Fertilization failure or low fertilization rate after ICSI is around 1–3% in IVF treatments. Several strategies have been studied in order to bypass the lack of activation. The aim of this study is to evaluate embryo morphokinetics and reproductive outcomes after intracytoplasmic sperm injection (ICSI) with assisted oocyte activation (AOA) using a calcium ionophore (CaI) in patients with previous fertilization failure or low fertilization rate (under 30%) and severe male factor.
MethodsMulticentric retrospective cohort study including 70 patients with fertilization failure or low fertilization rate and severe male factor (sperm concentration under 1million/mL) who underwent ICSI with CaI (756 oocytes), and 76 patients with severe male factor without previous fertilization failure who had standard ICSI (748 oocytes) between January 2011 and December 2016.
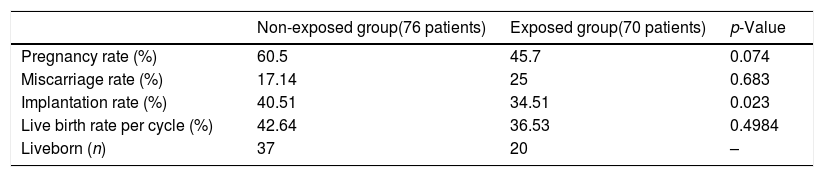
ResultsCaI Exposed and non-exposed groups differed significantly for normal fertilization rates, pronuclear disappearance timing (tPNf), time to 4cells stage, multinucleation at the 2- and 4-cell stages, and direct cleavage events. Implantation rate was higher in the non-exposed group (p=0.023). Other morphokinetic variables were similar between groups. Pregnancy (higher in the non exposed group), abortion, and live birth rates, were also not statistically different among groups.
ConclusionsAlthough the fertilization and implantation rates were higher in the non-exposed group, ICSI-CaI was associated with an increased fertilization rate compared to patient previous attempts and thus with increased pregnancy chances. Because of the specific patient populations involved (patients with severe male factor with or without previous fertilization failure), the results might not generalize for patients with different etiologies.
Trial Registration: 1506-VLC-045-MM.
El fracaso de la fertilización o la baja tasa de fertilización después de la ICSI es de alrededor del 1 al 3% en los tratamientos de FIV. Se han estudiado varias estrategias para evitar la falta de activación. El objetivo de este estudio es evaluar la morfocinética del embrión y los resultados reproductivos después de la inyección intracitoplasmática de espermatozoides (ICSI) con activación asistida de ovocitos (AOA) mediante un ionóforo de calcio (CaI) en pacientes con fracaso previo de fertilización o baja tasa de fertilización (menos del 30%) y factor masculino grave.
MétodosEstudio multicéntrico, retrospectivo, de cohorte, que incluye 70 pacientes con fracaso de la fecundación o baja tasa de fecundación y factor masculino grave (concentración de espermatozoides inferior a 1 millón/mL) que se sometieron a ICSI con CaI (756 ovocitos) y 76 pacientes con factor masculino grave sin fracaso previo de la fecundación que tuvieron ICSI estándar (748 ovocitos) entre enero de 2011 y diciembre de 2016.
ResultadosLos grupos expuestos y no expuestos al CaI difirieron significativamente en cuanto a las tasas normales de fertilización, el tiempo de desaparición pronuclear (tPNf), el tiempo hasta la etapa de 4 células, la multinucleación en las etapas de 2 y 4 células y los eventos de división directa. La tasa de implantación fue mayor en el grupo no expuesto (p = 0,023). Otras variables morfocinéticas fueron similares entre los grupos. Las tasas de embarazo (más altas en el grupo no expuesto), de aborto y de nacidos vivos tampoco fueron estadísticamente diferentes entre los grupos.
ConclusionesAunque las tasas de fertilización e implantación fueron más altas en el grupo no expuesto, el ICSI-CaI se asoció con una mayor tasa de fertilización en comparación con los intentos anteriores de la paciente y, por lo tanto, con mayores posibilidades de embarazo. Debido a las poblaciones de pacientes específicamente implicadas (pacientes con factor masculino severo con o sin fracaso previo de fertilización), es posible que los resultados no se puedan generalizar a pacientes con diferentes etiologías.
Registro de ensayos: 1506-VLC-045-MM.
Although intracytoplasmic sperm injection (ICSI) has a high success rate in in vitro fertilization (IVF) treatments, around 1–3% of cycles have total fertilization failure. A lack of oocyte maturation (nuclear and cytoplasmic), deficiencies in the main sperm–oocyte interaction events, and technical failures during the ICSI procedure have been proposed as reasons to explain fertilization failure (Swain and Pool, 2010; Sabetian and Shamsir, 2017; Duncan et al., 2016; Yeste et al., 2016). Calcium signals have a central role in fertilization process in all species. Its role as a second messenger, in meotic arrest resumption, and in the initiation of the Calcium-induced calcium release (CICR) process have been documented (Anifandis et al., 2019). Although major calcium reservoir is in the oocyte, and most of its effect happens in the female gamete, CICR and calcium triggering oscillations which launch fertilization process, depend on spermatozoa (Swann et al., 2001), what do not allow to clearly point out which is the fertilization failure cause, oocyte or spermatozoa.
Artificial oocyte activation (AOA) has been used in assisted reproductive technology for more than 15 years to address fertilization failure (Nasr-Esfahani et al., 2010; Vanden Meerschaut et al., 2014). Chemical AOA, the most common technique, relies on calcium ionophores (CaIs) such as calcimycin (A23187) and ionomycin. These molecules increase the intracytoplasmic calcium concentration and facilitate cellular calcium influx and intracellular calcium release, which increases the membrane calcium permeability of the oocyte (Anifandis et al., 2019).
Patients with a previous low fertilization rate (under 30%) or total fertilization failure may benefit from AOA (Heindryckx et al., 2005, 2008; Montag et al., 2012). A meta-analysis showed statistically significant improvement in fertilization, cleavage, blastulation, and implantation rates, as well as overall pregnancy and live birth rates after ICSI with AOA (ICSI-AOA), indicating the usefulness of this strategy for some patients (Murugesu et al., 2017).
Our aim was to assess embryo morphokinetics, oocyte fertilization, and clinical outcomes of patients with severe male factor and prior fertilization failure or low fertilization rate who underwent ICSI with AOA. We compared this group with patients who had severe male factor but no prior fertilization failure and who had ICSI without AOA.
Materials and methodsThis multicenter retrospective research was conducted at the Instituto Valenciano de Infertilidad (IVIRMA) in Vigo, Bilbao, and Valencia clinics, between January 2011 and December 2016, following in all the three clinics the same protocols, and employing for microinjection and embryo incubation the same equipment which is detailed below. We compared two patient groups: those with severe male factor and a fertilization failure or a low fertilization rate (under 30%) in a previous ICSI treatment who underwent ICSI-AOA (exposed group), and patients with a sperm concentration under 1million/mL and no previous fertilization failure or low fertilization rate and who underwent standard ICSI.
This study was approved by the institutional review board, which regulates and approves database analysis and clinical IVF procedures for research at IVI clinics (ref. 1506-VLC-045-MM). It also complies with the Spanish law governing assisted reproductive technologies (14/2006).
Patients received precise written information about the ICSI-AOA procedure and signed written consent for this experimental treatment, which was also approved by the institutional review board before starting ovarian stimulation. Informed consent was obtained from all individual participants included in the study. Detailed and clear information about the effects of CaIs (ionomycin from Streptomyces conglobatus; Sigma-Aldrich) on oocytes and the safety and cumulative experience with this product was provided. Patients were also informed about the limitations and unknown possible negative consequences of assisted oocyte activation, as previously suggested (Van Blerkom et al., 2015).
The time-lapse technology employed has an European Conformity Certificate (CE-certified) and thus meets the health and safety requirements for equipment in the European Union. In our study, it was used for its approved purposes. The CE certificate (#DGM-673) endorses the quality of the system from Vitrolife in terms of its manufacture and final inspection of the IVF incubators and accessories related to class II (including IVF incubators and the plates used for such incubators). The production, installation, and servicing of IVF incubators and accessories from Vitrolife are likewise certified (certificate #DGM-672).
Ovarian stimulation, oocyte retrieval, and oocyte decumulationGonadotropin-releasing hormone (GnRH) antagonist protocols were employed for controlled ovarian hyperstimulation, as previously described (Bosch et al., 2010). Briefly, once ovarian quiescence was confirmed by ultrasonography, ovarian stimulation with gonadotropins began, and the GnRH antagonist (Orgalutran, 0.25mg; Merck Sharp & Dohme Limited, Hertfordshire, UK) was added until the leading follicle was 14mm wide. Once three or more follicles were 17mm in diameter, a 0.2mg dose of GnRH agonist (0.1mg triptorelin; Decapeptyl; Ipsen Pharma) was administered for final oocyte maturation and ovulation triggering. Transvaginal oocyte retrieval was scheduled 36hours later, follicles were aspirated and oocytes were isolated and washed in Global w/HEPES (LifeGlobal). Afterwards, they were cultured in Global for fertilization (LifeGlobal) at 37°C, 6% CO2, and 20% O2 for 3h. Then, oocyte denudation was carried out by mechanical pipetting in a Global hyaluronidase (80IU/mL; LifeGlobal) and Global w/HEPES, making oocytes pass through denuding pipettes of descending diameter (from 300 to 145μm). Once granulosa cells were removed, oocyte maturity was confirmed under the inverted microscope, and only metaphase II oocytes were selected for sperm microinjection. Patients with less than four oocytes collected after oocyte retrieval were not included in this study.
ICSI-AOA and standard ICSI procedures and embryo cultureFour hours after oocyte collection, ICSI was carried out under an Olympus IX71 microscope.
For patients in the exposed group, the ICSI set-up was as follows. The CaI was diluted in cell culture-tested dimethyl sulfoxide (Sigma-Aldrich) to a 1mmol/L concentration. Then, it was diluted again with HEPES (CaI-HEPES drop) at 37°C and FERT media previously incubated at 37°C and 6% CO2 (CaI-FERT drop) to a 10μmol/L concentration, respectively. Each ICSI dish had a polyvinylpyrrolidone (PVP) (LifeGlobal) drop, a CaI drop, and a HEPES drop. Separately, a dish with CaI-FERT drops with underlying high-density oil was also set up. Oocytes were microinjected with spermatozoa inseminated in PVP (LifeGlobal). Approximately 0.01 million spermatozoa were inseminated in PVP, selected according to morphological criteria, and then immobilized by fracturing the flagellum with the ICSI needle. The chosen spermatozoa were later moved one by one to the CaI-HEPES drop, released in it, and then aspirated into the ICSI needle with about 5μL of solution.
Afterwards, sperm microinjection was carried out in the HEPES drop. A maximum of six oocytes were microinjected per dish in less than 10min. Once ICSI was carried out, oocytes were incubated in the dish containing CaI-FERT drops at 37°C, 6% CO2, and 20% O2 atmosphere for 10min. Then, microinjected oocytes were washed up in clean Global (LifeGlobal) media drops and placed in a pre-equilibrated culture embryo slides (Vitrolife). These slides have 12 straight-sided wells within a central depression. Each well was filled with 20μL of Global media, and the central depression was covered with a 1.2mL overlay of high-density oil (LifeGlobal) to prevent media evaporation, and incubated overnight at a 37°C, 6% CO2, and 5% O2 atmosphere. After pre-equilibration all the air bubbles were removed before the microinjected oocytes were individually transferred to each well and incubated in a time-lapse monitoring incubator (Embryoscope, Vitrolife, Sweden) in a 37°C, 6% CO2, and 5% O2 atmosphere. Images were captured at seven different equidistant focal planes every twenty minutes for each embryo, and recorded a 1280×1024 digital images in the EmbryoViewer, whereas they were analyzed later.
Standard ICSI was carried out in the non-exposed group, without any contact between gametes and CaI solutions. Once oocytes were microinjected, they were also cultured in the Embryoscope incubator, as explained above.
All ICSI-AOA and ICSI procedures were carried out by highly experienced senior embryologists in the three clinics.
Embryo scoring and embryo transferThe time of ICSI was annotated as the mid-time point from when sperm injection begins and ends for the cohort of oocytes (Ciray et al., 2014). Fertilization and embryo morphology were assessed according to the recommendations by Basile et al. (2015) using morphokinetics criteria.
Embryo selection for embryo transfer or cryopreservation was performed on day 3 or day 5 depending on embryo cohort characteristics and gynecological criteria. In the majority of treatments the number of embryos transferred was two. Elective single transfer was carried out when applicable. During this study, no triple embryo transference was carried out. Supernumerary embryos were vitrified for further frozen embryo transfers (Cobo et al., 2010).
Morphokinetics annotationsAll morphokinetic events were annotated according to Ciray et al.’s recommendations (2014), using EmbryoViewer software (Vitrolife, Sweden). Extrusion of polar body (tPB), pronuclei fade-in (tPNa) and fade-out (tPNf), the exact time of each blastomere cleavage (tn), multinucleation at the 2- and 4-cell stages, and direct cleavage events were recorded, as were some auto-calculated parameters such as the length of the S-phase (PNf-PNa), CC2 (t3-t2), and cc3 (t5-t3).
Clinical outcome assessmentβ-Human chorionic gonadotropin in peripherical blood was measured 13 days after embryo transference. Clinical pregnancy was confirmed when a gestational sac with a positive fetal heartbeat was detected by ultrasonography at 7 weeks of pregnancy.
Patients attended to our clinics until 12 weeks of pregnancy for the first pregnancy follow-up. Then, they continued follow-up appointments with their obstetrician.
Miscarriage rate was analyzed including early (before week 12 of pregnancy) and late abortion (after week 12). We registered early malformations, anembryonic pregnancies, or aneuplodies when possible. If we could not collect information from clinical records, we took advantage of telephone follow-up to ask for further details about miscarriages.
Live birth rate (birth of a baby alive after week 35), and abortion rate were also assessed. Live birth follow-up was carried out by phone calls, in which the following questions data were requested: delivery date, type of delivery, newborn length and weight, problems during the pregnancy, bleeding during pregnancy, and drug intake.
Statistical analysisPatients were split into two groups, those whose oocytes were injected with CaI (exposed group) and those whose oocytes were not (non-exposed group). Kolmogorov–Smirnov test was applied to test the normal distribution of data. ANOVA was employed to test for statistically significant differences in the average times for the continuous morphokinetics events. All variables studied in this research were normally distributed. Chi square tests were employed to compare categorical variables. The analysis included all microinjected oocytes.
Statistical analysis was performed using the Statistical Package for the Social Sciences 24 (SPSS, Chicago, IL).
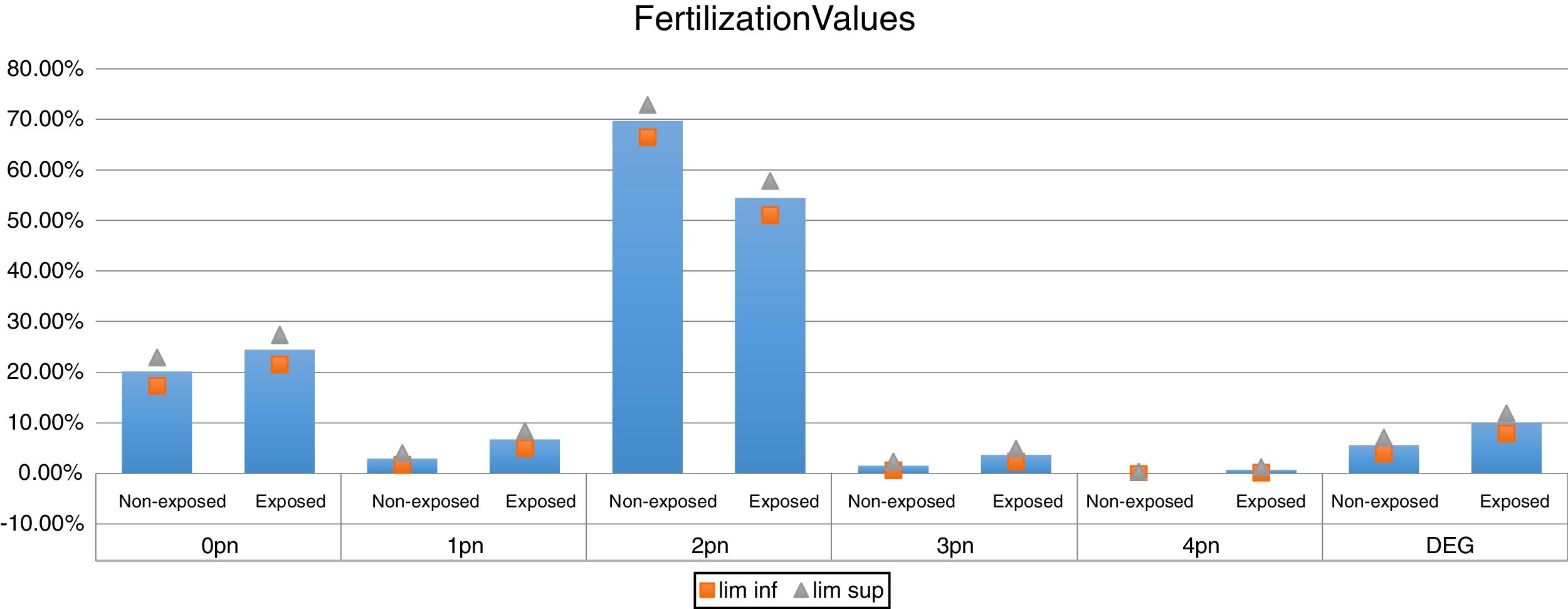
ResultsA total of 1504 microinjected oocytes were analyzed. Of these, 756 were from 70 patients in the exposed group and 748 from 76 in the non-exposed group. The mean age of patients in the exposed group was 36.8 years; and 35.4 years in the non-exposed group (no significant difference). Patients in the exposed group had a mean of 1.55 previous ICSI cycles with a mean fertilization rate of 11.1%. 25 patients (35.71%) in the exposed group had at least one previous total fertilization failure. The fertilization rate was 54.4% (95% confidence interval (CI), 51.08%–57.8%) in the exposed vs 69.7% (95% CI, 66.5%–72.9%) in the non-exposed group. Seven patients had a total fertilization failure after ICSI with CaI. These couples, also had had a previous total fertilization failure, which could not be bypassed by the AOA.
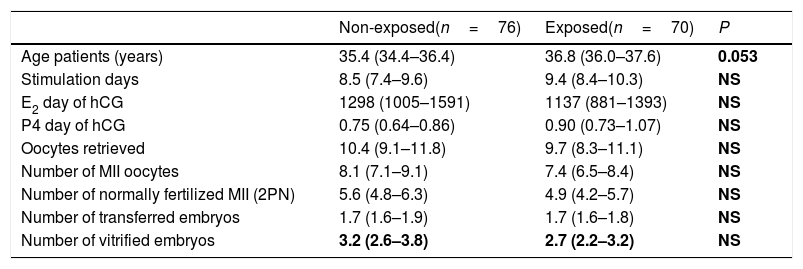
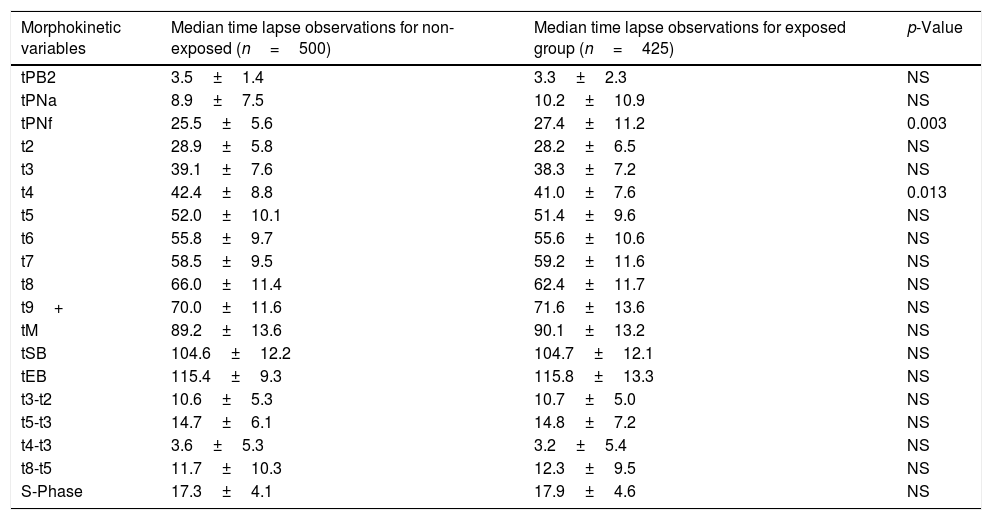
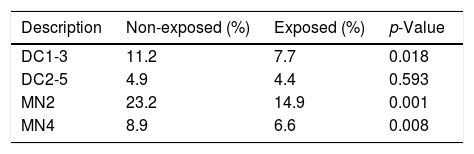
Table 1 shows the demographic data, which were comparable between groups. Fertilization values are shown in Fig. 1. Embryo morphokinetics were statistically different for tPNf and t4 (Table 2). Table 3 shows the incidence of direct cleavage and embryo multinucleation. Clinical pregnancy and implantation rate (p=0.023) were higher in the non-exposed group (Table 4).
Baseline characteristics by group.
| Non-exposed(n=76) | Exposed(n=70) | P | |
|---|---|---|---|
| Age patients (years) | 35.4 (34.4–36.4) | 36.8 (36.0–37.6) | 0.053 |
| Stimulation days | 8.5 (7.4–9.6) | 9.4 (8.4–10.3) | NS |
| E2 day of hCG | 1298 (1005–1591) | 1137 (881–1393) | NS |
| P4 day of hCG | 0.75 (0.64–0.86) | 0.90 (0.73–1.07) | NS |
| Oocytes retrieved | 10.4 (9.1–11.8) | 9.7 (8.3–11.1) | NS |
| Number of MII oocytes | 8.1 (7.1–9.1) | 7.4 (6.5–8.4) | NS |
| Number of normally fertilized MII (2PN) | 5.6 (4.8–6.3) | 4.9 (4.2–5.7) | NS |
| Number of transferred embryos | 1.7 (1.6–1.9) | 1.7 (1.6–1.8) | NS |
| Number of vitrified embryos | 3.2 (2.6–3.8) | 2.7 (2.2–3.2) | NS |
Mean timings from embryos analyzed by time-lapse.
| Morphokinetic variables | Median time lapse observations for non-exposed (n=500) | Median time lapse observations for exposed group (n=425) | p-Value |
|---|---|---|---|
| tPB2 | 3.5±1.4 | 3.3±2.3 | NS |
| tPNa | 8.9±7.5 | 10.2±10.9 | NS |
| tPNf | 25.5±5.6 | 27.4±11.2 | 0.003 |
| t2 | 28.9±5.8 | 28.2±6.5 | NS |
| t3 | 39.1±7.6 | 38.3±7.2 | NS |
| t4 | 42.4±8.8 | 41.0±7.6 | 0.013 |
| t5 | 52.0±10.1 | 51.4±9.6 | NS |
| t6 | 55.8±9.7 | 55.6±10.6 | NS |
| t7 | 58.5±9.5 | 59.2±11.6 | NS |
| t8 | 66.0±11.4 | 62.4±11.7 | NS |
| t9+ | 70.0±11.6 | 71.6±13.6 | NS |
| tM | 89.2±13.6 | 90.1±13.2 | NS |
| tSB | 104.6±12.2 | 104.7±12.1 | NS |
| tEB | 115.4±9.3 | 115.8±13.3 | NS |
| t3-t2 | 10.6±5.3 | 10.7±5.0 | NS |
| t5-t3 | 14.7±6.1 | 14.8±7.2 | NS |
| t4-t3 | 3.6±5.3 | 3.2±5.4 | NS |
| t8-t5 | 11.7±10.3 | 12.3±9.5 | NS |
| S-Phase | 17.3±4.1 | 17.9±4.6 | NS |
Values are mean±SD time in hours. NS=not statistically significant.
tPB2: extrusion of second polar body, tPNa: time of pronuclear appearance; tPNf: time of pronuclear fading; tn: time when embryo cleaves into n cells; tM: time of morula; tSB: time of blastulation start; tEB: time of blastocyst fully expanded; s-phase: tPNf-tPNa.
Frequency of embryonic features of embryos.
| Description | Non-exposed (%) | Exposed (%) | p-Value |
|---|---|---|---|
| DC1-3 | 11.2 | 7.7 | 0.018 |
| DC2-5 | 4.9 | 4.4 | 0.593 |
| MN2 | 23.2 | 14.9 | 0.001 |
| MN4 | 8.9 | 6.6 | 0.008 |
DC1-3: Direct cleavage from one cell to three cells.
DC2-5: Direct cleavage from two cells to five cells.
MN2: Multinucleation at 2 cells stage.
MN4: Multinucleation at 4 cells stage.
20 babies were born in the exposed group with an average weight of 2983.23g (95% CI, 2709.52–3256.94) and an average length of 49.04cm (95% CI, 47.83–50.26). 37 were born in the non-exposed group with an average weight of 2826.56g (95% CI, 2568.96–3084.18) and an average length of 48.34cm (95% CI, 47.30–49.38).
None of the parameters analyzed differed among clinics.
DiscussionICSI-AOA improved reproductive outcomes for patients with previous fertilization failure, although patients in the non-exposed had better global results. CaI had an effect on embryo morphokinetic, as only some early events (tPNf and t4) were statistically different between groups. Of note, multinucleation at 2 and 4cells and direct cleavage events seem to be diminished in the non-exposed group.
Normal fertilization was significantly higher in the non-exposed group at 69.7% (95% CI, 66.5%–72.9%) vs. 54.4% (95% CI, 51.1%–57.8%). These data must be considered in the context of the previous fertilization failure among patients in the exposed group who underwent an average 1.55 ICSI cycles with a mean fertilization rate of 11.1%. After ICSI-AOA, the normal fertilization rate increased to 54%. Thus, for these patients, AOA may help them to achieve a greater fertilization rate. This improvement, in turn, would increase the likelihood of their having a better embryo selection and better pregnancy chances.
Fertilization failure and the proportion of abnormally fertilized and degenerated oocytes were higher in the exposed group, but not statistically significant. In their study, Capalbo et al. (2016) evaluated the effect of CaI on chromosome segregation during second meiotic cleavage. They found that CaI may lead to discoordination between telophase II and second polar body extrusion, which could trigger retention of both chromosome sets in the oocyte and explain the greater abnormally fertilized oocyte rates. In some patients, this extra pronuclei became apparent during the period of pronuclei checking proposed by the Alpha/ESHRE Special Interest Group of Embryology (2011). This possibility reinforces the use of time-lapse monitoring incubators, which allow embryologists to check the second polar body extrusion, appearance of pronuclei, or unusually timed observations of fertilized oocytes. Being able to mark these events helps clinicians avoid transference of abnormally fertilized oocytes after ICSI-AOA. The higher proportion of degenerated oocytes could be related to a worse oocyte quality, which could explain previous fertilization failures.
Our study offers a detailed morphokinetics analysis that highlights the fact that CaI affects some early embryonic events, mainly tPNf and t4. We found no statistically significant differences between groups in any other morphokinetic events or in the auto-calculated variable S-phase, which is considered as the time that two sets of DNA take to merge and duplicate in the first cell cycle (Aguilar et al., 2014).
Calcium has key roles in fertilization. It leads the oocyte to exit meiotic arrest and regulates mitogen-activated protein kinase in promoting pronuclei formation. These crucial roles may explain the delay in the timing of pronuclear events in the exposed group. Calcium is also important in early cleavage events (Swanson et al., 1997; Goud et al., 1999; Berridge et al., 1998), so the most significant effect of a CaI would be expected at the early cleavage stages.
An algorithm that allows for improved embryo selection in patients with severe male factor also could be applied for those patients who undergo ICSI with CaI, but such an algorithm remains to be developed (Aparicio-Ruiz et al., 2018).
Statistically significant differences in blastomere multinucleation at the 2- and 4-cell stages were observed between groups. The events were more frequent in the exposed group and in the 2-cell stage vs the 4-cell stage in both groups. We recently assessed multinucleation at the second cell cycle in oocyte donation cycles and found that blastomere multinucleation seems to be more frequent at the 2-cell stage, and is reversible (Aguilar et al., 2016). Despite the greater frequency in the exposed group, this group also had more multinucleated blastomeres at the 2-cell stage, which also was reversible.
We suggest that the effect of CaI on mitogen-activated protein kinase and early fertilization is still active after the first mitosis, which could explain the increased multinucleation in the exposed group. In addition, Aurora kinase C is a component of the chromosomal passenger complex in human oocytes and blastomeres, along with the inner centrosomal proteins survivin and boralin. Some members of the Aurora kinase family can be modulated by calcium calmodulin binding (Santos et al., 2011), which also could lead to the kind of increased multinucleation observed in the exposed group.
Direct cleavage is not unusual in embryonic development. Embryos with these divisions have reduced implantation potential (Zhan et al., 2016; Cruz et al., 2012). Some authors have suggested sperm centriole status as a major contributor to direct cleavages (Schatten and Sun, 2009; Chatzimeletiou et al., 2007). However, maternal centrosomal proteins have a key role during spindle genesis, and any defects in oocyte maturity may influence the incidence of direct cleavage (Zhan et al., 2016). Here, we found a greater incidence of direct cleavage in the non-exposed group (p=0.018). It is possible that male factor could be directly related to this finding, along with a lack of calcium, which would have been bypassed by the AOA with CaI. Although this finding requires further investigation.
The number of embryos transferred was similar between the groups, 1.7 (1.6–1.9) vs. 1.7 (1.6–1.8) but pregnancy and implantation rates were higher in the non-exposed group (p=0.023 for implantation). As noted, patients from the exposed group had previous low fertilization rate or total fertilization failure, and their post-ICSI-AOA outcomes were much improved by comparison. Of interest, abortion rates were comparable between groups (p=0.683). All the miscarriages in the non-exposed group were early abortions. All but one in the exposed group was early. The exception was miscarriage of a twin pregnancy at around week 24, in which both fetuses died because of extreme prematurity. We did not identify the ploidy status in any of these cases.
After trying to contact all patients with ongoing pregnancy, we were unable to reach five in the non-exposed, group, so these cases were excluded from the analysis. Livebirth rate was higher in the non-exposed group at 42.64% vs 36.53%, although they were not statistically different, as well as the average weight and height of the newborns which did not differ statistically between groups either. We have not found an increased rate of congenital anomalies after ICSI with CaI. In addition, the abortion rate between groups was comparable, which agrees with previous results (Ebner et al., 2015). Nevertheless, this data must be handled with caution due to the low number of cases herein analyzed, for reaching conclusions on obstetric and perinatal information.
A recent randomized control trial evaluated the effect of ICSI-AOA with strontium chloride (SrCl2) and calcimycin compared with ICSI alone in patients with previous fertilization failure (Fawzy et al., 2018). This group observed improved clinical pregnancy, implantation, and live birth rates in patients who underwent ICSI-AOA, with calcimycin or SrCl2, compared with ICSI alone. Clinical pregnancy, implantation, and miscarriage rates in the calcimycin group were similar to our results for the exposed group in the current study. This agreement supports the idea that ICSI-AOA may benefit patients with previous fertilization failure. A recent study by Bonte et al. (2019), described the efficiency of AOA in a 17 years retrospective study in a serie of patients with total fertilization failure, or low fertilization rate, and their results are in line with ours, showing higher fertilization rate, and reproductive outcomes of those patients who were treated with AOA compared with their previous results.
There are several authors who had studied AOA in fertilization failure in recent years, but its use in assisted reproduction still remains controversial, and it should be handled with caution, albeit as far as we know, no damage in liveborn has been documented.
In conclusion, our study offers, to our knowledge, an analysis of the largest series of oocytes microinjected with ICSI-AOA and incubated in incubators with time-lapse monitoring system, compared to those of patients with similar sperm characteristics but without previous fertilization failure. Although we found statistically significant differences in some early cleavage event parameters (t4, tPnf, multinucleation at 2- and 4-cell stages, and direct cleavage events), the remaining morphokinetic parameters were comparable. This result may suggest that CaI does not affect embryonic developmental timing. AOA with CaI offers an alternative for patients with previous fertilization failure or low fertilization rate as in this study, they achieved reproductive outcomes similar to those of patients with severe male factor and normal fertilization history.
Ethics approvalIt is by the institutional review board, which regulates and approves database analysis and clinical IVF procedures for research at these clinics (ref. 1506-VLC-045-MM). It also complies with the Spanish law governing assisted reproductive technologies (14/2006).
Availability of data and materialsThe datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author's contributionsJA was involved in original conception of the manuscript, its design and drafting, collection of data. EM played a role in critical revision and drafting of the manuscript, interpretation of data. LA made substantial contributions to the acquisition and analysis of data. ZL took part in critical and grammatical revision of the article. JR played a role in the design of the work, critical revision. MM was involved in original conception, analysis and interpretation of data and gave final approval. All authors did participated in interpretation of data, and JA and EM were major contributors in writing the manuscript. All authors read and approved the final manuscript.
FundingNot applicable.
Conflict of interestsThe authors have no conflict of interest to disclosure.
The authors acknowledge the support of the embryologist staff from IVI Valencia, IVI Bilbao and IVI Vigo.