The aim of the study is to assess whether delaying frozen embryo transfer (FET) after pre-implantation genetic testing for aneuploidy (PGT-A) cycle provides any benefit for reproductive outcomes.
MethodsRetrospective cohort study including a total of 913 frozen embryo transfers related to 5104 PGT-A cycles performed between May 2016 and March 2017 at IVI clinics. We compared the FET performed the month following the ovarian puncture (OPU) (n=184) with the FET delayed one or more months (n=700). The main outcome was clinical pregnancy rate (CPR) and the secondary outcomes were implantation (IR) and miscarriage rates.
ResultsNo significant statistical differences were detected between groups as regards patient age and cycle parameters. Similar results were also observed concerning metaphase II oocytes (11±0.8 vs 10±0.4, p=0.03), number of blastocysts (4.3±0.4 vs 3.8±0.2, p=0.122), euploid embryos (2.1±0.3 vs 1.8±0.1, p=0.039), or transferred embryos (1.1±0.1 vs 1.1±0.2, p=0.52). Finally, no significant differences were found in CPR (52.7% vs 54.9%, p=0.33). The multivariate logistic regression showed that the number of euploid (OR=1.170, 1.062–1.288; p=0.001) and transferred embryos (OR=2.530, 1.703–1.509; P<.001) significantly affected the probability of getting pregnant, while the timing of the frozen embryo transfer (OR=1.090, 0.787–1.509; p=0.604) did not have a significant effect on reproductive outcomes.
ConclusionsDelaying frozen embryo transfer after PGT-A cycles does not seem to improve outcomes in terms of pregnancy rates.
El principal objetivo del estudio fue evaluar si realizar la transferencia inmediata tras la estimulación ovárica en ciclos de FIV con PGT-A afectaba o no a los resultados clínicos.
MétodosEstudio de cohortes retrospectivo que incluye los primeros 913 ciclos de transferencias de embriones congelados (TEC) de 5.104 ciclos de FIV con PGT-A realizados entre mayo de 2016 y marzo de 2017 en clínicas IVI. Comparamos las TEC realizadas en el ciclo inmediato de la punción ovárica (OPU) (n=184) con el TEC realizado descansando más de un mes tras la OPU (n=700). El resultado principal fue la tasa de embarazo clínico (TG) y los resultados secundarios fueron la implantación (TI) y las tasas de aborto espontáneo.
ResultadosNo se encontraron diferencias estadísticas con respecto a la edad de los pacientes y las variables del ciclo entre los grupos. Además, se observaron resultados similares con respecto al número de ovocitos de metafase II (11±0,8 frente a 10±0,4; p=0,03), número de blastocistos (4,3±0,4 frente a 3,8±0,2; p=0,122), embriones euploides (2,1±0,3 frente a 1,8±0,1; p=0,039) o embriones transferidos (1,1±0,1 frente a 1,1±0,2; p=0,52). Finalmente, no encontramos diferencias significativas en la RCP (52,7 frente al 54,9%; p=0,33). La regresión logística multivariada mostró que el número de embriones euploides (OR=1,170, 1,062-1,288; p=0,001) y transferidos (OR=2,530, 1,703-1,509; p<0,001) afectó significativamente la probabilidad de quedar embarazada, mientras que el momento de realizar la transferencia de embriones congelados (OR=1,090, 0,787-1,509; p=0,604) no tuvo un efecto significativo en los resultados reproductivos.
ConclusionesRetrasar la transferencia de embriones congelados después de los ciclos de PGT-A no parece mejorar los resultados en términos de tasas de embarazo.
Since the development of in vitro fertilization (IVF), controlled ovarian stimulation with gonadotropins has been used to develop multiple follicles in a single cycle and therefore more oocytes. This increased follicular recruitment that is associated with supraphysiological levels of estradiol and premature elevations of progesterone, has sometimes been described as detrimental to the endometrium (Labarta et al., 2011; Roque et al., 2013; Shapiro et al., 2011). An alternative to minimize these effects could be to cryopreserve all viable embryos and to transfer them in a subsequent frozen embryo transfer (FET) cycle (Basile and Garcia-Velasco, 2016). The freeze-all strategy, which is usually applied to prevent ovarian hyperstimulation syndrome, or in the presence of high progesterone levels in the follicular phase, has been associated to a better synchrony between embryo development and the endometrial window of implantation, to better pregnancy rates and to the avoidance of certain risks (Devroey et al., 2011). All these benefits are only be possible due to the establishment of reliable vitrification programmes yielding to good embryo survival and implantation rates after warming (Cobo et al., 2012).
On the other hand, the use of a preimplantation genetic test for aneuplodies (PGT-A) is increasing, with embryo biopsies been performed normally at the blastocyst stage (Forman et al., 2012; Schoolcraft and Katz-Jaffe, 2013). This policy, however, requires the cryopreservation of all the embryos while waiting for the genetic diagnosis and, therefore, delaying the ET procedure (Horcajadas et al., 2005; Scott et al., 2013).
Thus it is common practice to perform FET 1 or 2 months after embryo biopsy (Aflatoonian et al., 2010), causing a significant amount of stress and anxiety to the patients and increasing the time to pregnancy (TTP). For this reason some studies have focused on evaluating whether if waiting, or not, for one menstrual cycle or more, before performing embryo transfer (ET) can affect live-birth rates, suggesting that it is not necessary to wait (Santos-Ribeiro et al., 2016).
The objective of this retrospective study was to compare clinical outcomes when ET was performed immediately after ovarian stimulation, or after one (or more) menstrual cycles in patients undergoing PGT-A considering a bigger data set than previously published studies.
Material and methodsThis large, non-interventional, retrospective multicentre cohort study included 5104 cycles of patients>35 years old (5104 cycles) who underwent PGT-A cycles between May 2016 and March 2017. Patients were stimulated following antagonist stimulation protocols with different kinds of gonadotropins (FSHr, FSH/LHr or HMG) with dosages between 150 and 300UI and with 0.2mg GnRHa (Decapeptyl 0.1mg IPSEN PHARMA, Barcelona, Spain) triggering when more than 1 follicle achieved a size over>17mm. Briefly oocyte pick-up took place 36hs post trigger. Cumulus-corona complexes were denuded 3hours post retrieval and all MII oocytes were inseminated through intracytoplasmic sperm injection (ICSI) immediately afterwards. Fertilization check was performed 18–19 hs post injection and all fertilized oocytes were cultured individually. On day 5 or day 6 of development embryo biopsy (n=6423) was performed using Octax laser and the preimplantational genetic test was performer by NGS. Only cavitated, expanded, hatching or fully hatched blastocysts with a clear ICM were biopsied. After biopsy was confirmed embryos were vitrified following a standard vitrification protocol using an open system as described elsewhere.
FETs were scheduled once genetic results were available and the presence of euploid embryos was confirmed. FETs took place either in a natural or artificial cycle. All the procedures were approved by an institutional review board (1704-MAD-025-MC) in IVI Madrid and complied with the Spanish law on assisted reproductive technologies (14/2006). Only the first FET following the fresh cycle was included in the study. For that reason only 913 were analyzed, because we wanted to know if delay or not the transfer affect or not in results.
913 FET were included, 712 with an endometrial artificial preparation and 201 with natural cycle. The artificial preparation was doing with oral oestradiol valerate (Progynova®; Schering, Madrid, Spain) on day 2–3 of the cycle by the classic endometrium built-preparation protocol started with 2mg/day on days 1–4, 4mg/day on days 5–8 and 6mg/day from day 9 onwards. After 10–12 days of oestrogen treatment a transvaginal ultrasound was performed. Frozen embryo transfers were scheduled once endometrial thickness was at least 6mm and triple lined, there was neither a dominant follicle nor signs of ovulation, and serum P4 levels were confirmed below 1 ng/ml. Natural micronized progesterone was administered vaginally (Utrogestan®; Seid, Barcelona, Spain) at a dose of 400mg/12h during 5 complete days before ET. Progesterone and estradiol supplementation continued if pregnancy occurred and until week 12 of gestation.
Natural cycles (n: 201) were performed after spontaneous menses to detect the dominant follicle. Once the dominant follicle had a mean diameter of 17mm, and the endometrial thickness was at least 6mm and triple lined, hCG (Ovitrelle® 250μg; Merck, Madrid, Spain) was administered subcutaneously. Seven days later, embryos were warmed and transferred. Luteal phase support began 5 days before ET (embryo age+0 days) by administering micronized vaginal progesterone at a dose of 200mg/12h daily (Utrogestan®, 200mg; Seid, Barcelona, Spain) until week 5 of gestation.
The primary outcome of this study was clinical pregnancy rate (CPR), defined as the presence of a gestational sac with foetal heart beat observed by ultrasound determination. Secondary end-points included implantation rate (IR), calculated as the number of intrauterine gestational sacs observed by transvaginal ultrasound divided by the number of transferred embryos. And miscarriage rate (MR), which refers to the number of pregnancies lost before week 12 of gestation divided by the number of patients with positive β-hCG
All the procedures were approved by an institutional review board (1704-MAD-025-MC) in IVI Madrid and complied with the Spanish law on assisted reproductive technologies (14/2006).
Statistical analysisAll the calculations were done on a “per embryo transfer” basis. Values were expressed as means with standard deviations (SDs). One-way analysis of variance was applied for continuous variables and chi-squared test for categorical ones. All the data were analyzed by SPPS 13 (IBM Corporation, NY, USA), and p value<0.05 was considered statistically significant.
Considering the sample size of the study, the statistical power to detect a 5% improvement in a unilateral test was 80.4%, with a 95% significance level.
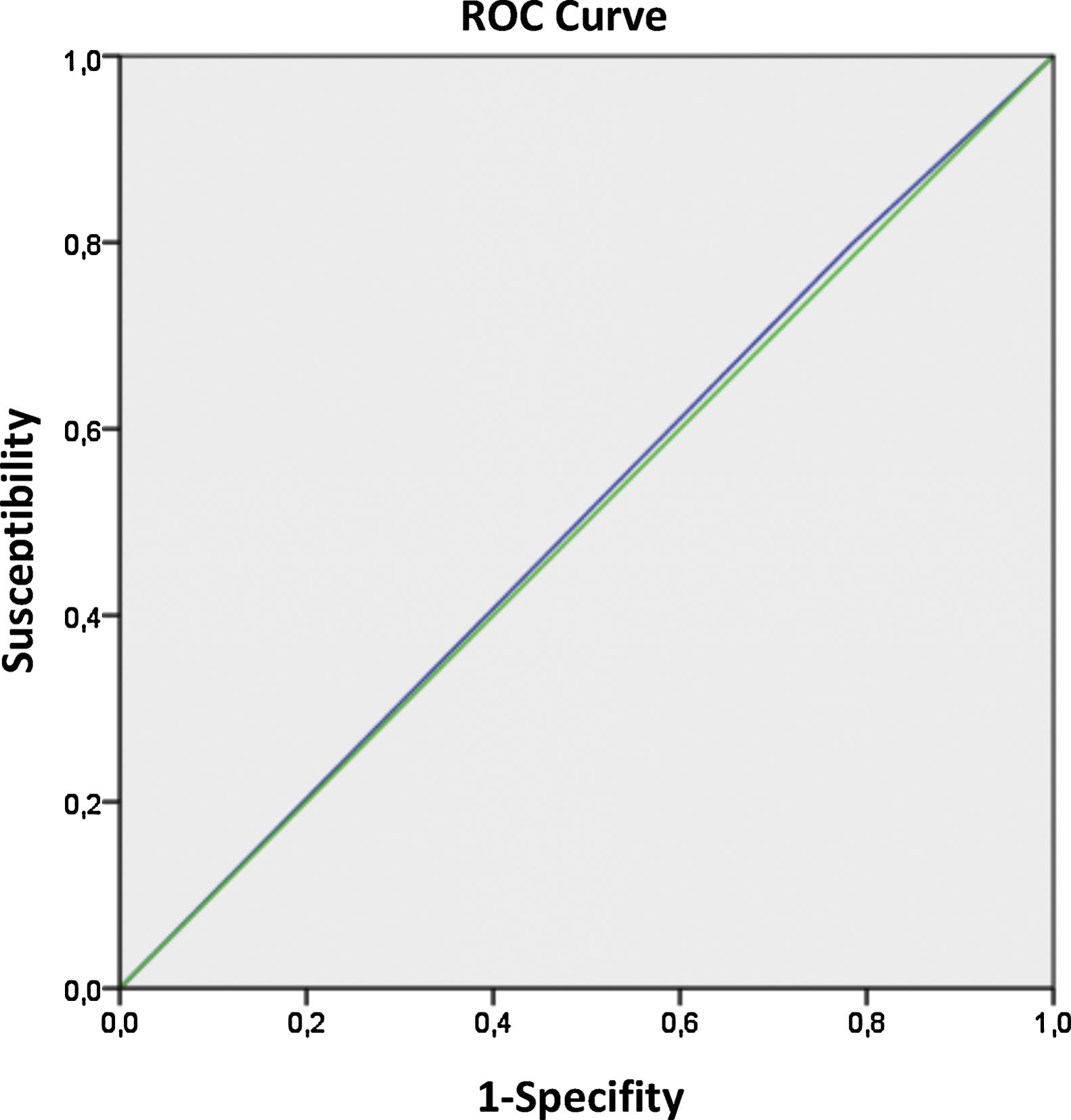
The odds ratio (OR) of all the variables generated for pregnancy were expressed in terms of 95% confidence intervals (CI). Multivariate logistic regression was performed to quantify the effect of the different variables on pregnancy. Furthermore, a receiver operating characteristic (ROC) curve was used to test the predictive value of “time to FET” and was included in the model compared to the CPR. The receiver operating characteristic (ROC) analysis provided values for the area under the curve (AUC), which were between 0.5 and 1.0, and can be interpreted as the measurement of the model's global classification ability.
ResultsA total of 913 patients underwent FET either in an artificial cycle (n=712) or natural one (n=201) FET occurred in any of the 13 clinics of our group in Spain.
Results were compared according to the first FET between patients whose transfer took place immediately after ovarian stimulation (n=198) and those who did it afterwards (n=715).
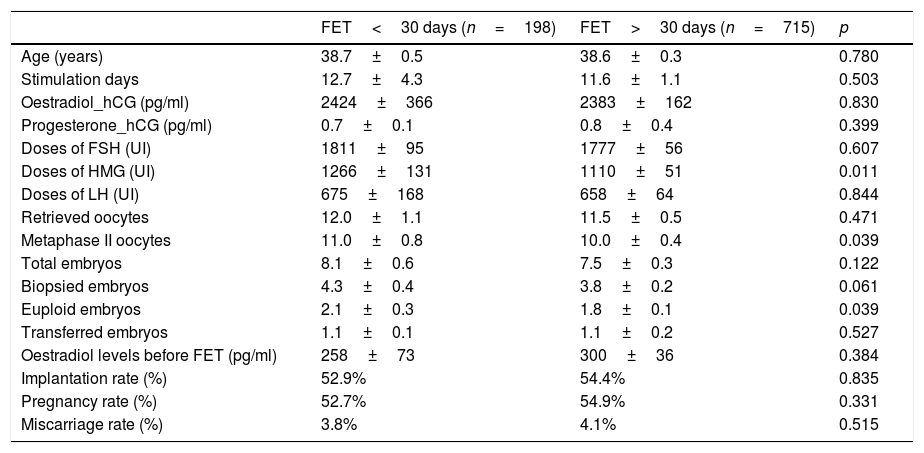
Baseline characteristics were similar in both groups (Table 1). Statistical differences were only observed in HMG doses and number of euploid embryos. There was no significant difference in cumulative pregnancy rate (CPR), implantation rate (IR) and miscarriage rate (MR) between both groups.
Baseline characteristics.
| FET<30 days (n=198) | FET>30 days (n=715) | p | |
|---|---|---|---|
| Age (years) | 38.7±0.5 | 38.6±0.3 | 0.780 |
| Stimulation days | 12.7±4.3 | 11.6±1.1 | 0.503 |
| Oestradiol_hCG (pg/ml) | 2424±366 | 2383±162 | 0.830 |
| Progesterone_hCG (pg/ml) | 0.7±0.1 | 0.8±0.4 | 0.399 |
| Doses of FSH (UI) | 1811±95 | 1777±56 | 0.607 |
| Doses of HMG (UI) | 1266±131 | 1110±51 | 0.011 |
| Doses of LH (UI) | 675±168 | 658±64 | 0.844 |
| Retrieved oocytes | 12.0±1.1 | 11.5±0.5 | 0.471 |
| Metaphase II oocytes | 11.0±0.8 | 10.0±0.4 | 0.039 |
| Total embryos | 8.1±0.6 | 7.5±0.3 | 0.122 |
| Biopsied embryos | 4.3±0.4 | 3.8±0.2 | 0.061 |
| Euploid embryos | 2.1±0.3 | 1.8±0.1 | 0.039 |
| Transferred embryos | 1.1±0.1 | 1.1±0.2 | 0.527 |
| Oestradiol levels before FET (pg/ml) | 258±73 | 300±36 | 0.384 |
| Implantation rate (%) | 52.9% | 54.4% | 0.835 |
| Pregnancy rate (%) | 52.7% | 54.9% | 0.331 |
| Miscarriage rate (%) | 3.8% | 4.1% | 0.515 |
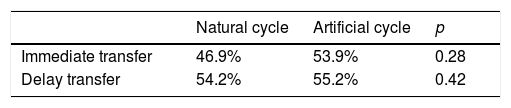
When analyze the results based on the type of endometrial preparation, natural or artificial cycles, we didn’t find differences between both (Table 2). The pregnancy rates in NC were 46.9% in the group of immediate transfer vs 54.8% in the group of delay transfer (p: 0.284); and in Artificial cycles, the PR were 53.9% vs 55.2% (p: 0.429). There were no differences based in the type of cycle used.
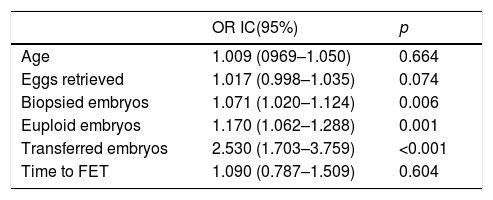
In order to assess if the CPR for FET remained unaltered after adjusting for the measured confounding factors, a mixed-effects multivariate regression analysis was performed (Table 3). For the CPR, the number of biopsied embryos, euploid embryos and transferred embryos significantly affected the probability of getting pregnant, which seemed normal in a PGT-A cycle. However, when analyzing the effect of the time to perform the FET, this was not significantly relevant.
Multivariate logistic regression to quantify the potential effect of different variables on pregnancy rate was performed; the odds ratio (OR) were expressed considering a 95% confidence interval.
| OR IC(95%) | p | |
|---|---|---|
| Age | 1.009 (0969–1.050) | 0.664 |
| Eggs retrieved | 1.017 (0.998–1.035) | 0.074 |
| Biopsied embryos | 1.071 (1.020–1.124) | 0.006 |
| Euploid embryos | 1.170 (1.062–1.288) | 0.001 |
| Transferred embryos | 2.530 (1.703–3.759) | <0.001 |
| Time to FET | 1.090 (0.787–1.509) | 0.604 |
Finally, the predictive value of the time to FET regarding the CPR was analyzed by a ROC curve (Fig. 1). The AUC was 0.507 (95%CI: 0.469–0.545), which indicates that the area under curve (AUC) analysis for this parameter was not conclusive, and the time that wait for the transfer didn’t affect the IR.
DiscussionFETs are frequently postponed (besides the necessary wait for genetic results) in an attempt to minimize effects derived from recent ovarian simulations on window implantation (Miravet-Valenciano et al., 2015). Although this empirical decision may be well intended, the elective deferral of FETs, with no proved clinical benefit, may unnecessarily frustrate couples who wish to get pregnant quickly. In this study we proved that there are no differences in CPR, IR and MR between patients that wait to have a FET versus those that don’t. Therefore, in our opinion, postponing FETs, would only represent a potential source of stress to the patients due to discontinuation of the treatment and an increased time to pregnancy.
There is no doubt that the percentage of “freeze-all” cycles has increased mainly due to: prevention of ovarian hyperstimulation syndrome (OHSS), an increase in genetic screening at the blastocyst stage, which forces clinics to freeze all embryos while awaiting genetic results (Garcia-Herrero et al., 2016), cases of high progesterone levels at triggering and women with implantation failure who may present a displaced window of implantation (Miravet-Valenciano et al., 2015); Given all these indications, the “freeze-all” (Shapiro et al., 2011) strategy is common practice these days. Therefore, the question whether is it necessary or not to wait to schedule a future FET is worth answering.
There are 3 studies that have evaluated the impact of the time of the first FET on clinical outcomes. Two of them (Santos-Ribeiro et al., 2016; Ozgur et al., 2018) analyzed it after patients having a failed fresh embryo transfer. The first one, with a “good sample size” reported benefits for the immediate FET group versus the delayed one while the second one, included over 1000 patients, showed no benefit at all.
The third one, a retrospective study based on 333 FETs, analyzed the same question but with patients undergoing elective freeze-all cycles, an approach that the authors considered crucial taking in consideration that the luteal phase is considered to be substantially reduced after GnRH agonist triggering (Beckers et al., 2003; Acevedo et al., 2006) thus dispelling the empirical notion that an artificially shortened luteal phase would have a negative impact on immediate FETs (Santos-Ribeiro et al., 2016; Lattes et al., 2017).
Our study, compared to this last one presents a similar rationale considering that patients undergoing genetic screening do electively freeze-all their embryos, although this indication was not included in Santos Ribeiro study (Santos-Ribeiro et al., 2016).
Finally, the regression model and the data that derive from the ROC curve confirm that the time to perform ET does not influence the chances of getting pregnant. So we assume that ovarian stimulation does not affect endometrial receptivity, regardless of the stimulation protocol with an antagonist protocol, ovulation triggering with GnRHa or endometrial preparation, most cycles with an artificial protocol and oestrogens, and less with a natural cycle. These facts allow patients to choose when they wish to accomplish their FET when it is most convenient for them.
It is important to believe in the future of our patients and to plan strategies that maximize success. In this context, the impact of psychosocial aspects should be taken in account (among many other things). It is important to identify possible causes of burden across patients, treatments and clinic domains, and to develop tailored interventions that can be easily included in the routine practice of a fertility clinic. In our group, and until quite recently, doctors were unaware about how long it was advisable to wait after oocyte retrieval before performing FET. However, the results of this study demonstrate that it is not necessary to delay FET to obtain good clinical outcomes, and this situation will relieve negative psychological impacts on couples. The strategy of cutting waiting times should imply better treatment experiences for patients, improved and adequate time planning, and lower stress alive (Aarts et al., 2012; Van Empel et al., 2011).
To the best of our knowledge, we have evaluated the largest data set by analysing the effect of the first FET timing of euploid embryos in PGT-A cycles, which reinforces our study. It is also important to emphasize the relevance of these results, especially if we consider that couples are increasingly delaying childbearing with the consequent higher demand of pre-implantation genetic diagnosis procedures. Apart from waiting, it is important to mention that these patients also suffer from anxiety from the genetic test itself. In short, performing FET in the immediate subsequent cycle favours them because we diminish their anxiety. The retrospective nature of this study is a limitation as not all risk factors may have been identified and subsequently recorded. Another limitation is the difference between the number of FET performed by natural or artificial cycle, is big (201 vs 712), but although there weren’t differences between them. Measuring risk factors and outcomes in a complete database may not be consistent or accurate enough. Therefore, only association, and not causation, can be inferred from the results.
In conclusion, performing the FET of euploid embryos immediately after a freeze-all cycle does not vary significantly from delaying it in terms of pregnancy rates, but improves the IVF experience as it minimizes patient treatment and clinic sources of burden that can have a negative psychological impact on IVF couples.
FundingThe present investigation has not received specific aid from public sector agencies, commercial sector or non-profit organizations.
Conflict of interestNo conflict of interest exists.