Kartagener syndrome is an autosomal recessive genetic disorder associated with male infertility. Mutations in genes that encode a protein called dynein affect sperm motility.
A good option for these patients is to enhance the sperm selection for ICSI that could lead to improve the reproductive outcomes. For this purpose, different strategies have been used successfully in isolated cases.
In the present case report, we successfully applied MACS technique to a semen sample. This immunomagnetic method of sperm selection was aimed at reducing apoptosis manifestations, including DNA fragmentation, to optimize the outcomes of the ICSI procedure. By doing so, we increased the chances of selecting spermatozoa with superior quality and higher fertilization potential in the presence of total asthenozoospermia, even after incubation with pentoxifylline. Six of ten oocytes (60%) were appropriately fertilized. Two good-quality embryos were transferred on day 3 resulting in a pregnancy and a healthy baby born and one cavitated blastocyst was frozen on day 5.
MACS technique used as a compliment of sperm preparation technique may be a promising approach in routine IVF practice for men with Kartagener syndrome to improve reproductive outcomes optimizing the outcomes of the ICSI procedure.
El síndrome de Kartagener es un desorden genético autosómico recesivo asociado con la infertilidad masculina. Las mutaciones en los genes que codifican una proteína llamada dinaina afectan la motilidad del esperma.
Una buena opción para estos pacientes es mejorar la selección de espermatozoides para el ICSI que podría llevar a mejorar los resultados reproductivos. Para ello se han utilizado con éxito diferentes estrategias en casos aislados.
En el presente informe de casos, aplicamos con éxito la técnica MACS a una muestra de semen. Este método inmunomagnético de selección de espermatozoides tenía por objeto reducir las manifestaciones de apoptosis, incluida la fragmentación del ADN, para optimizar los resultados del procedimiento de ICSI. De este modo, aumentamos las posibilidades de seleccionar espermatozoides de calidad superior y con mayor potencial de fecundación en presencia de astenozoospermia total, incluso después de la incubación con pentoxifilina. Seis de diez ovocitos (60%) fueron fertilizados apropiadamente. Se transfirieron dos embriones de buena calidad el día 3, lo que dio lugar a un embarazo y al nacimiento de un bebé sano, y un blastocisto cavitado se congeló el día 5.
La técnica MACS utilizada como complemento de la técnica de preparación de esperma puede ser un enfoque prometedor en la práctica rutinaria de la FIV para hombres con síndrome de Kartagener para mejorar los resultados reproductivos optimizando los resultados del procedimiento ICSI.
Kartagener syndrome (KS) is an autosomal recessive genetic disorder, affecting approximately one in every 15,000 live births. This congenital disease belongs to ciliary dyskinesia syndrome (CDS), which is characterized by the presence of several symptoms such as chronic sinusitis, bronchiectasis, situs inversus, and infertility, secondary to structural or functional defects in the action of the motile cilia (Brush et al., 2007; Fawcett et al., 1977).
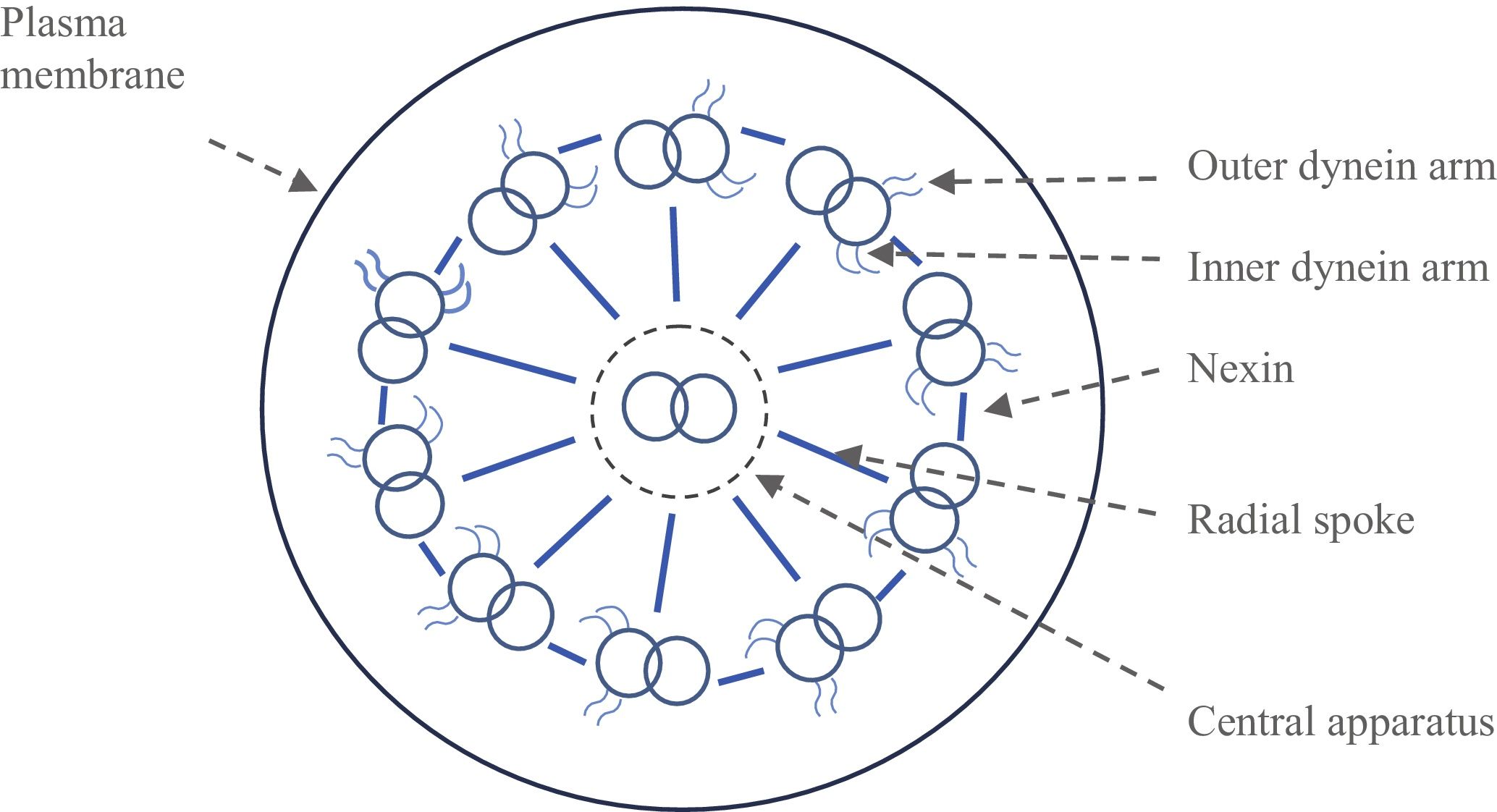
In male patients, infertility is due to partial or total immobility of the spematozoon flagellum because of mutations on genes that control the synthesis of radial spokes or inner and outer dynein arms. These structures form temporary cross bridges between adjacent ciliary filaments and are believed to be responsible for generating movement in cilia and flagellum spermatozoa. Previous reports indicated mutations or deletions on these genes such as DNAI1, DNAI2, DNAH5, DNAH11, CCDC103, ARMC4, KTU/DNAAF2, LRRC50/DNAAF1, LRRC6, DYX1C1, ZMYND10, CCDC39, CCDC40, CCDC164, HYDIN, RSPH4A and RSPH1. Even a X-linked inheritance pattern was published in cases of CDS and KS (Raidt et al., 2014; Horani and Ferkol, 2018). Currently, 40 genes have been associated with disease, and >70% of patients tested have mutations in one of these genes.
Nowadays, intracytoplasmic sperm injection (ICSI) is considered to be the only reproductive strategy available in male patients affected by this syndrome (Nagy et al., 1998; Nijs et al., 1996). There have only been a few reports on successful fertilization and pregnancy using inmotile ejaculated sperm for ICSI where the main problem has been differentiating between live and dead sperm (Hattori et al., 2011; Ved et al., 1997; Soares et al., 2003).
In this line, magnetic-activated cell sorting (MACS), or magnetic separation by columns of annexin V, is applied in reproductive medicine in order to improve the sperm selection of assisted reproduction techniques. This technique is based on the ability of pre-apoptotic and dead spermatozoa with damaged plasma membranes and externalization of phosphatidylserine (PS) to bind to annexin V. This protein, when conjugated with super-paramagnetic microbeads, is retained along the separation column allowing non-apoptotic spermatozoa with intact membranes to go through the column when under the influence of a magnetic field (Paasch et al., 2007). Therefore, the selection of nonapoptotic spermatozoa can be used for enhancing assisted reproduction outcomes (Miltenyi et al., 1990; Grunewald et al., 2001, 2003; Paasch et al., 2004).
Over the past decade, several therapeutic approaches have been proposed to optimize the reproductive outcomes of infertile couples where the man is affected with primary ciliary dyskinesia (PCD). This study describes the case of a patient with Kartagener syndrome who achieved an ongoing pregnancy after MACS technique was applied to his ejaculated sperm. This immunomagnetic method is aimed at reducing apoptosis manifestations including DNA fragmentation, increasing the chance of selecting immotile sperm with superior quality.
Materials and methodsA couple with two years of primary infertility and without previous in vitro fertilization treatments (IVF) came to our fertility center.
The male, a 36-year-old man with no family history of the disease, was under suspicion of having Kartagener syndrome because of the presence of situs inversus (dextrocardia), recurrent upper respiratory tract infections and abnormal sperm analysis, characterized by total asthenozoospermia and teratozoospermia.
The morphology of sperm flagella was checked in another center under scanning electron microscopy, looking for alterations of dynein arms.
Subsequently, a genetic test was performed on blood samples, searching for the presence of mutations in the main genes associated with PCD.
After that, a karyotype and sperm-FISH (fluorescence in situ hybridization analysis on the sperm) were carried out. In the sperm-FISH analysis, chromosome anomalies were assessed in 2303 spermatozoa for chromosomes 18, X, and Y whereas 2018 spermatozoa were evaluated for chromosomes 13 and 21.
The basic semen analysis procedure was performed according to the World Health Organization (WHO) 2010 guidelines. Total asthenozoospermia was verified in spite of incubation with pentoxifilline (PF) (Sigma-Aldrich Quimica, Spain) 1.76mM for 30min (Yovich et al., 1990) and sperm viability was assessed by the eosin–nigrosin staining. Approximately equal volumes of semen and stain were mixed and incubated for 30seconds (s) at room temperature. Then, a droplet was transferred to a slide where it was smeared. At least 200 spermatozoa were assessed at a magnification of 100× under oil immersion with an optical microscope (not phase contrast). White sperm (unstained) were alive and those that showed any pink or red color were classified as dead (WHO, 2010).
In this study, MACS technique was performed to eliminate apoptotic spermatozoa and obtain high quality sperm for the injection. The sperm vitality and DNA damage (spermatozoa with DNA fragmentation) was assessed before and after applying MACS.
Before beginning treatment, the couple received genetic counseling regarding the implementation of the indicated assisted reproduction techniques.
Controlled ovarian stimulation
The female, a 35-year-old woman, underwent controlled ovarian stimulation using a GnRH antagonist protocol (Orgalutran®, Merck, Spain) for pituitary suppression. Ovarian stimulation was carried out with a starting dose of 200IU/day of recombinant follicle-stimulating hormone (rFSH) (Puregon®, Organon, Spain) for 11 days. A GnRH antagonist was added when one or more follicles of 13-mm mean diameter were visualized. Ovulation induction was performed with recombinant human chorionic gonadotropin (rhCG) 250μg (Ovitrelle®, Merck, Spain) when at least 3 follicles reached a mean diameter of 17mm, and oocyte retrieval was carried out 36h later. The luteal phase was supported with 400mg/day of natural micronized vaginal progesterone (Utrogestan®, Seid, Spain), starting 1 day after oocyte retrieval.
Sperm preparation for ART
After liquefaction at room temperature (25°C), the ejaculated spermatozoa were prepared by centrifuging at 300xg and the pellet re-suspended with Glofert medium (Global® for fertilization, LifeGlobal, Belgium) in order to assess sperm concentration before magnetic separation. One aliquot of the sperm suspension served as a control and the other aliquot was subjected to MACS.
Sperm selection using the magnetic activated cell sorting (MACS) technique
For sperm magnetic selection, 5×106 sperm cells were incubated with 100mL annexin V-conjugated MicroBeads (MACS® microbeads, Miltenyi Biotec, Spain) in the dark, at room temperature, and under continuous agitation for 30minutes (min). The column containing iron globules was placed in the magnet (MiniMACS™, Miltenyi Biotec, Spain) and set up by rinsing 3 times with 500ml of Binding Buffer (Miltenyi Biotec, Spain) before use. After the incubation period, 300mL of Glofert were added to the sperm microbead suspension and, subsequently, the cell suspension was applied to the column. The annexin V negative fraction, containing nonapoptotic sperm, was eluted through the column and the annexin V positive fraction is retained in the separation column and collected (Said et al., 2005). Then, an aliquot of the annexin V negative fraction was washed and re-suspended in Glofert specific medium prior to the ICSI procedure.
Measurement of DNA fragmentation by TUNEL assay and flow cytometry analysis
DNA fragmentation was assessed before and after MACS technique using the TUNEL (terminal deoxynucleotidyltransferase-mediated deoxyuridine triphosphate-biotin nick-end labeling) assay (Roche, Spain) as in the report (Liu et al., 2004), with minor modifications. Briefly, aliquots of the sperm samples were washed twice in phosphate-buffered saline (PBS) (Gibco® PBS, Life technologies, Spain) for 5min, followed by centrifugation for collecting spermatozoa at 200×g. The spermatozoa were then treated with a solution containing 0.1% Triton X-100 (Sigma-Aldrich Quimica, Spain) and 0.1% sodium citrate (Sigma-Aldrich Quimica, Spain) for 2min on ice. A 30-ml TUNEL mixture consisting of terminal deoxynucleotidyl transferase (TdT) and fluorescein dUTP was added to the same volume of each sample. The samples were incubated for 60min at 37°C in a moist chamber in darkness, washed three times with PBS and then analyzed in a FACScan flow cytometer (Becton Dickinson, Spain). At least 10,000cells were counted. The presence of green fluorescent signals was regarded as positive. For positive controls, spermatozoa were processed in the same way, except for a prior incubation with DNase I (1mg/ml, D-4263; Sigma) for 10min at room temperature to induce DNA fragmentation before the addition of the TUNEL mixture. For negative controls, the spermatozoa were similarly processed, but the TUNEL mixtures were added without the presence of TdT. The percentage of sperm with damaged DNA were calculated in a sperm aliquot before and after MACS technique. The established reference value of <20% TUNEL-positive spermatozoa to be predictive for ICSI outcomes, was incorporated.
Oocyte retrieval and Intracytoplasmic Sperm Injection
All mature oocytes were micro-injected as described by Palermo et al. in 1992 with immotile spermatozoa after MACS. Sperm selection was also carried out according to the morphology and rigidity of the sperm flagellum. A rigid tail was considered a sign of non viability (Marques de Oliveira et al., 2004). After microinjection, oocytes were transferred into 50μl drops of Glofert under mineral oil (LifeGlobal, Belgium) in a plastic dish and incubated at 37°C under a 6.5% CO2 atmosphere.
Fertilization was assessed 16–19h after ICSI and only zygotes with the presence of two pronuclei and two polar bodies were considered normal. Only the successfully fertilized oocytes were cultured in dishes with drops of 50μl of Global culture medium (Global®, LifeGlobal, Belgium).
Standard embryo evaluation was based on embryo development and morphological characteristics.
ResultsBefore the treatment, a genetic test was performed on blood samples, searching for the presence of mutations in the main genes associated with PCD, including DNAH5 and DNAI1, on chromosomes 5 and 9, respectively. Such mutations were not found on these regions. Up to now, many genetic PCD variants remain undiscovered, not all cases can be identified genetically. So that, negative genetic testing does not exclude diagnosis (Horani and Ferkol, 2018; Raidt et al., 2014).
On the other hand, karyotype and sperm-FISH analyses were normal for the analyzed chromosomes.
The morphology of sperm tails was checked under scanning electron microscopy to visualize the axoneme ultra-structure showing the absence of dynein arms (Fig. 1).
Urogenital examination showed no pathology and endocrine parameters were as follows: serum leves of follicle stimulating hormone (FSH) 3.91mIU/mL, luteinizing hormone (LH) 4.14mIU/mL and testosterone 378.2ng/mL.
On the day of oocyte retrieval, the fresh semen evaluation showed a volume and concentration of 1.3ml and 32×106/ml, respectively. Subsequently, 0.5ml of the ejaculate (EJ) was washed and the pellet re-suspended in 0.2ml Glofert. Total absence of motility in the sperm sample was confirmed, even after incubation with PF. The eosin-nigrosin test revealed 55% live spermatozoa.
Finally, 0.5ml of the sperm sample concentrated at 0.2×106/ml was used for micro-injection after applying MACS technique. ICSI procedure was carried out with totally immotile spermatozoa avoiding those with completly rigid tail. Appliying TUNEL, the initial sperm fragmentation index in washed sperm was 23% (spermatozoa with fragmented DNA) and after MACS technique it was reduced to 11%. In the same way, the viability results in ejaculated spermatozoa before and after treatment were 55% and 60% respectively.
The female revealed no pathology and her basal endocrine assessment on the third day was normal: FSH 7.5mIU/mL, LH 4.8mIU/mL and estradiol 48pg/mL.
After retrieval, 10 of the 14 oocytes were in metaphase II (71.4%). From sixteen to nineteen hours after ICSI, six of these 10 oocytes (60%) were appropriately fertilized. The presence of 3 pronuclei and 2 polar bodies was only observed in 1 zygote (10%), one of the zygotes (10%) with only 1 polar body and 0 pronuclei and 2 (20%) were degenerated. With regard to embryonic development on day 2, 4 of 6 embryos presented with 4 even cells with <10% fragmentation, and 1 had multinucleated blastomeres. Two less developed embryos (with 2 and 3cells) were multinucleated and the second one had 25% fragmentation and was uneven. Embryo transfer was carried out on day 3. Two even 8- and 6-cell-embryos were transferred into the uterus. Three poor quality embryos with slower development were cultured until day 5. Of those, one cavitated blastocyst was frozen.
Thirteen days after the embryo transfer, the value of the woman's β-hCG blood test was 112.6IU, and 10 days later the transvaginal ultrasonography showed the existence of one intrauterine gestational sac with heart activity at 7 weeks gestation. At this present moment, we know a healthy baby girl was born with normal pediatric developmental rate without situs inversus and recurrent respiratory infections. Nevertheless, there is a possibility that medical problems and other fertility problems will be diagnosed later. All patients must be informed about these risks and receive appropriate genetic counseling before the use of ICSI is considered.
DiscussionPreviously, the main function of spermatozoa in fertilization and embryo development was reduced to being a carrier that transports DNA to the oocyte. But now, it is well known that the quality of both gametes play an important role that extends beyond the fertilization and embryo development directly influencing in the results (Said and Land, 2011; Sirard et al., 2006).
Literature states that total asthenozoospermia can arise from several mechanisms such as ultrastructural defects in the sperm tail related to genetic disorders, genital infections, antisperm antibodies, environmental pollutans, delayed epididimal transport and oxidative stress (Ortega et al., 2011). PCD is an autosomal recessive genetic disorder that impairs the action of cilia in the respiratory tract, fallopian tube and in the flagella of spermatozoa, leading to infertility problems in 50% of men with this pathology (Brush et al., 2007; Fawcett et al., 1977). Although there are different levels of sperm motility in these patients, most of them only present immotile spermatozoa in the ejaculate (Kay and Irvine, 2000; Von Zumbusch et al., 1998; Abu-Musa et al., 1999; Papadimas et al., 1997), as reflected in this case.
Even with the improvements in IVF techniques and the introduction of ICSI into routine laboratory procedures, total asthenozoospermia still exerts a negative effect on reproductive outcomes (Kahraman et al., 1996; Nagy et al., 1995).
In daily lab routin the embriologyst has inevitably to face the use of immotile spermatozoa. Therefore quick and easy laboratory methods to differentiate between dead and viable but immotile spermatozoa are required. For the purpose of sperm selection for ICSI, several studies have been published which investigated alternative techniques to implement in in vitro fertilization (IVF). Different strategies have been used in isolated cases with a wide range of results, such us the hypo-osmotic swelling test, pentoxifylline to enable movement, mechanical touch technique to test the flexibility of the sperm tail and laser-assisted immotile sperm selection, (Yildirim et al., 2009; Gerber et al., 2008; Ved et al., 1997; Marques de Oliveira et al., 2004; Hattori et al., 2011).
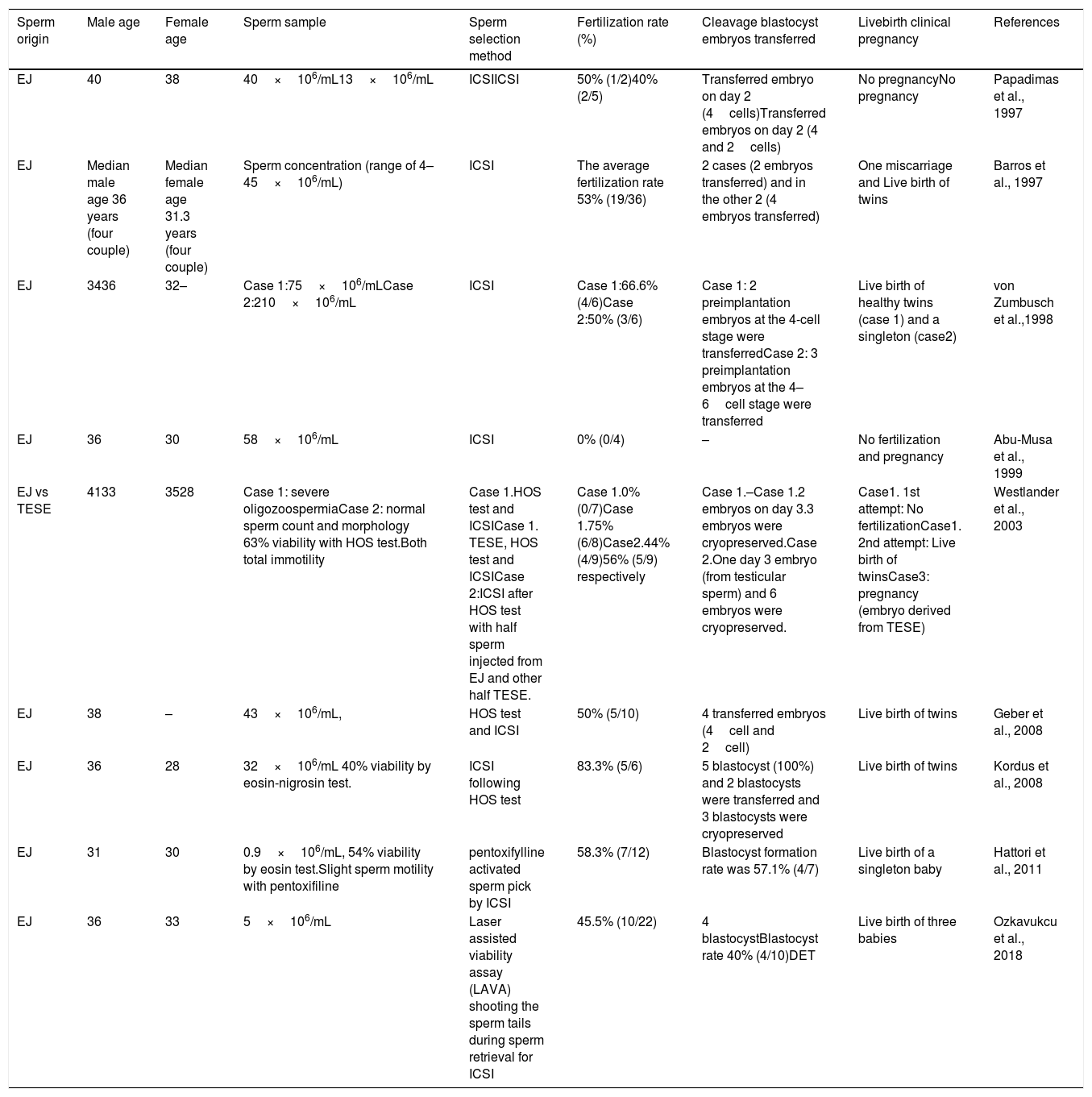
The first child born live after ICSI with immotile sperm from a male patient with Kartagener's syndrome was reported by Stalf et al. in 1995. Even though, we know that microinjection with these sperm provide poor outcomes, there have been several cases of asthenozoospermia related to dynein arm deficiency in which ICSI achieved healthy offspring (Table 1).
Reproductive results of ICSI using totally immotile ejaculated sperm (a brief chronological summary).
| Sperm origin | Male age | Female age | Sperm sample | Sperm selection method | Fertilization rate (%) | Cleavage blastocyst embryos transferred | Livebirth clinical pregnancy | References |
|---|---|---|---|---|---|---|---|---|
| EJ | 40 | 38 | 40×106/mL13×106/mL | ICSIICSI | 50% (1/2)40% (2/5) | Transferred embryo on day 2 (4cells)Transferred embryos on day 2 (4 and 2cells) | No pregnancyNo pregnancy | Papadimas et al., 1997 |
| EJ | Median male age 36 years (four couple) | Median female age 31.3 years (four couple) | Sperm concentration (range of 4–45×106/mL) | ICSI | The average fertilization rate 53% (19/36) | 2 cases (2 embryos transferred) and in the other 2 (4 embryos transferred) | One miscarriage and Live birth of twins | Barros et al., 1997 |
| EJ | 3436 | 32– | Case 1:75×106/mLCase 2:210×106/mL | ICSI | Case 1:66.6% (4/6)Case 2:50% (3/6) | Case 1: 2 preimplantation embryos at the 4-cell stage were transferredCase 2: 3 preimplantation embryos at the 4–6cell stage were transferred | Live birth of healthy twins (case 1) and a singleton (case2) | von Zumbusch et al.,1998 |
| EJ | 36 | 30 | 58×106/mL | ICSI | 0% (0/4) | – | No fertilization and pregnancy | Abu-Musa et al., 1999 |
| EJ vs TESE | 4133 | 3528 | Case 1: severe oligozoospermiaCase 2: normal sperm count and morphology 63% viability with HOS test.Both total immotility | Case 1.HOS test and ICSICase 1. TESE, HOS test and ICSICase 2:ICSI after HOS test with half sperm injected from EJ and other half TESE. | Case 1.0% (0/7)Case 1.75% (6/8)Case2.44% (4/9)56% (5/9) respectively | Case 1.–Case 1.2 embryos on day 3.3 embryos were cryopreserved.Case 2.One day 3 embryo (from testicular sperm) and 6 embryos were cryopreserved. | Case1. 1st attempt: No fertilizationCase1. 2nd attempt: Live birth of twinsCase3: pregnancy (embryo derived from TESE) | Westlander et al., 2003 |
| EJ | 38 | – | 43×106/mL, | HOS test and ICSI | 50% (5/10) | 4 transferred embryos (4cell and 2cell) | Live birth of twins | Geber et al., 2008 |
| EJ | 36 | 28 | 32×106/mL 40% viability by eosin-nigrosin test. | ICSI following HOS test | 83.3% (5/6) | 5 blastocyst (100%) and 2 blastocysts were transferred and 3 blastocysts were cryopreserved | Live birth of twins | Kordus et al., 2008 |
| EJ | 31 | 30 | 0.9×106/mL, 54% viability by eosin test.Slight sperm motility with pentoxifiline | pentoxifylline activated sperm pick by ICSI | 58.3% (7/12) | Blastocyst formation rate was 57.1% (4/7) | Live birth of a singleton baby | Hattori et al., 2011 |
| EJ | 36 | 33 | 5×106/mL | Laser assisted viability assay (LAVA) shooting the sperm tails during sperm retrieval for ICSI | 45.5% (10/22) | 4 blastocystBlastocyst rate 40% (4/10)DET | Live birth of three babies | Ozkavukcu et al., 2018 |
To the best of our knowledge, this is the second time that the use of MACS was reported in an attempt to improve ICSI procedures, particularly in males with Kartagener's syndrome. In the present report, selection of high-quality sperm including improvements in DNA integrity, resulted from the application of an advanced sperm selection technique (MACS) that represents another option to optimize the reproductive outcomes in males with an immotile sperm population. A previous research with sixteen patients showed that the combined use of density gradient centrifugation (DGC) and MACS is an efficient method for isolating viable spermatozoa, with lower level of DNA fragmentation and normal morphology (Zhang et al., 2018).
Several studies attempted to link apoptotic alterations in sperm (fragmentation) with conventional seminal parameters finding a significant negative correlation between the proportion of apoptotic cells and sperm motility, viability and normal morphology in ejaculated semen (Han-Ming et al., 2002).
Programmed cell death is an ongoing physiological phenomenon that has been documented to play a role in male infertility if deregulated (Avendaño et al., 2009; Boe-Hansen et al., 2006). Caspase activation, externalization of phosphatidylserine, alteration of mitochondrial membrane potential, and DNA fragmentation are markers of apoptosis found in ejaculated human spermatozoa. DNA damage consists of the presence of breaks in the sperm's genetic material, affecting the integrity and quality of the sperm sample. The greater the damage, the lower the integrity of the genetic material and the lower the likelihood of achieving an ongoing pregnancy. Recently, increased sperm DNA fragmentation was shown to be related to recurrent fertilization failure in a case of Kartagener syndrome (Nuñez et al., 2010). In human spermatozoa, typical apoptosis starts with the externalization of the PS phospholipid on the surface of the plasma membrane and finishes with DNA fragmentation (Glander and Schaller, 1999).
Taking this into account, the selection of non-apoptotic spermatozoa from the ejaculated may also prove to be beneficial in patients with this syndrome. This sperm selection technique, MACS, based on the high affinity of annexin V for phosphatidylserine, has been used as a non-invasive method in different fields in order to reduce the percentage of apoptotic cells (Said et al., 2006). The sperm selection process has become a good approach to better selection of good quality spermatozoa from ejaculated sperm to improve clinical outcomes in assisted reproductive treatments (Rawe et al., 2010). Many researches have proved the ability of MACS employing annexin V microbeads to enrich vital and motile sperm with inactivated apoptosis signaling and lower DNA fragmentation rate (Grunewald et al., 2001, 2006; Paasch et al., 2003, 2004). Moreover, Clinical studies and reports have showed improved ability to fertilize oocytes (Miltenyi et al., 1990; Paasch et al., 2004) and superior implantation and pregnancy rates, integrating this method as a unique molecular preparation technique that can compliment conventional sperm preparation protocols (Dirican et al., 2008; Rawe et al., 2010).
In the present case report, we successfully applied this sperm selection technique to a semen sample from a patient with Kartagener syndrome, aimed at reducing the percentage of damaged cells to optimize the outcomes of the ICSI procedure. By doing so, we increased the chances of selecting spermatozoa with superior quality and higher fertilization potential in the presence of total asthenozoospermia. The percentage of spermatozoa stained with eosin-nigrosin was slightly lower after MACS. This observation may be explained by the fact that the spermatozoa with apoptotic markers, susceptible to annexin V binding at an early stage of apoptosis, are not always related to dead sperm while the staining is being carried out (Kotwicka et al., 2011).
Although, this particular case has shown clear benefits appliying this technique in a male with this pathology, further comparative studies with a larger number of patients would be recommended to confirm these findings.
Taking this into consideration and based on our good results, having achieved an almost average fertilization rate, we conclude that MACS may be a promising approach in routine IVF practice for men with Kartagener syndrome, improving reproductive outcomes. This immunomagnetic method is aimed at reducing apoptosis manifestations including DNA fragmentation, increasing the chance of selecting immotile sperm with superior quality.
Conflict of interestsThe authors declare that they have no conflict of interest.