In the last decades we have experienced, a worldwide steep increment on the demand of assisted reproductive treatments (ART). Therefore, the necessity to improve the performance of ART has been crucial to increase the pregnancy outcomes. Although research has pronominally focused on identifying the proper ovarian stimulation improving condition in IVF laboratories, luteal phase has possibly been the field in which research has been unproportionally deficient. Despite the strong evidence on the importance of the luteal phase support in IVF fresh and frozen cycles, there is still a lack of homogeneity on the best route of progesterone (P4) administration, the dose, and the surveillance of the luteal phase, suggesting that future research may be needed in this field. Independently on the ovarian stimulation (OS) or the frozen embryo transfer (FET) techniques, mostly vaginal and parenteral P4 are prescribed nowadays. However, in recent years the oral and subcutaneous administration of P4 were introduced and demonstrated to be safe and as efficient as the other routes. At this time point, the decision on which P4 route and dose should be used, in a tailored patient-approach, is still controversial, especially considering the safety and the tolerability of the P4 treatment. The aim of this narrative review is to provide evidence on the latest innovation on the luteal phase surveillance and management and to describe the different treatment options for the luteal support, not only in fresh, but also in frozen embryo transfer cycles which demonstrate an exponential increase the last decade.
En las últimas décadas se ha producido un fuerte incremento de la demanda de tratamientos de reproducción asistida (TRA) en todo el mundo. Por lo tanto, la necesidad de mejorar el rendimiento de las TRA ha sido crucial para aumentar los resultados del embarazo. Aunque la investigación se ha centrado principalmente en la identificación de la estimulación ovárica adecuada para mejorar las condiciones en los laboratorios de FIV, la fase lútea ha sido posiblemente el campo en el que la investigación ha sido desproporcionadamente deficiente. A pesar de las sólidas evidencias sobre la importancia del soporte de la fase lútea en los ciclos de FIV en fresco y congelados, todavía existe una falta de homogeneidad sobre la mejor vía de administración de progesterona (P4), la dosis y la vigilancia de la fase lútea, lo que sugiere que pueden ser necesarias futuras investigaciones en este campo. Independientemente de las técnicas de estimulación ovárica (SO) o de transferencia de embriones congelados (FET), hoy en día se prescribe principalmente P4 vaginal y parenteral. Sin embargo, en los últimos años se ha introducido la administración oral y subcutánea de P4 y se ha demostrado que es segura y tan eficaz como las otras vías. En este momento, la decisión sobre qué vía y dosis de P4 debe utilizarse, en un enfoque adaptado al paciente, sigue siendo controvertida, especialmente si se tiene en cuenta la seguridad y la tolerabilidad del tratamiento con P4. El objetivo de esta revisión narrativa es proporcionar evidencia sobre las últimas innovaciones en la vigilancia y el manejo de la fase lútea y describir las diferentes opciones de tratamiento para el apoyo lúteo, no sólo en fresco, sino también en los ciclos de transferencia de embriones congelados que demuestran un aumento exponencial en la última década.
The luteal phase (LP), described as the second phase of the menstrual cycle, begins after the ovulation and goes on until the instauration of the pregnancy or, in case of failed conception, until the beginning of a new cycle, with the first day of the next menstrual bleeding. It is characterised by the switch of the steroidogenic activity of the ovaries centred more on the production of progesterone (P4). In nature, the leading follicle of the follicular phase (FP), becomes the corpus luteum (CL) in the LP. The CL is a transient ovarian gland of the LP and early pregnancy, capable of producing significant amount of P4, E2, androgens, growth factors, and non-steroid hormones. The production of P4 reaches maximum levels during mid-luteal phase with 40–60 nmol/L in a natural cycle (Soules et al., 1988). The steroidogenic capacity of the CL depends on a pulsatile and continuous LH secretion from the hypophysis. The produced P4 sees its main target in the uterus and specifically it interacts with the endometrium to start the process of decidualisation, being growth and coiling of the spiral arteries, secretory transformation of the glands and decidualisation of the stromal compartment (Gellersen and Brosens, 2003; Gellersen et al., 2007). This morphological and biochemical reprogramming of the endometrial stromal compartment is entirely dependent on the convergence of the cyclic adenosine monophosphate (c-AMP) and P4 signalling pathways that drives integrated changes at both the transcriptome and the proteome level. As a result, decidualising stromal cells acquire the unique ability to regulate trophoblast invasion, to resist inflammatory and oxidative insults, and to dampen local maternal immune responses (Gellersen et al., 2007).
In normo-ovulatory menstrual cycle, the CL works producing P4 until 9–11 days after ovulation. If the CL is not rescued by the production of hCG from the trophoblast, menstrual bleeding occurs within 14 days from ovulation. If implantation of an embryo occurs, the hCG produced by the placenta interacts with the LH receptors and maintain the luteal support until the placental production of P4 is fully established, around 6 weeks of gestation (Csapo et al., 1972).
Luteal phase deficiencyLuteal phase deficiency (LPD) is a condition where there is insufficient endogenous P4 for embryo implantation, which can be associated with subfertility, infertility, first trimester pregnancy loss, short menstrual cycle, and pre-menstrual spotting. LPD can be therefore defined as insufficient temporal LP duration, detected clinically if LP <10 days or as insufficient quantity of P4 produced, and is detected biochemically by serum P4 assessment (Practice Committees of the American Society for Reproductive Medicine and the Society for Reproductive Endocrinology and Infertility, 2021). LPD can be associated with different pathologic conditions that can alter the gonadotropin releasing hormone (GnRH) and LH pulpability, for instance hypothalamic amenorrhea, eating disorders, extreme weight conditions (obesity or significant weight loss) excessive exercise, stress, endocrinological disorders, and advanced reproductive age. However, it is widely accepted that the use of the ovarian stimulation in ART is one of the main causes of LPD (Fig. 1). Different hypotheses have been proposed to explain LPD in ovarian stimulation ART cycles: (1) removal of granulosa cells at oocyte retrieval, which could express a sudden drop in the P4; (2) hCG suppressing the pulsatile LH activity; (3) high E2 suppressing LH activity; (4) use of GnRH agonist or GnRH antagonist; (5) combination of these factors. Beckers et al. (2000) showed different P4 profiles in the luteal phase, in case of long agonist suppressed stimulations, ovulation induction with hCG and luteal support with or without P4.
Progesterone administrationProgesterone can be administered throughout multiple routes: vaginally, intramuscularly, rectally, orally, or subcutaneously. Table 1 shows the different type of P supplementation present in the market.
Different routes of P supplementation.
| Route | Composition | Dose/day |
|---|---|---|
| Vaginal | Gel | 90 mg natural Progesterone |
| Vaginal or Oral | Capsules | 600–800 mg micronised Progesterone |
| Vaginal | Tablets | 300–600 mg natural Progesterone |
| Oral | Tablets | 30 mg synthetic progestin (dydrogesterone) |
| IM | Vials | 50–100 mg micronised Progesterone |
| SC | Pre-filled syringes | 25 mg lyphilised Progesterone |
The most commonly used is the vaginal way, however the parenteral way (subcutaneous or intramuscular) is generally accepted in the clinical practice (Racca et al., 2020; Ramos et al., 2021) in some countries [e.g. USA using predominantly intramuscular P4 (IM P4) especially in FET cycles]. An important aspect to consider when talking about P4 is that the pharmacokinetics is dependent on the route of administration and consequently the serum concentration (Miles et al., 1994). In particular, the advantage of the vaginal P4 is undeniably the uterine first pass, meaning that although the circulating P4 levels remain quite low, the uterine concentration is way higher compared to the IM where the endometrial concentration is very low, and the circulating concentration very high (Miles et al., 1994). Table 2 summarises the main characteristics and pharmacodynamics of different types of P4.
Pharmacokinetics and side effects of P4.
| Form | Posology | Cmax(ng/mL)/T1/2(h) | Side effects | |
|---|---|---|---|---|
| Vaginal tablets 200 mg | Tablets | 3/day | 11.3/13.7 | Discharge, vaginal infections, vaginal discomfort, vaginal itching |
| Vaginal gel | Gel | 2/day | 10.5/25.9 | Perineal pain, bloating, cramps |
| IM | In Oil | Daily | 20/28 | Pain, local soreness, sterile abscess |
| SC | Acqueous | Daily | 57.8/13 | Bruising, oedema, pain |
| Oral | Tablets | 3/day | 2.1/5–7 h | Migraine, headache, nausea |
Ovarian stimulation (OS) takes place with the objective to increase the number of oocytes yield in a single cycle and therefore, increase the chance of pregnancy. Due to the multi-follicular growth, OS cycles show unphysiological levels of steroid hormones. Specifically, the serum estradiol levels at the end of a stimulated follicular phase can be 10–20 folds increased resulting into an extensive suppression of the natural LH pulsatility (negative feedback). Moreover, the usage of GnRH agonist and antagonist may also alter the LH secretion and lastly the performance of the oocyte’s retrieval can affect the P4 production due to the aspiration of granulosa cells together with the follicular fluid (Pabuçcu et al., 2020). As a results, a fresh IVF cycle is well known to be associated with a iatrogenic luteal phase defect (Yanushpolsky, 2015). The luteal support of a fresh IVF cycle is basically provided by the luteotropic action of the hCG injected for the trigger of the ovulation, however the long effect of the hCG last 5–6 days and then, consequently to the hCG clearance, the P4 production decreases consistently. Therefore, a fresh IVF cycle should be supported with P4, directly after the oocyte retrieval (Beckers et al., 2000, 2003). While there is consensus on the need of P4 supplementation in fresh IVF cycles, the type, the best dose, and the time of discontinuation are still matter of debate; especially considering the new products introduced lastly in the market.
Comparison of different routes of administrationWhile there is consensus on the need for progesterone supplementation after a fresh IVF cycle, when to start the supplementation, how it can be supplemented, which route of administration, and for how long, are still a matter of debate. Supplementation can start on the day before the oocyte pick-up (OPU), the day of OPU or the day after and it can last several weeks: until a positive hCG or until heartbeat is confirmed or longer until 8–12 weeks of pregnancy. Beside the variability in the timing of the P supplementation, also there is still a lack of consensus on different preparations and different ways of administration that can be used (Table 1). Evidence from the literature shows a global tendency through the vaginal route in tablets that are administered from the day of OPU until week 8–10 of pregnancy (Vaisbuch et al., 2014; Mohammed et al., 2019; Di Guardo et al., 2020). As described earlier, Table 2, one important aspect to considerer is that different routes of administration have different pharmacological profiles. The vaginal route is associated with higher uterine concentration of P, despite the drawbacks of being administered multiple times a day and the vaginal discharges and irritation that are reported quite frequently. Nonetheless, the vaginal route is preferred to the IM route, which is associated to higher serum concentration of P, at the expense of an higher incidence of side effects reported, more pain and irritation due to the injection (Mohammed et al., 2019).
To the extent of injectable progesterone, currently an increase of subcutaneous administration has occurred. Latest studies have demonstrated that the aqueous preparation of P4 for subcutaneous administration is effective in priming the endometrium and as luteal phase support (de Ziegler et al., 2013; Sator et al., 2013). As with IM progesterone, with subcutaneous P4 the serum concentration of P4 is higher and the incidence of low values of serum P4 during the luteal phase is lower (Ramos et al., 2021). However, important drawbacks of the injectable P4 are the elevated costs, especially considering the length of the treatment, and pain, irritation due to the injection (Mohammed et al., 2019). In the last years, important RCTs have been performed to study the safety and efficacy of the oral synthetic progestin, dydrogesterone. In particular Lotus I (Tournaye et al., 2017) and Lotus II (Griesinger et al., 2018) showed that there was no difference in terms of pregnancy outcomes, nor in terms of side effects and congenital anomalies when comparing with vaginal (600 mg/day) or vaginal gel (90 mg/day), respectively Lotus I and II. Although very promising results, in several counties, including Spain, oral dydrogesterone is still out of label.
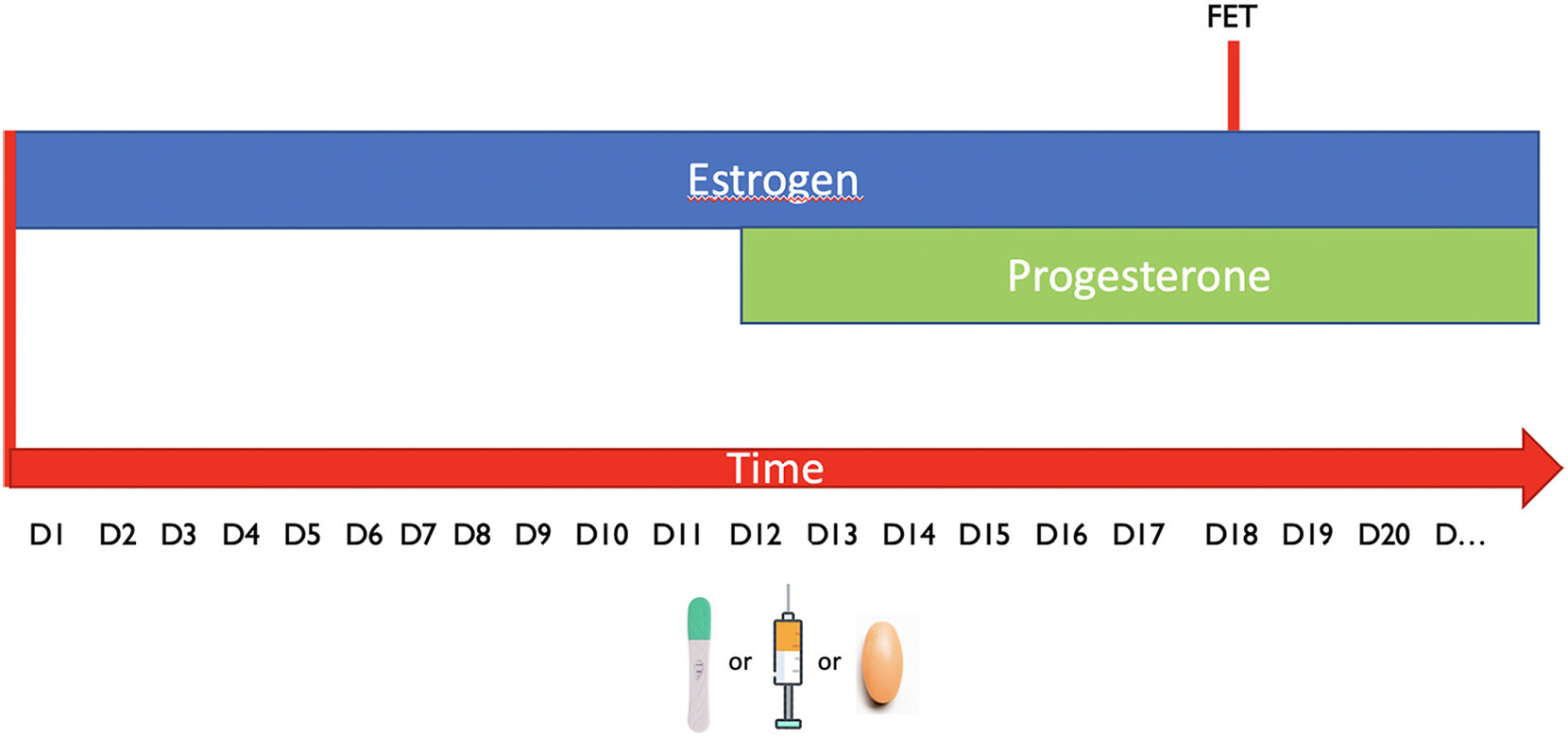
FET cyclesDue to the improvement in the vitrification techniques and the indication for elective and non-elective indications, the transfer of cryopreserved embryos is the most common procedure in all the IVF clinics. The ovarian cycle has a specific pattern with a sequence of oestrogens and progesterone, which is fundamental for the preparation of the endometrium; in fact, the endometrium can allow a successful implantation only in a specific moment of the cycle called “window of implantation”. A FET can be performed either in a natural, semi-natural or artificial cycle respectively, depending on the exposure to the endogenous hormones (and the LH peak or the administration of hCG for the ovulation induction) or the sequential administration of exogenous hormones (Fig. 2). The decision between one or the other protocol depend mostly on patient characteristics and needs. In fact, according to the results of recent studies (Groenewoud et al., 2016; Ghobara et al., 2017) the efficacy of the different techniques is comparable; however, HRT allows a minimal cycle monitoring, easy scheduling and more importantly is applicable to any woman age and stage.
D1 is referring to the first day of menstruation. FET (frozen embryo transfer). LH means spontaneous ovulation. Injection refers to hCG ovulation trigger while the pills represent artificially prepered endometrium. Regardless on the tecnhique the sequence oestrogens/Progesterone needs to be respected and the FET is planned in the specific time point called »window of implantation».
For the implantation to happen and the achievement of pregnancy, it is necessary a decidualised, receptive endometrium and adequate serum levels of P4 during the luteal phase. In the natural cycle, the transformation of the endometrium and the pregnancy maintenance are achieved by endogenous P4. The P4 is secreted by the CL and induces the secretory transformation of the endometrium necessary for the implantation. About 30 mg of P4 a day are secreted from the women while approximately 1 mg is secreted by the adrenal glands (Fritz and Speroff, 2011). The metabolism of P4 is mainly happening in the liver, and the P4 elimination half-life is about 5 min (Di Renzo et al., 2016).
The evidence on the repercussions of P4 deficiency are clearly demonstrated by earlier studies where a luteectomy was performed during the first 7 weeks of pregnancy and an inability to maintain the pregnancy was shown in these patients (Csapo et al., 1972). While in artificially prepared FET cycle the use of P4 is mandatory due to the absence of the CL, in natural FET cycles this is not required (Groenewoud et al., 2016); however, due to the last years research results showing the importance of serum P4 levels around the time of embryo transfer in artificially prepared cycles, whether there is a minimum serum threshold of P4 to reach to improve reproductive outcome in natural cycles became a very important point. To this extent, Gaggiotti-Marre et al. (2020), showed that a serum P4 levels of at least 10 ng/mL are required to improve live birth rate of FET in a natural cycle, supporting previous evidence on the negative impact of LPD on pregnancy outcomes. These results implies that when P4 is lower than a specific threshold of around 10 ng/mL could be necessary to supplement the luteal phase with exogenous P4. Although some patients due to the complete absence of medicacions required prefer the NC-FET, nowadays in the majority of the clinics worldwide, the HRT-FET is the most commonly used due the wide applicability (regardless of the women age and stage), and the easy scheduling.
HRT-FETThe artificial cycle is universally the most elected method to perform a frozen embryo transfer. The common scheme is with 10–12 days of oestrogens followed by vaginal P4 administered with a dose of 200 mg three to four times a day (Mackens et al., 2017). Up until now, the formal monitoring of the HRT cycle has been an ultrasound on day 10–12 of the oestrogen intake to determine endometrial thickness, while there is no need for the hormonal evaluation during the administration of oestrogen as there is no threshold of estradiol above which the likelihood of pregnancy increases (Mackens et al., 2020). One important finding of the last years is the role of the CL which produces P4 but also relaxin and other vasoactive substances essential to help the adaptation of the mothers’ cardiovascular system in the early instaurations of the pregnancy (Conrad, 2011; von Versen-Höynck et al., 2019; Dall’Agnol and García Velasco, 2020; Singh et al., 2020). This flow of research is in line with evidence showing different pregnancy and neonatal outcomes according to the FET protocol used. In fact, altered trophoblastic invasion, either decreased after IVF with fresh embryo transfer or exacerbated after FET, are described. In most cases, the placenta can compensate for original injury throughout pregnancy, which results in a healthy newborn; however, if compensation is overwhelmed, the consequences could be adverse obstetrical and neonatal outcomes (Choux et al., 2019). Moreover, according to von Versen-Höynck et al. (2019) the FET-HRT cycles are the ones with the higher risk of pre-eclampsia and gestational hypertension, and the explanation lies on the role of the vaso-active substances produced by the CL; however, although promising results on the necessity to prevent pre-eclampsia and hypertension in women undergoing FET cycles, the question whether we should stop HRT is not yet answered, as the HRT is still considered the most practical and widely used way to prepare the endometrium (Lawrenz et al., 2020).
The latest evidence shows the need for high serum P4 levels for the implantation and evolution of the pregnancy, specifically, P4 is highly important not only locally, at the level of the uterus allowing decidualisation and myometrial quiescence (Macklon and Brosens, 2014; Fanchin et al., 2000), but also systemically, with its role as immunomodulator that induces tolerance at the maternal–foetal interface (Norwitz et al., 2001; Shah et al., 2019).
Like for the fresh IVF cycles, in FET-HRT we have different route of administration of P4 (Table 1). Progesterone serum levels are described to reach higher values when the route of administration is parenteral compared to local (Ramos et al., 2021; Miles et al., 1994). To this extend, there is evidence suggesting that the vaginal absorption of P4 can be limited in some patients due to patients intrinsic characteristics (Cédrin-Durnerin et al., 2019; González-Foruria et al., 2020). In fact, there are some factors associated to the pharmacokinetics, like age, weight, and history of low progesterone in previous FET cycles, which can affect the P4 serum concentration.
Individualised LPS based on progesterone levelsProgesterone plays the main role in the endometrial preparation for embryo transfer for both fresh and frozen embryo transfers, therefore, reaching adequate levels of P4 is crucial. In fact, as demonstrated by the previous publications on the topic, with the common doses of 600 or 800 mg P4 a day up to respectively 40% and 30% of the women have inadequate (<9–10 ng/mL) serum levels of P4 when assessed before performing the ET (Labarta et al., 2017; Cédrin-Durnerin et al., 2019; Melo et al., 2021; Álvarez et al., 2021).
According to previously published data (Filicori et al., 1994; González-Foruria et al., 2020), another important aspect to consider is the variability of the P4 levels during the day and the days; in fact serum P4 levels are subject to large fluctuations because of pulsatile hormone release (Knobil, 1980).
Results of the latest years studies on the P4 assessment in the luteal phase and pregnancy outcome showed a very high correlation between the two, regardless of the moment P4 was measured. Specifically, the latest studies have shown that low serum levels of P4 (below 9–10 ng/mL) are associated with lower pregnancy and live birth rate, and higher miscarriage rate (Labarta et al., 2017, Labarta et al., 2021b; Alsbjerg et al., 2018; Gaggiotti-Marre et al., 2020; Álvarez et al., 2021). Therefore, P4 assessment in the luteal phase is of utmost importance as well as the innovative idea of progesterone supplementation (PS), the so-called “rescue protocol” (Labarta et al., 2017, 2021b; Alsbjerg et al., 2018; Gaggiotti-Marre et al., 2020; Álvarez et al., 2021). Table 3 summarises all studies looking at the association between P4 during the luteal phase (with slightly different time of measurement) and pregnancy outcomes.
Studies demonstrating the association between P4 during the luteal phase and pregnancy outcomes. The black line divides the different timing in P4 assessment.
| Author | Design | N | P4 route/dose | Day of assessment | P4 threshold | Embryo origin | FET technique | Outcome |
|---|---|---|---|---|---|---|---|---|
| Gaggiotti-Marre et al., 2019 | Retrospective | 244 | Vaginal ovulos/200 mg/8 h | Day before ET | 10.64 ng/mL | Own oocytes with PGT-A | HRT | LBR 47.5 vs 62.3% (respectively for P4< and >10.64 ng/mL) |
| Gaggiotti-Marre et al. (2020) | Retrospective | 294 | None | Day before ET | 10 ng/mL | Own oocytes with or without PGT-A | NC | LBR 25.7 vs 41.1% (respectively for P4< and >10 ng/mL) |
| Ramos et al. (2021) | Retrospective | 320 | Vaginal 200 mg/8 h + SC 25 mg/day | 1–2 days before ET | Different quartiles | Own oocytes, PGT-A, oocyte donation | HRT | OPR |
| Álvarez et al. (2021) | Prospective | 574 | Vaginal ovulos/ 200 mg/8 hSupplemented: 25 mg/day SC P4 (if Prog<10.6) | Day before ET | 10.6 ng/mL | Own oocytes, PGT-A, oocyte donation | HRT | OPR 49.1 vs 52.3 (respectively if P4<10.6 and not supplemented vs supplemented) |
| Brady et al. (2014) | Retrospective | 229 | IM50–100 mg/day | Day of ET | >20 ng/mL | Fresh own oocytes and oocyte donacion | HRT | LBR (54 vs 51%) |
| Kofinas et al. (2015) | Retrospective | 213 | IM50–75 mg/day | Day of ET | >20 ng/mL | FET, own oocytes with PGT-A | HRT | LBR (49 vs 65%, respectively for low and high P4) |
| Yovich et al. (2015) | Retrospective | 529 | Vaginal (400 mg/8 h) | Day of ET | 22–31 ng/mL | FET, own oocytes and oocyte donation | HRT | LBR (50 vs 42%) |
| Labarta et al. (2017) | Prospective | 211 | Vaginal (400 mg/12 h) | Day of ET | >11 ng/mL | Own oocytes, PGT-A, oocyte donation | HRT | OPR (53 vs 43%, respectively for high and low P4 levels) |
| Cédrin-Durnerin et al. (2019) | Retrospective | 227 | Vaginal ovulos/ (200 mg/8 h) | Day of ET | 11.4 ng/mL | Own oocytes | HRT | LBR 17% versus 31%, respectively for low and high P4 |
| Labarta et al. (2021a) | Prospective | 1205 | Vaginal (400 mg/12 h) | Day of ET | <8.8 ng/mL | Own oocytes, PGT-A, oocyte donation | HRT | OPR (36.6% vs 54.4%) and live birth rate (35.5% vs 52.0%), respectively for low and high P4 |
| Basnayake et al. (2018) | Retrospective | 1580 | Vaginal (various) | 16 days after P4 intake | >15 ng/mL | Own oocytes and oocyte donation | HRT or NC | LBR (27 vs 11%) |
| Alsbjerg et al. (2018) | Retrospective | 244 | Vaginal (gel)/ 90 mg/8 h | Day of hCg test | 11 ng/mL | Own oocytes | HRT | OPR (51 vs 38) Respectively if P4 > or < of 11 ng/mL) |
The assessment of P4 before the ET, allows the possibility to perform an individualisation of care by “rescuing” the cycle with low P4 values with extra P4 supplementation, increasing pregnancy outcomes in FER-HRT of euploid embryos and in oocytes donation program (Labarta et al., 2021b; Álvarez et al., 2021). Considering the evidence on P4 variability and the inter-women difference in P4 absorption, the assessment of P4 before the ET represents an important milestone in the direction of the individualisation of the luteal phase, based on P4 values.
New frontiers for the luteal phase evaluation could be the assessment of P4 before the embryo transfer and another time in the late luteal phase, given the high serum P4 variability, the high correlation with pregnancy rate and given the opportunity to adjust low levels of P4 (<9–10 ng/mL) by supplementation at different time points of the LP.
Future researchThe incidence of low serum P4 levels ranges at the level of 40–50% when vaginal P4 is administered, while lower when SC P4 is used. Accumulating results suggest that low systemic serum P4 levels have been associated with lower live birth rates and increased pregnancy loss in women undergoing FET cycles. To this extent, addition of subcutaneous P4 to the vaginal in FET cycles relates excellent pregnancy rates in women with low serum levels prior to ET, opening the door for future research with the objective of understanding the best route of P4 administration for each patient for a perfect individualisation of care. Although SC P4 has shown in several studies that could rescue the luteal phase in FET cycles supplemented with vaginal P4 when serum P4 levels are low, future research is needed to investigate whether other routes of administration (i.e. oral) could have the same effect or if in the end whether combined routes of administration should be adopted from the first day of LPS in HRT FET cycles.
ConclusionsIn conclusion, this review on the luteal phase shows that in the last years some innovative tools have been introduced, as the introduction of new preparations of P4 for oral and subcutaneous use and the assessment of luteal P4 around the day of the ET (mid-luteal phase) or even later in the late-luteal phase. Luteal P4 has been demonstrated to be a very strong predictor of cycle outcome in FRET HRT and very interestingly, the incidence of inadequate levels of P4 is about 30% throughout different timing of assessment and with slightly different dosages of vaginal P4. Considering these findings an individualisation of P4 supplementation in ET-HRT and eventually the adoption of a “Rescue Protocols” in any moment of the luteal phase could be considered beneficial in terms of increasing the pregnancy outcomes.
The management and evaluation of the Luteal phase have seen a big improvement in the last years, with the introduction of new products in the market and with the beginning of the P4 assessment; however, we expect soon the possibility to standardise different protocols and to discover which one can be better applied for different patients and indications.