousineau and Domar, 2007; Hammarberg et al., 2001). In fact, the primary reason for treatment discontinuation is that patients are too distressed to continue (Hammarberg et al., 2001).
During the last decade, increased attention has been paid to the development of new treatment approaches, more simple and patient-friendly, in an attempt to reducing patient treatment stress and the high drop-out rate that occurs after a failed treatment (Verberg et al., 2008). Therefore, new regimens with improved efficacy and user convenience had to be investigated. Accordingly, the GnRH agonist long protocols have been gradually displaced by the GnRH antagonist protocols, due to the shorter duration of the treatment, lower risk for cyst formation, reduced risk of ovarian hyperstimulation syndrome (OHSS) and greater safety for patients (Al-Inany et al., 2016).
During OS is necessary to suppress the endogenous luteinizing hormone (LH) secretion to avoid untimed ovulation. Recently, a new form of controlling endogenous LH secretion has been proposed based upon the effects of Progesterone (P): either the endogenous P secreted by the corpus luteum during the luteal phase or the exogenously administered P during the follicular phase (progestin primed ovarian stimulation (PPOS). Consequently, exogenous progesterone has been studied as an effective alternative for the prevention of premature LH surge in women undergoing controlled OS.
Several studies have shown that the use of oral P does not affect the number of oocytes collected or the quality of the embryos obtained (Crha et al., 2018; Kuang et al., 2015; Massin, 2017). Compared to GnRH agonist OS cycles, PPOS cycles showed no differences in pregnancy rates, whilst effectively controlling the endogenous LH increase, without the need for daily injections of the GnRH analog (Crha et al., 2018; Kuang et al., 2015). In in vitro fertilization (IVF)-PPOS cycles, deferred embryo transfer is mandatory, due to the endometrium inadequacy. In case of recipients of donated oocytes, endometrial preparation can be performed in a synchronized manner with the donor PPOS and fresh embryo transfer is allowed.
To further decrease patient burden treatment, while keeping safety and efficacy, the use of a long-acting FSH (Corifollitropin-alfa, CTP) would reduce the number of injections. A single dose maintains the level of circulating FSH above the threshold necessary to support multi-follicular growth for a whole week, diminishing patient visits during the first week of controlled ovarian stimulation (COS) making OS more convenient (Pouwer et al., 2016).
The combination of CTP with oral Desogestrel (DSG) is a novel stimulation protocol that has the advantage of being more simple, comfortable and extremely patient-friendly. This treatment may improve the overall patient experience and treatment adherence and may also reduce errors during drug administration and the anxiety that this can cause to the patient (Huang et al., 2018). This new and simple regimen deserves more studies and it is gaining increasing interest in daily practice.
1AimsThe present study aimed to evaluate the level of acceptability in oocyte donors who had been stimulated with CFT in a PPOS cycle with oral DSG and who had a previous OS cycle with CFT and GnRH-antagonist (ANT) for LH suppression.
The primary outcome was the degree of satisfaction of the donor with the new protocol. The secondary outcome was the comparison of the ovarian response during the DSG and ANT cycles, regarding total extra dose of gonadotropin consumption, days of stimulation, total number of injections, peak estradiol, and number of oocytes and MII retrieved.
2Material and methods2.1Study designThis is a retrospective, cohort study, including oocyte donors from the Oocyte Donation Program at a private infertility clinic, treated between April 1st 2016 and April 1st 2017.
2.2Eligibility criteriaOocyte donors that underwent two cycles of OS in a single private infertility clinic within one year (between April 1st 2016 and April 1st 2017) were included. All patients included were healthy oocyte donors, between 18 and 35 years, with body mass index between 18 and 28kg/m2, regular menstrual cycles, and no history of any relevant personal or family antecedents. Normal karyotype was obtained, and all had a negative screening for sexually transmitted diseases. Patients were invited to fulfill a satisfaction questionnaire (EFESO questionnaire (Roca de Bes et al., 2013)) regarding the degree of satisfaction with the medication used during the second treatment cycle. The exclusion criteria included oocyte donors refusing to fulfill the questionnaire, presence of polycystic ovary syndrome based on Rotterdam criteria, hypersensitivity to the active substance or to any of its excipients, abnormal vaginal bleeding of unknown cause, presence of ovarian cyst or enlarged ovaries and history of hyperstimulation syndrome (OHSS).
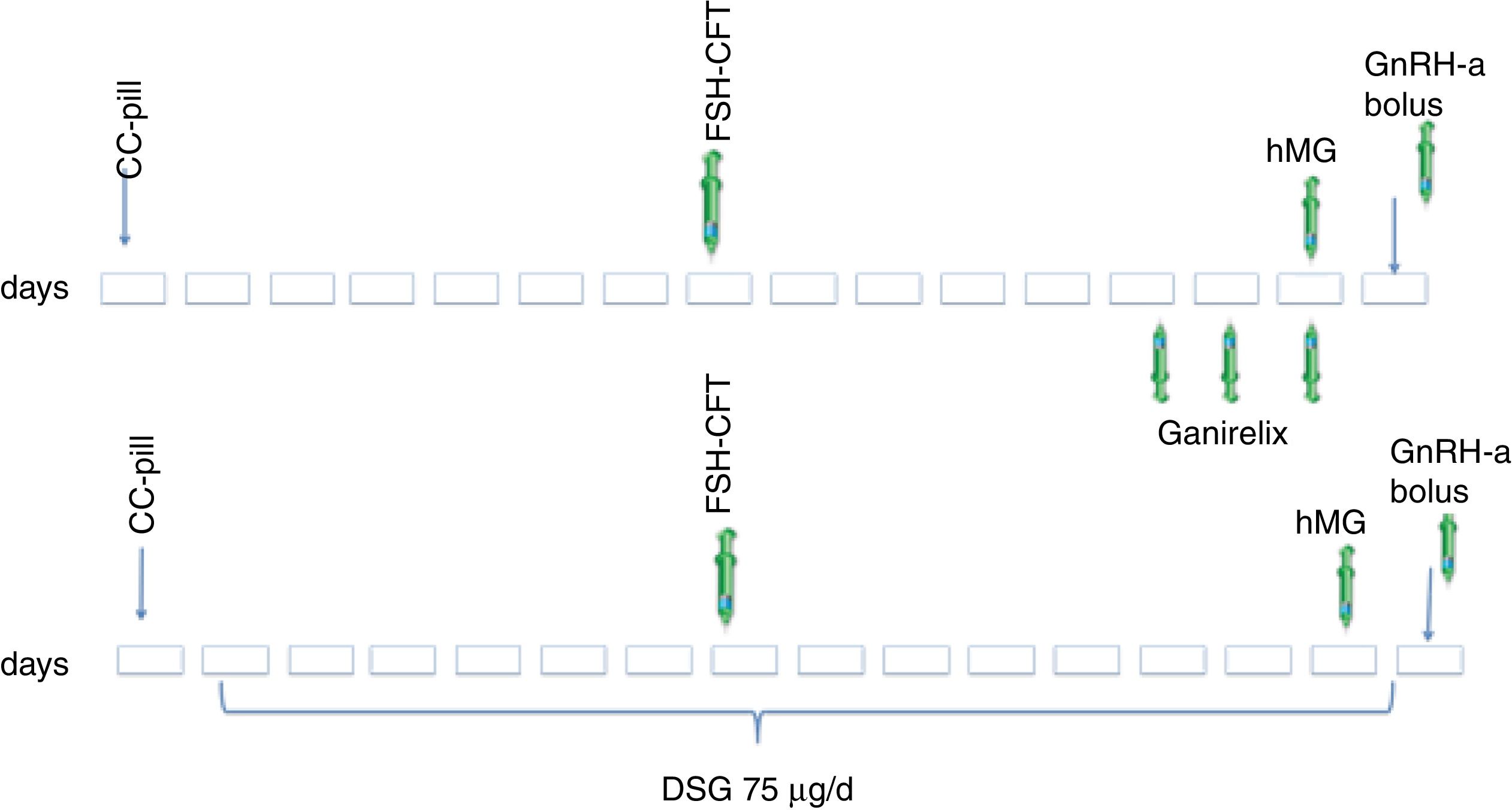
2.3Ovarian stimulation protocolAll oocyte donors received combined contraceptive pills (CCP) for 14–22 days. After a washing period of 7 days (Pérez-Calvo et al., 2017) the stimulation cycle was started with a single dose of CFT (100 or 150IU) administered at the clinic 7 days after CCP discontinuation.
In the first cycle, to control endogenous LH rise, ANT, Ganirelix sc. was administered at a daily dose of 0.25mg, starting when one or more follicles >14mm were visualized during the monitoring, and continued until the day of final oocyte maturation.
In the second cycle, daily oral DSG was used to prevent a premature LH surge, initiated after stopping CCP, maintaining it until triggering ovulation.
In both cycles, endocrine monitoring with serum estradiol evaluation and ultrasound scans were performed for controlling response. On day 8 of stimulation, daily doses of gonadotropins were administered if needed, until triggering criteria were met (more than three leading follicles greater than 18mm). Final trigger was performed with a GnRH agonist bolus. Oocyte retrieval was performed 36h later (Fig. 1).
After the second retrieval, donors were asked to fulfill a satisfaction questionnaire (EFESO questionnaire (Roca de Bes et al., 2013)). This opinion poll included 16 questions regarding donor's satisfaction with the medication used for ovarian stimulation, the process, and the information received from the members of the clinic. Answers ranged from a very high degree of satisfaction (score=5) to a very low degree of satisfaction (score=1).
2.4Statistical analysisContinuous variables were compared with t-test or Wilcoxon Mann–Whitney test as needed. To compare means between the first and the next cycle in the same patient, the t–test for paired data was used. The statistical analysis was performed using the IBM SPSS Statistics v22 software.
3Results42 donors were included in this study with a mean age of 26.19±4.64years, body weight of 58.7±8.5kg, antral follicle count (AFC) 18.34±4.76 and plasma anti-Mullerian hormone (AMH) levels of 3.34±1.74ng/ml.
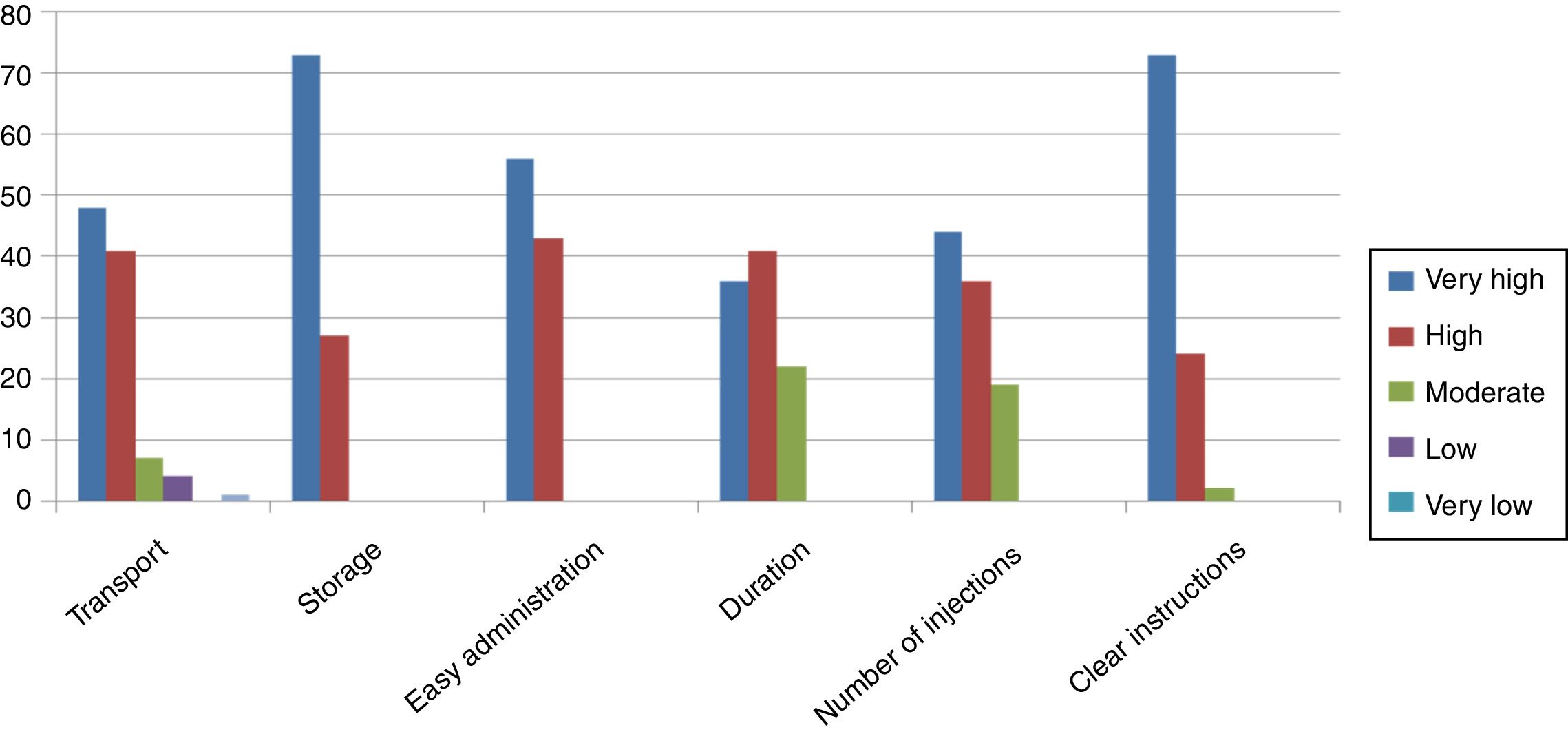
To evaluate the degree of acceptability of the DSG treatment cycle, a satisfaction questionnaire was used (EFESO questionnaire (Roca de Bes et al., 2013)). Six responses specifically related to the degree of satisfaction with the new treatment protocol out of the 16 items of the questionnaire were selected for the evaluation. Answers ranged from a very high degree of satisfaction (score=5) to a very low degree (score=1). The degree of satisfaction observed with the novel stimulation protocol was high. Mean degree of satisfaction with the 16 items of the questionnaire ranged between 4.05 and 4.73. Lower-scoring items were interference with daily activities (4.05) and local secondary effects (4.12) and higher-scoring items were easy-to-store (4.73) and understanding instructions of the medication (4.70). Donors scored treatment as easy to administer (4.52), handling (4.56), and exact dosing (4.39) (Fig. 2).
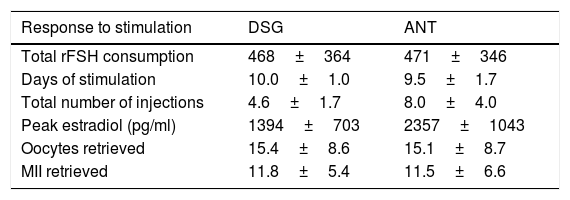
Table 1 displays data of ovarian response during DSG and ANT treatment cycles.
Characteristics of response to stimulation for first and second treatment cycles.
| Response to stimulation | DSG | ANT |
|---|---|---|
| Total rFSH consumption | 468±364 | 471±346 |
| Days of stimulation | 10.0±1.0 | 9.5±1.7 |
| Total number of injections | 4.6±1.7 | 8.0±4.0 |
| Peak estradiol (pg/ml) | 1394±703 | 2357±1043 |
| Oocytes retrieved | 15.4±8.6 | 15.1±8.7 |
| MII retrieved | 11.8±5.4 | 11.5±6.6 |
DSG: Desogestrel; ANT: Ganirelix.
There were no statistically significant differences between both treatments regarding total rFSH consumption, defined as the total supplementary dose used to reach the desired follicular development, duration of stimulation, and total number and MII oocytes obtained (p>0.05).
Compared to ANT cycles, donors received during DSG cycles nearly half number of injections (p=0.038). There were no cycles canceled due to low ovarian response and no cases of ovarian hyperstimulation.
4DiscussionIn the present study a patients’ preference for CFT in combination with oral DSG was expressed, without finding significant differences between both groups of treatment related to clinical variables. These findings are in agreement with previously published trials (Huang et al., 2018; Requena et al., 2013) and support the option of using CFT and DSG in IVF programs (Mahmoud Youssef et al., 2012).
Although there are already studies that value the acceptability of CFT as an OS treatment (Bouloux et al., 2001; Devroey et al., 2009; Duijkers et al., 2002), this is believed to be the first one that values acceptability in a group of donors undergoing treatment with CFT and oral DSG that have previously performed a stimulation cycle with daily GnRh antagonist protocol.
In our study, to avoid the premature LH surge, antagonist injections were substituted by daily oral progesterone, with an optimal control of the endogenous increase of LH. Oral progestin-induced suppression of LH surge has been demonstrated in several studies, without affecting the results of ART (Crha et al., 2018; Kuang et al., 2015; Massin, 2017).
It is important to design safe and efficient stimulation programs for all patients undergoing an ART cycle, but it is even more important in oocyte donors, being an ethically complex process by submitting young and healthy patients to treatments for which they do not receive benefits (Kalfoglou, 2001).
Many stimulation treatment protocols have been proposed over the past few years, but all of them consisted in daily injections of gonadotropins. Simplifying treatment protocols could help reduce physical demands and minimize disruption for the patients. The need for daily subcutaneous injections in infertility treatment can impair treatment adherence, and leads to patient anxiety surrounding whether the correct dose has been delivered and regarding the possibility of making unconscious mistakes. Innovations introduced to simplify treatment protocols can decrease stress during OS and enhance patient's wellbeing and satisfaction (Verberg et al., 2008). In fact, over half of patients report impact in its everyday life because of daily injections (Huisman et al., 2009).
The advantage of this protocol is that many injections of FSH and ANT can be replaced by one shot of CFT and oral DSG co-treatment. According to previous literature, the reduction in number of injections and visits translates into less discomfort and mental and physical stress in our patients, keeping effectiveness and clinical outcomes of the treatment (Huang et al., 2018).
However, its medical and economic significance remains to be demonstrated (Barrenetxea et al., 2018; Cruz et al., 2017; Melo et al., 2010). More data is needed to conclude about the economic impact that this novel treatment could have in our daily practice.
Regarding safety, there is some concern about the risk of hyperstimulation ovarian syndrome (OHSS) with CFT. Previous studies have shown that OHSS rates are similar using CFT or recombinant FSH (Corifollitropin alfa Ensure Study Group, 2010; Ledger et al., 2011; Tarlatzis et al., 2012). In our study no cases of OHSS were registered, probably due to routine use of GnRH agonist trigger in our center in oocyte donors.
With the combination of oral progesterone for controlling LH rise, and a single injection of CFT for OS, a novel treatment is presented, effective and extremely patient friendly, which manages to reduce the number of punctures that the patient must be administered, maintaining the same efficacy as conventional treatments. Ovarian donors treated with this novel regimen showed a high degree of acceptance, without apparent negative impact on the number of mature oocytes retrieved. Further well-designed clinical trials are mandatory prior to a shift in our current ART practice.
5Conflict of interestsThe authors declare no conflict of interests.