Early life events can exert a powerful influence on both the pattern of brain architecture and behavioral development. The paper examines the nature of nervous system plasticity, the nature of functional connectivities in the nervous system, and the application of connectography to better understand the concept of a functional neurology that can shed light on approaches to instruction in preschool and primary education. The paper also examines the genetic underpinnings of brain development such as synaptogenesis, plasticity, and critical periods as they relate to numerosity, language and perceptual development. Discussed is how the child's environment in school and home interact with and modify the structures and functions of the developing brain. The role of experience for the child is to both maintain and expand the child's early wiring diagram necessary for effective cognitive as well as neurological development beyond early childhood.
Los primeros acontecimientos vitales pueden ejercer una enorme influencia tanto en el patrón de arquitectura cerebral como en el desarrollo del comportamiento. En este trabajo exploraremos la naturaleza de la plasticidad del sistema nervioso, la naturaleza de sus conexiones funcionales y la aplicación de la tractografía, para lograr una mejor explicación del concepto de neurología funcional que pueda arrojar luz sobre las teorías de la instrucción en la enseñanza preescolar y primaria. El trabajo analiza también los fundamentos genéticos del desarrollo del cerebro tales como la sinaptogénesis, la plasticidad y los periodos críticos en lo que respecta a su relación con el desarrollo numérico, lingüístico y perceptivo. Se aborda cómo interactúa el entorno del niño en la escuela y en casa con las estructuras y funciones del cerebro en desarrollo y las modifica. El papel de la experiencia temprana será tanto mantener como expandir los circuitos neurales necesarios para un desarrollo efectivo (tanto cognitivo como neurológico) más allá de la temprana infancia.
From Camillo Golgi and Santiago Ramón y Cajal in the late 1890s, with their extensive observations, descriptions, and categorizations of neurons throughout the brain and the formation of the neuron doctrine and the start of modern Neuroscience, we have come a long way in understanding the nature of the nervous system in the control of human behavior. Little of that work has actually wound its way into the classroom and even less into public policy in education.
Basic principles have emerged that allow application to educational practice, especially in the early years from birth to five years that place great responsibility for brain development in the hands of parents and early childhood teachers. These principles include the following:
- 1.
The human brain develops from conception to the early twenties from the bottom up with vital and autonomic functions and control coming first and cognitive-motor sensory and perceptual processes later and integration and decision making last (Melillo & Leisman, 2009).
- 2.
The child's brain is influenced by the combined roles of genetics and experience (Leisman, Machado, Melillo, & Mualem, 2012; Leisman & Melillo, 2012; Melillo & Leisman, 2009).
- 3.
The brain's capacity for change decreases with age (Leisman, 2011).
- 4.
Cognitive, emotional, and social capacities are inextricably intertwined throughout the life course (Leisman, Braun-Benjamin, & Melillo, 2014).
- 5.
Motor and cognitive functions interact with our brains, being the direct result of bipedalism (Melillo & Leisman, 2009).
- 6.
Toxic stress damages developing brain architecture, which can lead to life-long problems in learning, behavior, and physical and mental health.
- 7.
The child's environment directly affects synaptogenesis and allows for neurological optimization (Gilchreist 2011; Leisman, Rodriguez-Rojas et al., 2014).
Early life events can exert a powerful influence on both the pattern of brain architecture and behavioral development. Both early as well as later experiences contribute to the wiring diagram of the child's brain, but experiences during critical periods establish the basis for development beyond the early years. The role of the kindergarten and nursery teachers becomes critical in establishing the solid functional footing of the developing child and the neurological adult.
The foundations of brain architecture are established early in life through a continuous series of dynamic interactions between genetic influences, environmental conditions, and experiences (Friederici, 2006; Majdan & Shatz, 2006). We have come to learn that the child's environment significantly impacts the timing and nature of gene expression directly affecting the child's brain architecture.
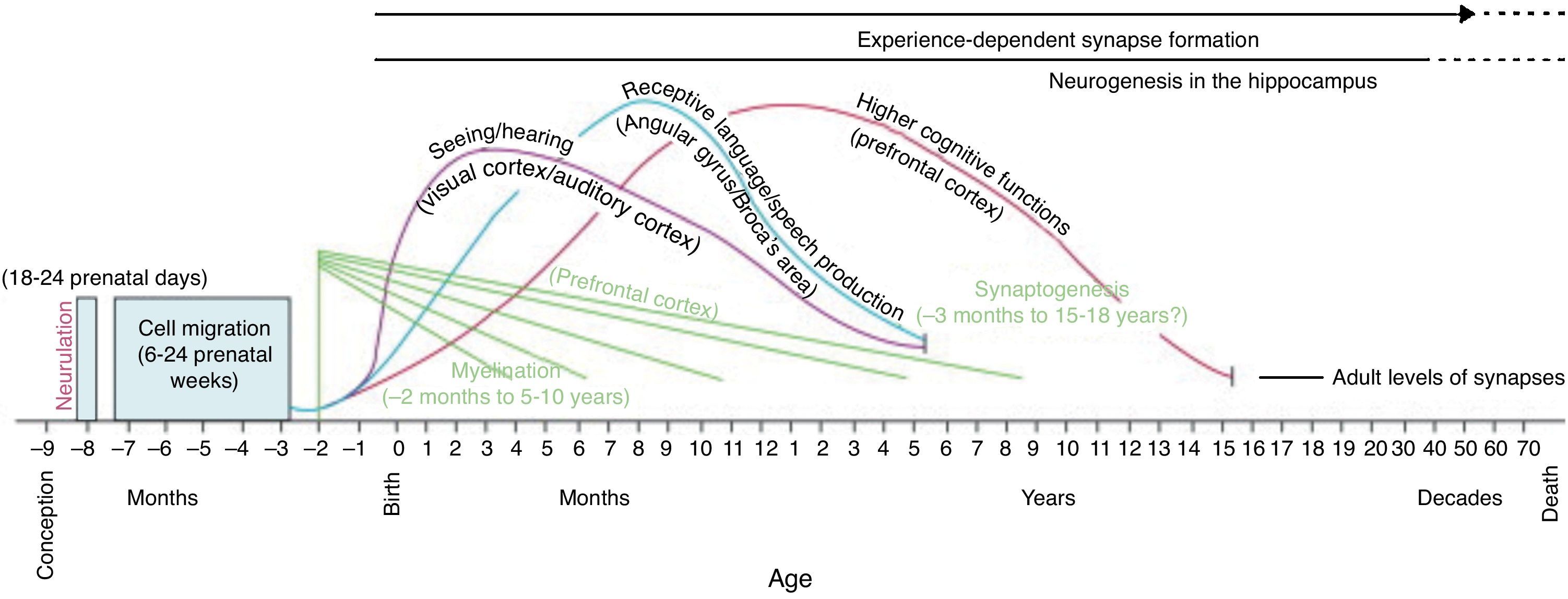
Because specific experiences potentiate or inhibit neural connectivity at key developmental stages, these time points are referred to as critical periods (Knudsen, 2004). Brain, cognitive, sensory, and perceptual development does not occur simultaneously but rather at different developmental stages as represented below in Fig. 1. Each one of our perceptual, cognitive, and emotional capabilities is built upon the scaffolding provided by early life experiences. Examples can be found in both the visual and auditory systems, where the foundation for later cognitive architecture is laid down during sensitive periods for basic neural circuitry.
The capacity to perceive stereoscopic depth requires early experience with binocular vision, (Crawford, Pesch, & von Noorden, 1996), which at a later point in development may have implications for perceptual and cognitive development. Likewise, the capacity to perceive a range of tones requires variation in the tonal environment, and exposure to such variation later leads to language processing and proficiency (Kuhl, 2004; Newport, Bavelier & Neville, 2001; Weber-Fox & Neville, 2001). The absence of tones associated with a given language will eradicate the discrimination of those developmentally unheard tones by the time the infant is one-year-old (Werker & Tees, 1983). Second language acquisition obtained early enough will have the same brain representation as the first language throughout the lifespan, but that second language, learned later in development, even when spoken at native level, will be represented differently in the brain relative to the first language (cf. Leisman, 2012; Leisman & Melillo, 2015).
Although early experiences are reflected in behavior, behavioral measures tend to underestimate (in part because of a lack of sensitivity and specificity) the magnitude and persistence of the effects of early neuronal development (Knudsen, 2004). In order to explore the role of timing and quality of early experiences on later cognitive function, we must therefore have a genetic framework of the developing brain.
We see no fundamental difference between the task of the educational system, rehabilitation after neurological insult or developmental disabilities, the task of parenting, the effects of social interaction, the effects on the nervous system of sport, or even the ability to intervene in the natural consequences of cognitive aging. The term education can be used interchangeably with rehabilitation as all directly relate to measurable dynamic plastic changes in neural connectivities.
Education has been grabbing at straws for a long time. Often when a preliminary finding is reported in the neuroscience literature or presented at a conference, it is grabbed and expounded upon with little consideration of the fundamental nature of biological processes that underlie those changes. For better or worse, over the last 10 years, education has been actively and aggressively looking to the biological sciences in order to inform education policy and practice.
A good example is that of the 1998 decision in Georgia to fund an expensive program, to provide CDs of Mozart's music to all new mothers. In establishing this policy, the governor of Georgia drew heavily on work in cognitive neuroscience conducted at the University of California, Irvine. The actions were taken in the hope of “harnessing the ‘Mozart effect’ for Georgia's newborns – that is, playing classical music to spur brain development.” Despite what the program implied, Mozart effect research, upon close examination, had little to offer education. One study, reported in Nature (Rauscher, Shaw, & Ky, 1993), found that listening to Mozart raised the IQs of college students for a brief period of time. Another study found that keyboard music lessons boosted the spatial skills of three-year-olds (Schlaug, Norton, Overy, & Winner, 2005). Cognitive neuroscientists responsible for this work, were baffled by Georgia's program and actions based on their work. Since this debacle, major figures in the sciences have published articles emphasizing caution and care as scientists, educators, and practitioners proceed down this exciting, but pitfall-laden road. These cautionary articles have laid the groundwork for relationships between neuroscience and education. However, there is a paucity of publications that systematically examine an area of research where conservative but confident claims can be made of the benefits of interdisciplinarity.
Most currently prevailing patterns of education are heavily biased towards left cerebral functioning and are antithetical to right cerebral functioning. Reading, writing, and arithmetic are all logical linear processes, and for most of us are fed into the brain through our right hand. Most educational policies have tended to aggravate and prolong this one-sidedness. There is a kind of damping down of fantasy, imagination, clever guessing, and visualization in the interests of rote learning, reading, writing, and arithmetic. Great emphasis is placed upon being able to say what one has on one's mind clearly and precisely the first time. The atmosphere emphasizes intra-verbal skills, “Using words to talk about words that refer to still other words” (Bruner, 1971).
If there is any truth in the assertion that our culture stresses left hemisphere skills and discriminates against the right hemisphere, this is especially true of school systems. Our society's overemphasis on “propositionality” at the cost of “appositionality” does not only result in adjustment difficulties but also in a lopsided education for the entire student body. Our students are not being offered the education they require to understand the complex nature of the world and themselves, an education for the whole brain. Sperry wrote: “Our education system and modern society generally (with its very heavy emphasis on communication and on early training in the three R's) discriminates against one whole half of the brain. I refer, of course, to the nonverbal, non-mathematical, minor hemisphere, which we find has its own perceptual, mechanical, and spatial mode of apprehension and reasoning. In our present school system, the attention given to the minor hemisphere of the brain is minimal compared with training lavished on the left, or major hemisphere.” (Sperry, 1975)
Educational institutions have placed a great premium on the verbal/numerical categories and have systematically eliminated those experiences that would assist young children's development of visualization, imagination and/or sensory/perceptual abilities. The over-analytic models so often presented to children in their textbooks emphasize linear thought processes and discourage intuitivity, analogical, and metaphorical thinking. These factors of neural functioning among children have been left to modification by random environmental, rather than systematic, institutional means. Education, which is predominantly abstract, verbal and bookish, does not have room for raw, concrete, esthetic experience, especially of the subjective happenings inside oneself.
Education imposes a structure of didactic instruction, right-wrong criteria, and dominance of the logical-objective over the intuitive-subjective on the learning child so early in the course of emergent awareness of his world and of himself that, except in rare cases, creative potential is inhibited, or at least diminished (cf. Melillo & Leisman, 2009). This leads us to affirm that our system of education is one, which leads to the underdevelopment of the right hemisphere. As a result of excessive emphasis on intellectualizing, verbalizing, analyzing, and conceptualizing processes, ‘curriculum’ has become equated with mere ‘understanding’. This imposes a ‘neurotogenic limitation’ and binds mental processes so tightly that they impede the perception of new data. In the words of Gazzaniga (1975) a long time ago, curriculum is “inordinately skewed to reward only one part of the human brain leaving half an individual's potential unschooled.” The traditional preoccupation with formal intellectual education effectively blocks the possibility for the students to recognize and cultivate creativity and transcendence. It has been the adaptation by educators of applications of brain sciences into the classroom and the culture of dichotomies of the Behavioral Sciences over the past 150 years that have placed undo reliance by our educational systems on functional brain models that may be irrelevant at best and damaging at worst to children's classroom performance and its evaluation.
What emerges as the central proposition of this paper is that (A) the examination and study of regional cerebral differences in brain function as a way of explaining and evaluating the learning process within the educational system is irrelevant (cf. Figs. 6A & B); (B) the evaluation of students by standardized aptitude and achievement tests is not sufficient although probably still necessary; and (C) the educational systems had better examine student performance and teach towards “cognitive efficiency” rather than simply mastery vs. non-mastery with methods that employ both psychophysics that examine person-environment interaction and mathematical means of examining optimization and the strategy used to get there as well as how far or close a student is functioning from a mathematically derived optimization regression line or, in fact, how quickly the learner is progressing in that direction.
Educators, although perhaps not palatable to conceive of early childhood education as such, are producing a product and production management techniques that should be useful for evaluating not just the product but the process or “manufacture” of that product as well.
Genetic and Environmental Interplay in the Developing BrainThe uniquely large number of cells and their potential for association as well as asymmetry is directly the result of bipedalism along with genetic mutations (cf. Melillo & Leisman, 2009). Once the large cell assemblies were established and pressures for bi-symmetry were released, humans then could develop asymmetric functions in their brains that were not directly tied to motor or autonomic control. Hemispheric specialization then could develop different control centers consistent with the previous function of that hemisphere, creating most of the unique human characteristics. The other demand that bipedalism would place on the brain would be the need to be more precise and complex in the synchronization of muscles to be able to walk, run, and jump. This increased synchronization would require greater frequency of oscillation of control centers within the inferior olive and cerebellum and their feedback to the intralaminar nucleus of the thalamus and its reciprocal thalamo-cortical projections. This increase in oscillation into the 40-Hertz range is thought to be required to achieve binding within various cortical sites into one continuous conscious percept of the world. This appears to be the foundation of human consciousness, which is thought to be unique in humans and due to unique connectivities in the human brain.
Therefore, a proposal of an increase in neuroblast proliferation in the human brain is consistent with the concept of neoteny in the human evolution. This concept states that certain characters are delayed in their development with respect to others (pedeogenesis) (cf. Fig. 1) (Bjorklund, 1997). This resulted in changes in adult morphology during evolution. This is thought to be the process in the human skull in which infantile dimensions are comparable to other primates. This first factor explains the increase in cell size that concurs with minimal genetic change. However, the maintenance of these cells would not continue without the appropriate activity, presynaptically and postsynaptically. In essence, they require a power source as well as they would, in turn, require connections to expanded areas sub-cortically. Bipedalism would provide both by increasing exponentially the amount of temporal and spatial summation within sensory motor networks, especially cerebellum, thalamus, and cortex. This would require expanded areas of cerebellum and thalamus that would evolve in parallel with the expanded areas of cortex and could provide a site for connection to these increased numbers of neurons. This would take place because although the genetic change would increase cell number, it would do so with a non-directional force, which would not specify any specific shape. Posterior epigenetic reorganization (synaptic stabilization) would determine the shape and configuration of the networks within the brain itself. Therefore, genetic factors would produce the density of cells required but environmental factors would trim and shape it in a specific fashion.
Therefore, it can be speculated that there are no genes specifying particular types of neuronal networks involved in higher cognitive function. The human brain is about four times larger than those of primates, because the brain cells or neurons are spread about (Nieuwenhuys, 1999). The thicker cortices of the large mammals and humans as well seem to be primarily a function of larger nerve cell bodies, more extensive dendritic and axonal systems, and more numerous glial cells. Although neurons do not reproduce after birth, glial cells can. They reproduce based on increased metabolic demand of the neurons or increased stimulation. This increase in growth of glial cells allows the neurons to make more connections, which increases the ability and speed of the cell to transmit signal. The increase in size and strength of connections allows both to happen more efficiently. The growth in size and complexity of the human brain comes from the number of supporting cells which in essence feeds the neurons with more fuel and encourages the growth of new connections. It is not the increased number of neurons, but the increase in connections between the cells and the increase in separating and supporting cells that accounts for the large growth of the human brain. This is the very definition of “plasticity.”
Plasticity is the ability of the brain to grow and whether it is growing on a short-term basis or on a long-term basis in the case of evolution, the facts of plasticity are consistent. This can only mean that there was some increase in the frequency, duration, and intensity of stimulation of the human brain over time for it to have evolved as uniquely as it has. There are two things that make humans unique among other organisms: 1) we have a larger cortex and, 2) we stand upright (bipedal).
Refinements in the neural circuits that mediate sensory, emotional, and social behaviors are driven by experience (Feldman & Knudsen, 1998; Leisman et al., 2012). Specifically, postnatal experiences drive a protracted process of maturation at the structural and functional level, but the very ability of such developmental processes to occur successfully is dependent in large part on the prenatal establishment of the fundamental brain architecture that provides the basis for receiving, interpreting, and acting on information from the world around us (Hammock, 2006).
While the term “blueprint” has been utilized in the past to describe a fixed set of genes with inflexible interactions, the term is used here as an analogy to a rough draft, or design – the framework from which a more defined structure will evolve, alternatively, an operating system in which programs have yet to be laid down. The emergence of the architecture in all vertebrate species begins early; in humans, this occurs within the first two months post-fertilization (Levitt, 2003).
The cerebral cortex has garnered substantial attention in defining key developmental features across species. This is due in part to the technical advantages of studying a well-organized, layered structure, and the functional relevance of linking typical and atypical maturation of complex behaviors and neurodevelopment.
The neocortex in all mammalian species is comprised of six layers of neurons, the architecture, connectivity and chemistry of which are distinct depending upon their location. The neocortex is organized to receive information from the organism's surrounding environment, typically through connections with the thalamus. It does so by integrating information within and across architecturally distinct functional domains, and then relays this information to other brain centers that generate an appropriate functional response.
There are two major organizing principles of the neocortex influenced by gradients of gene networks that have developed evolutionarily. First, the precursors of different functional areas emerge during roughly the first and second trimester of pregnancy in the human (cf. Leary, Chou, & Sahara, 2007). Regional specification is not absolute, but involves networks controlling the expression of axon guidance molecules that control the initial input and output wiring plan. Expansion of the size of the neocortex during evolution (e.g., 1000-fold between mouse and human) occurs mostly in this period (Rakic, 2005).
The ‘inside-out’ pattern of neuron production and migration provides the basis for building cell connectivities forming functional areas, with small variations in the ratio of excitatory to inhibitory neurons in different regions. In fact, this organization provides a framework for later-developing refinement of circuits influenced extensively by patterns of physiological activity through experience and training.
Experiments in genetically manipulated mice demonstrate that altering the expression of just one genetic transcription factor, cortical regions can be changed (Cholfin & Rubenstein, 2007). For example, the genetic factor emx2 controls the expression of the Fgf8 factor near the anterior end of the cerebrum. Fgf8 alone is sufficient to specify the cortical regions that will eventually receive connections that are typical of frontal and somatosensory cortices (Fukuchi-Shimogori & Grove, 2003). This type of early genetic re-specification is functionally relevant. For example, the Fgf17 is responsible for initial patterning of different frontal cortex areas (Cholfin & Rubenstein, 2007).
It is not our function here to pursue this notion in detail other than to indicate that the early specification and re-specification of the neocortex by genetic factors is powerful because additional axon guidance molecules serve as important chemical cues for getting axons to grow into their correct target region prior to beginning the extended process of synapse formation (cf. Alcamo et al., 2008). Gene regulatory networks also can influence the initial size of cortical areas by modulating the number of neurons produced. The long-distance circuit projections that help to define functional cortical areas, and even functional differences in superficial and deep projecting neurons, are altered when the disruption of early gene networks modifies guidance cues so that atypical connections are made.
Though we tend to think that genetic mechanisms are immutable, it is important to stress that expression of early gene networks can be perturbed not only by catastrophic genetic mutations that disrupt important regulatory genes, but also by prenatal environmental influences, such as drugs, alcohol, toxins, and inflammatory responses. These may have less profound impacts on brain patterning, but nonetheless can result in long-term disruption of cellular differentiation and behavioral development (Stanwood & Levitt, 2008).
In all mammalian species, this early period of specified patterning to generate a unique architecture is followed by an extended period of synapse formation, adjustment, and pruning that typically extends from the last quarter of gestation through puberty (Bourgeois, Goldman-Rakic, & Rakic, 1999).
Experience-based Adjustments to Neural Architecture in Early ChildhoodAlthough genetics provides an important foundation for early development, it is only a framework upon which the early childhood environment can influence future structure and function. This can best be illustrated through studies of the sensory systems, which demonstrate the crucial role of environment in the early development and maintenance of the nervous system (Leisman, 2011). Such work also demonstrates the need for patterned physiologic activity during development, as well as refinement and maintenance of detailed sensory maps.
Synaptic reorganization takes place most predominantly during childhood and adolescence (Blakemore, 2012). During these periods the brain becomes sensitive to change which allows it to develop in unique ways dependent upon the individual age, gender, and environment along with many other variables (Andersen, 2003). The concept of “self-organization” indicates that the brain actually organizes itself based on the individual's experiences. Environment stimulation and training can affect how the brain develops and at what pace (Andersen, 2003; Leisman, 2011). The environment can include factors like location and surroundings, home, parenting, and of course the classroom, as well as circumstances in each of those environments (Blakemore, 2012; Tau & Peterson, 2010). Environment can also be identified as a child's emotions or responses to certain stimuli, in this case, the concept of self-organization which postulates that the brain organizes itself based on each child's unique experiences.
The fact that humans have a greater capacity than rats or even chimps for self-organizing, plastic, or flexible behavior provides no implication that we are either all stereotyped or flexible in our behavior and brain organization. Stereotypy creates for efficiencies but plasticity or flexibility allow for adaptation due to the exigencies of one's environment. We, given the notions of stability and flexibility (Leisman, 1980), have a basis for rehabilitation and effective adaptive function. The concept of the interplay between stability and flexibility and its implications for the education of the normally developing child's brain needs to be viewed as a relativistic notion, viewed against the features of the organism that are not plastic. In order to identify flexibility or plasticity, one must be able to identify the invariant and constant. The identification of plasticity requires us to be able to know the constraints of the system. The fact, however, that we are more plastic than other organisms is expressed even in our adult lives as organisms. This suggests that our capacity for systematic change and the fact that we retain flexibility across our later developmental periods allows application of rehabilitation thinking and the measurement of optimization throughout the life span.
Hebb had postulated in 1949 that when one cell excites another repeatedly, a change takes place in one or both cells such that one cell becomes more efficient at firing the other (Hebb, 1949). It is this view that is not only limited to a particular cell and its arborized neuronal connections but to definable anatomical regions. It is this notion that forms the basis of our concept of plasticity.
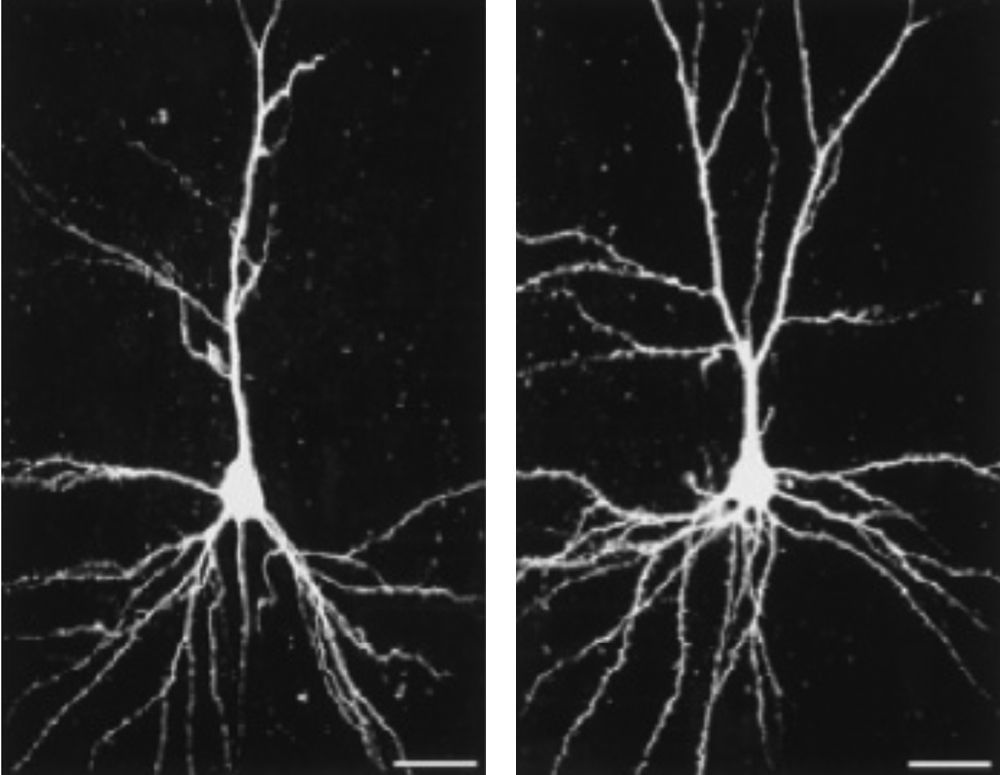
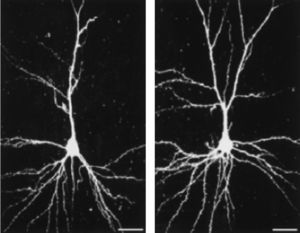
Hebb was the first to propose the ‘enriched environment’ as an experimental concept. He reported anecdotally that laboratory rats that nurtured at home as pets were behaviorally different than their littermates kept at the laboratory. Hebb was not the only one who conceptualized the effects of enriched nurturance having an effect on nervous system structure and function. Hubel and Wiesel examined the effects of selective visual deprivation during development on the anatomy and physiology of the visual cortex (Hubel & Wiesel, 1970; Wiesel & Hubel, 1965) and Rosenzweig and colleagues (Rosenzweig, 1966; Rosenzweig & Bennett, 1996; Rosenzweig et al., 1978) introduced enriched environments as a testable scientific concept by measuring the effects of environment on ‘total brain weight,’ ‘total DNA or RNA content,’ or ‘total brain protein’. Numerous researchers have demonstrated a significant linkage between enrichment and neurological plasticity that have included biochemical changes, gliogenesis, neurogenesis, dendritic arborization, and improved learning and memory (Greenough, West, & DeVoogd, 1978; Kempermann, Kuhn, & Gage, 1997). An example is provided below in Figure 2.
Dendritic morphology of pyramidal neurons in layer III of the somatosensory cortex in rat housed in (left) standard and (right) enriched environments. Bar=25μm. The enrichment significantly increases dendritic branching as well as the number of dendritic spines (cf. Johansson & Belichenko, 2001).
In an experimental setting, an enriched environment is ‘enriched’ in relation to standard laboratory housing conditions in that experimental animals in larger cages than their non-enriched peers have greater opportunity at social interaction with nesting material, toys and food locations frequently changed. The enriched animals were also given opportunities for voluntary activity on treadmills. These experiences have allowed researchers to formulate a definition of enrichment as “a combination of complex inanimate and social stimulation” (Rosenzweig et al., 1978).
In the landmark studies of vision by Wiesel and Hubel (1965), it was demonstrated that kittens reared with normal visual experience resulted in each eye having sole access to alternating columns of neurons in layer IV of the striate cortex. At birth, however, both eyes synapsed on all neurons in layer IV. In order to assure that a neuron is stimulated by experience coming from only one eye, a competitive process occurs in which activation and neighboring inhibition result in an alternating pattern of connectivity between columns of neurons in layer IV and each eye (Wiesel & Hubel, 1965).
When kittens were reared with one eye closed for a period of time after birth, the occluded eye became essentially functionally blind. This blindness is due to the elimination of connections of the closed eye to layer IV and the lack of exposure to patterned activity. If occlusion extends beyond a certain time period, the typical pattern of ocular representation cannot be recovered despite the restoration of visual input to both eyes (Wiesel & Hubel, 1965). It has been hypothesized that the initial ingrowth of axons from the thalamus to ocular dominance columns in visual cortex is governed by molecular cues (Crair, Horton, Antonini, & Stryker, 2001; Crowley & Katz, 2000). It has recently been shown, for example, that the decreased visual acuity seen in the adult rat suffering from chronic monocular deprivation is reversed if the adult rat is treated with dark exposure prior to removal of the occlusion (He, Ray, Dennis, & Quinlan, 2007). The increased plasticity induced by the dark environment may be due to a lack of input to visual cortex through the functioning eye, and therefore a reduction in the strength of previously established connections.
A similar restoration of visual acuity can also be induced with chronic administration of fluoxetine (Maya Vetencourt et al., 2008). Such dramatic changes in sensory system connectivity suggest that activity-dependent potentiation of these initial axons is required to maintain connections among cortical regions. In the case of primary visual cortex, local circuit neurons have been implicated in activity-dependent plasticity through GABAergic inhibition over a wide range of neighboring axonal paths (Fagiolini et al., 2004; Hensch & Stryker, 2004). An altered pattern of activity through one circuit can thus radically change neighboring circuits through an increase or decrease in inhibition of mediating cells.
The early development of visual pathways may be likened to the laying of a foundation and scaffolding for a building. If the scaffolding pattern is changed, the building may not be constructed in its original form, though a functional alternative may be reached. Thus, irreversible changes at the synaptic level do not necessarily translate into irreversible changes in a complex behavior (Feldman & Knudsen, 1998). For example, we now understand that the sensitive period for visual representation reflects, predominantly, the critical period for thalamic input to layer IV (Pascual-Leone, Amedi, Fregni, & Merabet, 2005), but that plasticity of other sensory systems may allow a blind person to demonstrate normal – and possibly enhanced – spatial awareness (Amedi et al., 2007). Plasticity in higher regions involved in spatial awareness feeds back upon lower pathways, thus compensating for an abnormal visual representation.
Advanced perceptual processes are also dependent upon the normal development of basic visual systems. For example, early visual deprivation due to congenital cataracts can lead to subtle but persistent deficits in face processing, even when the cataracts are removed in the first months of life (LeGrand, Mondloch, Maurer, & Brent, 2001). Similarly, experience with specific faces, such as same vs. different species, powerfully shapes subsequent face specialization. For example, monkeys deprived of viewing faces since birth are capable of discriminating both monkey and human faces following the selective restoration of faces in the visual environment, but what kind of faces determines whether that same monkey will be able to subsequently discriminate human or monkey faces – thus, monkeys selectively exposed to human faces can only discriminate human faces not monkey faces, and monkeys selectively exposed to monkey faces can only discriminate monkey faces, not human faces (Sugita, 2008).
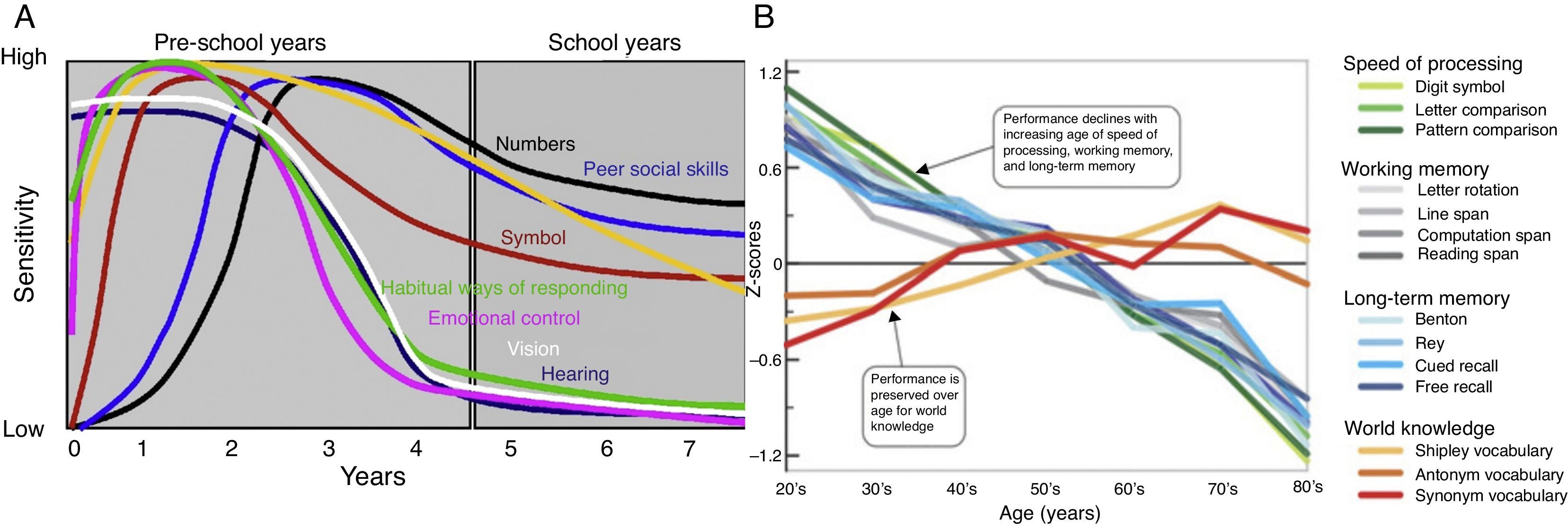
Critical and Sensitive Periods in the Early Development of Higher Cognitive ProcessesCritical periods are important stages in the lifespan of the child as he or she acquires a particular developmental skill that is indispensable and which can influence later development. If the child does not receive appropriate stimulation during a given critical period to learn a given skill or trait, it may be difficult, ultimately less successful, or even impossible, to develop some functions later in life. This is fundamentally different from the sensitive period, which is a more extended period of time during development when the child or adolescent is more receptive to specific types of environmental stimuli, usually because the nervous system development is especially sensitive to certain sensory stimuli at that given time. For example, the critical period for the development of a human child's binocular vision is thought to be between three and eight months, with sensitivity to damage extending up to at least three years of age. Further critical periods have been identified for the development of hearing and the vestibular system (Melillo & Leisman, 2009; Robson, 2002). Confirming the existence of a critical period for a particular ability requires evidence that there is a point after which the associated behavior is no longer correlated with age and ability stays at the same level. Sensitive periods of the child's cognitive development associated with the development of his or her nervous system is represented in Figs. 3 below. Here we can see the learning sensitivity for numerous cognitive as well as social skills.
Hubel and Wiesel's experiments involving visual deprivation brought about the concept of “sensitive” and “critical” periods in early cognitive development. “Sensitive” periods are defined as a time in development during which the brain is particularly responsive to experiences in the form of patterns of activity (Daw, 1997). Further, this time point may be termed a “critical” period if the presence or absence of an experience results in irreversible change (Newport et al., 2001; Trachtenberg & Stryker, 2001). Those factors that allow a circuit underlying cognition to be plastic – or render it unchangeable – are not yet well understood. In the area of speech and language, the “maturational hypothesis,” predicts that native language proficiency cannot be obtained when learning begins after puberty (Werker & Tees, 2005). Studies supporting this theory have correlated the degree of accent in a second language to age at the time of acquisition of that language (Birdsong & Molis, 2001).
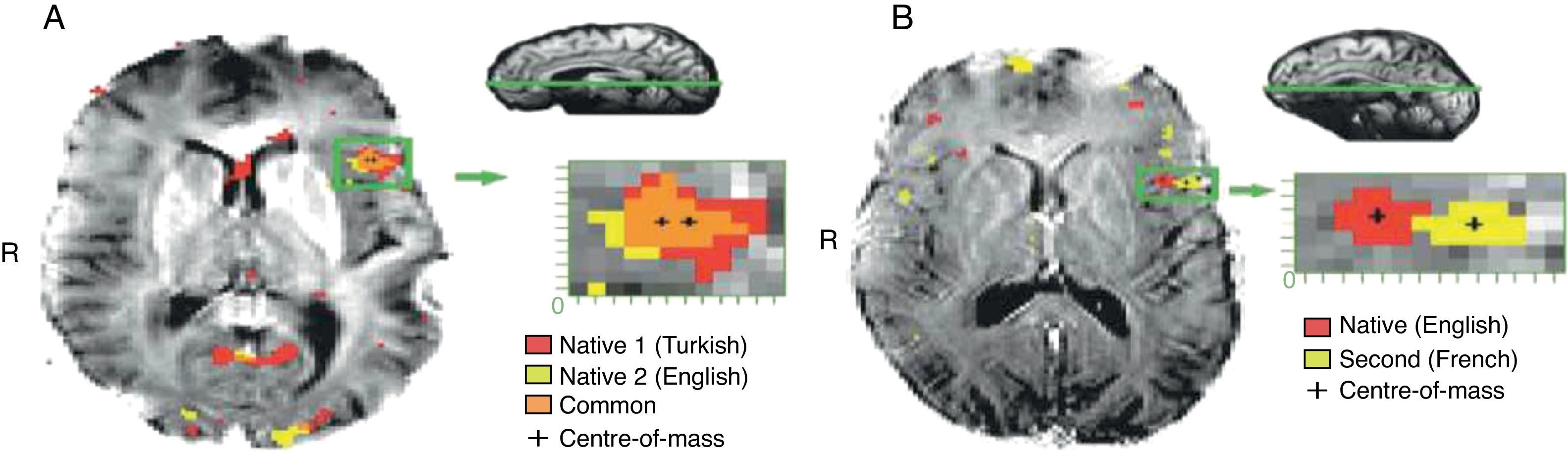
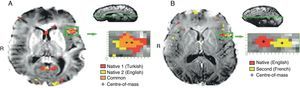
Adults exposed to a second language in early childhood were found to have native-like accents and pattern of tone (Gordon, 2000; Stein et al., 2006). Other researchers have also found a negative correlation between age at acquisition and grammaticality judgments (Komarova & Nowak, 2001). However, as seen in Figs. 4 below, brain areas representing early bilingual language acquisition overlap as compared to late bilingual language acquisition.
(A) and (B) represent the effect of brain on early as opposed to late exposure to a second language. The figures clearly indicate the nature of the optimization and efficiency of brain function connections when notions that related to early training and critical periods are applied.
Several investigators have used the theory of neural networks, originally developed for vision research, to model the activity of individual neurons and/or groups of neurons in the brain during learning (Morton & Munakata, 2005). These neural network models are particularly useful for comparing the experience-independent and experience-based accounts of sensitive periods, because the network can be kept constant with regard to features affected by maturation, motivation, and amount of exposure. Returning to the work of Hubel and Wiesel, it is important to note that the loss of binocular function in the kitten did not arise simply because of the absence of input to the occluded eye. Occluding both eyes during the same time period of development was proven not to result in loss of binocular vision (Cynader & Mitchell, 1980). It is necessary for one eye to have access to layer IV of the visual cortex while the other eye is denied access, allowing exclusive connectivity of the unoccluded eye to striate cortex. The irreversible loss of binocular vision during development must therefore be due to a combination of environmental experience and cortical learning processes (Knudsen, 2004).
The fact that the existence of a sensitive period can depend upon occurrence of a particular environment suggests that in early development, portions of networks become perceptually biased, making future modifications more difficult. For example, in the literature on both speech and face perception, the perceptual window through which faces and speech is initially processed is broadly tuned, then narrows with experience. For example, Pascalis et al. (2005) demonstrated that six- and nine-month-old infants and adults can readily discriminate two human faces, but only 6-month-old infants can discriminate two monkey faces. Similarly, six month olds given three months of experience viewing monkey faces can readily discriminate monkey faces at nine months of age, whereas nine-month-old infants not afforded such experience cannot (Pascalis et al., 2005).
As a rule, circuits that process lower level information mature earlier than those that process higher level information (Scherf, Behrmann, Humphreys, & Luna, 2007). For example, in the neural hierarchy that analyzes visual information, low-level circuits that analyze the color, shape, or motion of stimuli are fully mature long before the high-level circuits that analyze or identify biologically important stimuli, such as faces, food, or frequently used objects (Knudsen, 2004; Scherf et al., 2007). The process by which initial learning leads to a constraint on later learning is termed entrenchment, and is equally apparent in the development of speech (Munakata & Pfaffly, 2004; Seidenberg & Zevin, 2006). Several studies have shown, for example, that adults are often better at discriminating non-native phonetic contrasts when they differ substantially from phonemes of their native language (Kuhl, 2004).
Adults are poorer at discriminating when the phonetic contrasts are similar to phonetic contrasts of their native language. This is akin to the nature of the developing auditory system, which is more capable of discriminating tones outside of the tonal environment of hearing. At both the level of tone, and of speech phonetic discrimination, there is evidence for a fixed bias of the neural network. As discussed in the case of visual networks, however, neurons may be constantly modifying connectivity, allowing learning from new environments to compete against already existing tendencies. The role of environment and inputs to the brain may therefore be seen as critical in the bias of network formation during early life.
Altered patterns of enhancement and inactivity are thought to be the basis for neural plasticity and have been suggested in humans by studies of tactile and auditory perception in the blind, where such systems may even activate “visual” cortex (Merabet, Rizzo, Amedi, Somers, & Pascual-Leone, 2005). It is likely that changes in experience have a greater impact on an untrained ‘young’ network as compared to the same experience on an ‘older’ trained network. This biasing feature is suggested by studies on aphasia that show that words learned earlier in life are more resistant to loss, and are more easily accessed in naming tasks as compared to words learned later (Greenough, Black, & Wallace, 1987).
It has been suggested that learning through experience leads to the capacity to understand specific environments and the responses needed for these environments (Anisman, Zaharia, Meaney, & Merali, 1998). Similarly, changes in the environment – particularly when they are dramatic and pervasive – may have the power to alter neural connectivity and cognitive processing between systems. Examples can be found in studies of sensory deprivation, such as blindfolding, as well as sensory enhancement through technology. In studies of deaf children receiving cochlear implants, it is clear that language learning improves with earlier correction (Tomblin, Barker, Spencer, Zhang, & Gantz, 2005). It remains to be determined, however, whether this effect upon learning is due to actual changes in cognitive capacity or changes in the learning environment brought about by the ability to interact with others through spoken language.
Global Development: Higher-level Functions Build on Lower-level FunctionsThe nature of the child's experiences, particularly during a time-limited period in early development, can profoundly affect the mental framework we use to understand the world around us. Sensitive periods in child development are of interest because they represent a timeframe in which our capabilities can be modified and perhaps enhanced. The quality of experiences during such periods – be they adverse or enhancing – is also of importance in understanding why it may be difficult to restore normal function once development has been altered. While explanatory models for the timing of early experiences have generally been based at the genetic or neural circuit level, our direct observations of the effects of early environments are often made at the behavioral level. Through the study of sensitive periods, we are better able to understand the impact that early experience may have upon development. To cite but one example, it has recently been demonstrated that otherwise-typically developing young children institutionalized at birth have IQs in the low 70s. However, placing such children in high quality foster care before the age of two years leads to a dramatic increase in IQ (Nelson et al., 2007).
A similar trend also occurs for language (Windsor, Glaze, & Koga, 2007) and the development of the EEG (Marshall, Reeb, Fox, Nelson, & Zeanah, 2008), although in the case of the former, the sensitive period occurs around 16-18 months.
It is important to note recent work suggesting that the brain retains the capacity to adapt and change throughout the lifespan (Keuroghlian & Knudsen, 2007). However, the foundation of brain architecture must lie in the early developmental years, and that the influence of childhood environment is much more salient in such basic cognitive processes as sensory perception (Amedi et al., 2007; Knudsen, 2004; Pascual-Leone et al., 2005). Each sensory and cognitive system reaches a unique sensitive period (Daw, 1997), and thus identical environmental conditions will result in very different cognitive and emotional experiences for a child, depending upon his or her age (Amedi et al., 2007; Trachtenberg & Stryker, 2001; Tritsch, Yi, Gale, Glowatski, & Bergles, 2007).
Behavioral analysis can demonstrate the value of early experiences in the development of the brain. It must be remembered, however, that information is processed in a series of networks, each reflecting the effects of environment at varying time points. Higher level processing may mask modifications in lower levels networks (Daw, 1997; Feldman & Knudsen, 1998; Trachtenberg & Stryker, 2001). Thus, behavioral outcomes may be shaped by later experience, even though circuits at the lowest levels in a pathway remain irreversibly altered. In addition, studies of the plasticity of sensory processing reveal that similar information can be derived from alternative pathways (Akins, 2006; Pascual-Leone et al., 2005; Melchner, Pallas, & Sur, 2000). For example, when using sound devices to assess space, blind individuals have been shown to activate lateral occipital cortex in the same manner as sighted individuals do through vision (Amedi et al., 2007).
It has been suggested that loss of sensory input – such as occurs in late blindness – may in fact lead to the unmasking and strengthening of alternative pathways stemming from multisensory integration regions of the brain (Pascual-Leone et al., 2005). These pathways may not only substitute for the original sensory inputs, but may enhance previously existing capabilities. This form of sensory enhancement can often be seen in the highly tuned auditory and tactile perception of blind. High-level neural circuits that carry out sophisticated mental functions depend on the quality of the information that is provided to them by lower level circuits. Low-level circuits whose architecture was shaped by healthy experiences early in life provide high-level circuits with precise, high-quality information.
High-quality information, combined with sophisticated experience later in life, allows the architecture of circuits involved in higher functions to take full advantage of their genetic potential. Thus, early learning lays the foundation for later learning and is essential (though not sufficient) for the development of optimized brain architecture. Stated simply, rich early experience must be followed by rich and more sophisticated experience later in life, when high-level circuits are maturing, in order for full potential to be achieved (DeBello & Knudsen, 2004; Karmarkar & Dan, 2006; Sabatini et al., 2007).
Elevated cerebral glucose metabolism can be observed during ages 3-10 yrs., which, corresponds to an era of exuberant connectivity that is needed for energy needs of neuronal processes. In childhood it is measurably greater by a factor of 2 compared to adults. PET scans show the relative glucose metabolic rate. We see the complexity of dendritic structures of cortical neurons consistent with the expansion of synaptic connectivities and increases in capillary density in the frontal cortex. During early childhood cross-modal plasticity is more evident (Bavelier & Neville, 2002) with, as seen in Figure 4, exuberant connectivities between auditory and visual areas that will gradually decrease in most children between 6 and 36 months of age (Neville & Bavelier, 2002).
PET and fMRI studies have shown that elderly people are more less “optimized,” activating greater regions of the brain than younger individuals for a variety of motor tasks including simple one. Accuracy is not affected, but the results of greater areas of brain involved in motor tasks among the elderly is highly associated with increases in reaction time, with greater surface area activation, and with the recruitment of additional cortical and subcortical regions as compared to that found in younger individuals (Ward & Frackowiak, 2003).
Mind, Brain, and EducationNumerosityKnowing what we do about the neuroscience of plasticity and development under normal and enriched environments, we can understand that much of the knowledge base is predicated on a fair amount of research in lower organisms. This is not to say that there is no validity in the ability to extrapolate to normal child development. We know that brain networks, not necessarily structure, support cognitive function in the examples provided in the previous section. Classroom-based educational practice is supported by the knowledge base of Cognitive Psychology and it has been applied in the classroom to the analysis of reading by studying the component skills of word recognition, grammar and syntax text analysis, and metacognition. Additionally, the encoding of visual and auditory information from the printed words has been extensively examined as well as lexical access, which can determine if the visual representation matches a word in the reader's language. The tools of Cognitive Psychology have allowed the educator to understand better the component processes, skills, and knowledge structures underlying reading, mathematics, writing, and science (Bruer, 1993; Leisman, Machado, & Mualem, 2013; Skemp, 1987). The result of Cognitive Psychology's presence in the classroom has directly led to numerous instructional tools and technologies and this is not the forum in which to enumerate those advances (Carver & Klahr, 2013).
The missing piece is in the application of the Cognitive Neurosciences and engineering methods and methodology in the classroom. While applications in brain imaging have revealed much about the nature of thinking, problem solving, reading, sensory and perceptual processes and understanding, because of the lack of temporal resolution in these technologies, it is difficult to apply the findings in practical ways in classroom performance and in its evaluation.
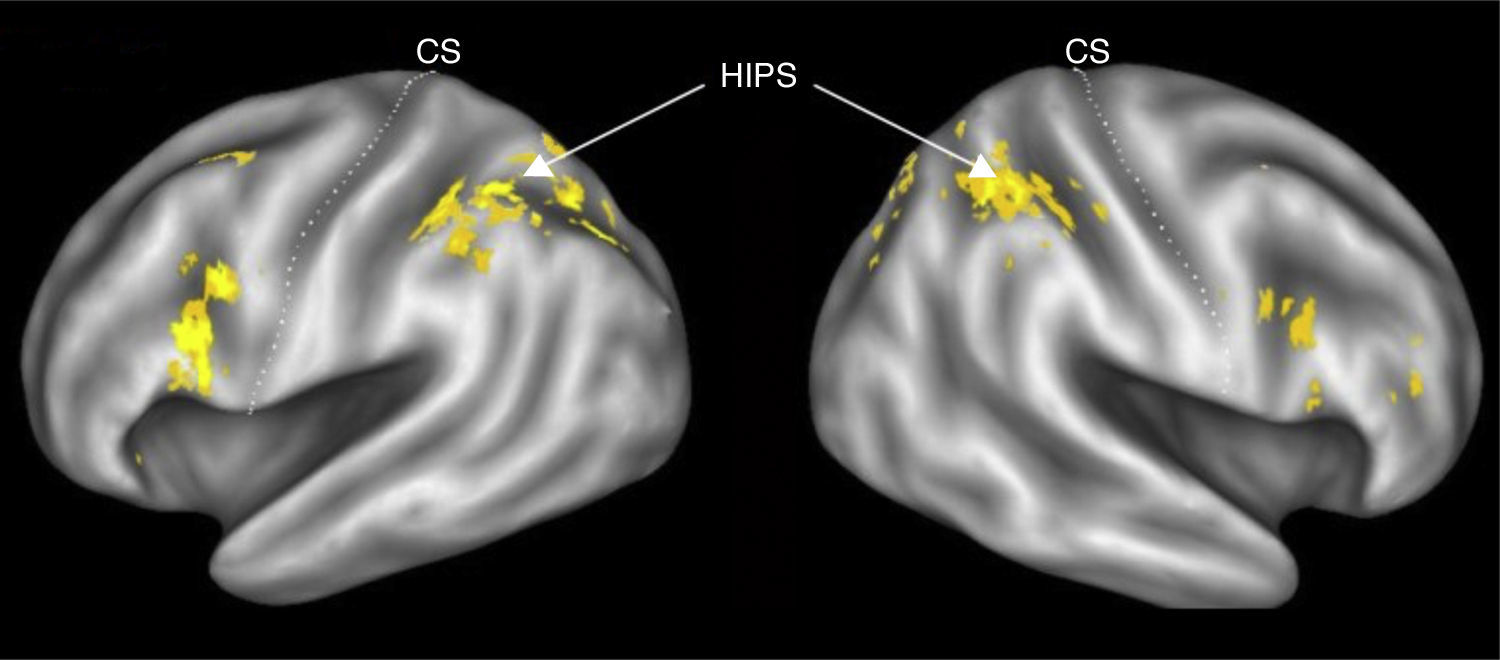
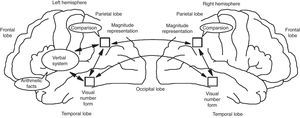
Stanislas Dehaene and Jean-Pierre Chageux (Dehaene & Changeux, 1993) developed a neuronal network model of number processing, which made the prediction that the parietal cortex contain “numerosity detectors” (cf. Fig. 5) These detectors are neurons tuned to a specific number, and thus firing preferentially for instance to sets of 3 objects. While it is very easy to fall into the trap of phrenology, with specific brain sites controlling specific function, Dehaene and colleagues have actually strongly argued for network approaches to understand brain and cognition. And it is those networks that need development inside the framework of formal education. An example of that type of network may be seen in Figure 6.
Neuronal Modeling for Numerosity (Dehaene & Changeux, 1993).
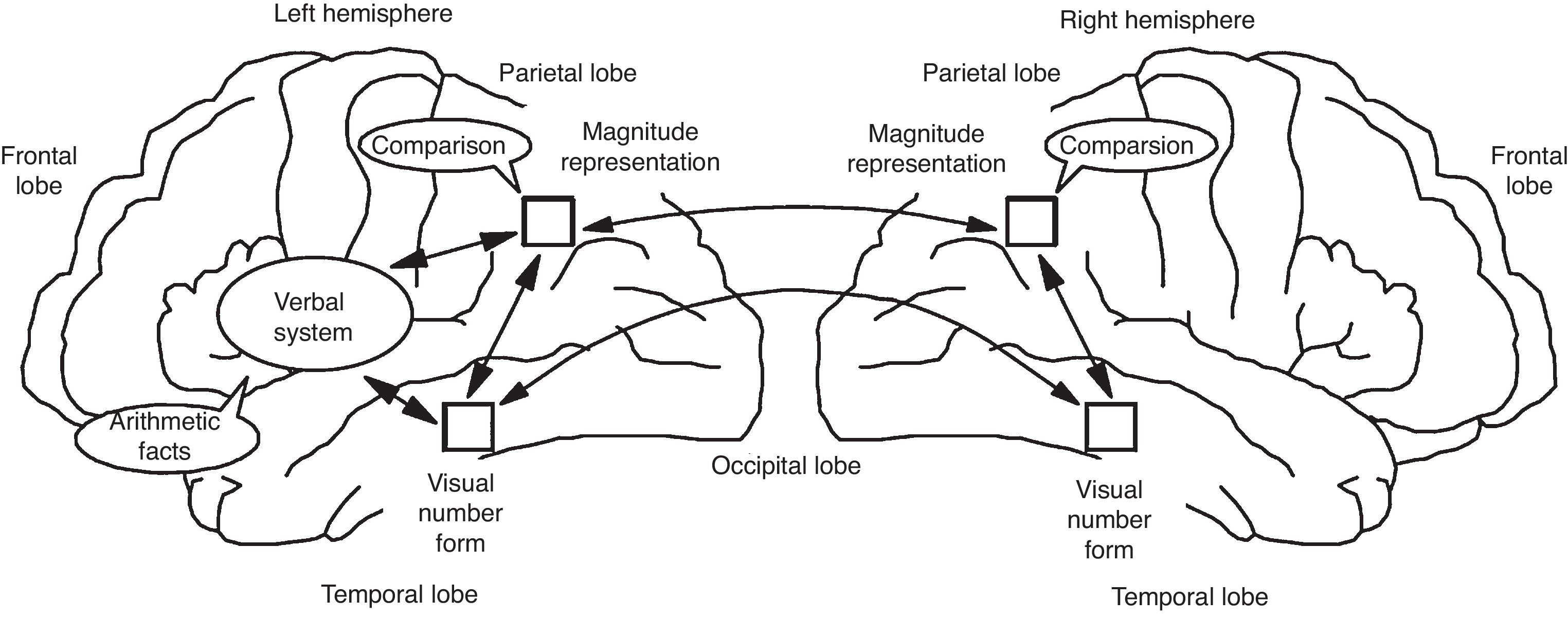
Schematic Functional and Anatomical Architecture of the Triple-code Model (Dehaene & Cohen, 1995). The localization of the main areas thought to be involved in the three numerical codes is depicted on a lateral view of the left and right hemispheres. The arrows indicate a functional transmission of information across numerical codes and are not meant as a realistic depiction of existing neural fiber pathways, whose organization is not fully understood in humans.
Dehaene argues that multiple brain areas contribute to the cerebral processing of numbers; the inferior parietal quantity representation is only one node in a distributed circuit. The triple-code model of number processing (Dehaene & Cohen, 1995) makes explicit hypotheses about where these areas lie, what they encode, and how their activity is coordinated in different tasks (Fig. 6). Functionally, the model rests on three fundamental hypotheses. First, numerical information can be manipulated mentally in three formats: an analogical representation of quantities, in which numbers are represented as distributions of activation on the mental number line; a verbal format, in which numbers are represented as strings of words (e.g., thirty-seven); and a visual Arabic representation, in which numbers are represented as a string of digits (e.g. 37). Second, transcoding procedures enable information to be translated directly from one code to the other. Third, each calculation procedure rests on a fixed set of input and output codes. For instance, the operation of number comparison takes as input numbers coded as quantities on the number line. Likewise, the model postulates that multiplication tables are memorized as verbal associations between numbers represented as string of words, and that multi-digit operations are performed mentally using the visual Arabic code.
Dehaene (Dehaene, 1996) designed an experiment to test a serial model of numerical comparison. In his experiment, right-handed college students had to decide if a number flashed on a computer screen was larger or smaller than five, then press a key to indicate their response. Dehaene manipulated three independent factors, where each factor was assumed to influence processing within only one of the model's stages. For the stimulus identification stage, he contrasted subjects’ performance when given Arabic (1, 4, 6, 9) versus verbal notation (one, four, six, nine). For the magnitude comparison stage, he compared subjects’ performance on close (4, 6 and four, six) versus far (1, 9 and one, nine) comparisons to the standard 5. His reason for choosing this factor is the well-established distance effect (Moyer & Landauer, 1967).
The distance effects showed that it takes subjects longer, and they make more errors, when asked to compare numbers that are close in numerical value than when asked to compare numbers that are farther apart in numerical value. In Dehaene's experiment, half the comparisons were close comparisons and half were far comparisons, a factor that should affect only the magnitude comparison stage. Finally, on half the trials, subjects had to respond “larger” using their right hand and “smaller” using their left hand, and on half the trials, “larger” with their left and “smaller” with their right. This factor should influence reaction times only for the motor preparation and execution stage.
When Dehaene analyzed subjects’ reaction times on the numerical comparison task, he found that the overall median (correct) reaction time was around 400 milliseconds. Subjects needed less than one half second to decide if a number was greater or less than 5. Furthermore, he found that each of the three factors had an independent influence on reaction time. Reactions to Arabic stimuli were 38 milliseconds faster than those for verbal notation, far comparisons were 18 milliseconds faster than close comparisons, and right-hand responses were 10 milliseconds faster than left-hand responses. Finally, the three factors had an additive effect on subjects’ total reaction times, just as one would expect if subjects were using the serial-processing model.
Dehaene's experiment, however, went beyond the typical cognitive experiment that would have stopped with the analysis of reaction times. Dehaene also recorded event-related potentials (ERPs), while his subjects performed the number comparison task. His ERP system measured electrical currents emerging from the scalp at 64 sites, currents that presumably were generated by the electrical activity of large numbers of nearby neurons. ERPs have relatively poor spatial resolution, but relatively precise temporal resolution. Significant changes in the electrical activity recorded at each of the 64 sites as subjects compared numbers might give general indications about where the neural structures were in the brain that implemented the three processing stages. The ERPs’ more precise temporal resolution might indicate the time course of the three processing stages. Together, the spatial and temporal data would allow Dehaene to trace, at least approximately, the neural circuitry that is active in numerical comparison. A cognitive model together with brain recording techniques created the possibility of mapping sequences of elementary cognitive operations onto their underlying neural structures and circuits.
This first significant ERP effect Dehaene observed occurred 100 milliseconds after the subjects saw either the Arabic or verbal stimulus. This change in brain activity was not influenced by any of the experimental factors. It appeared to occur in the right posterior portion of the brain. Based on this and other imaging and recording experiments, early activation in that part of the brain is most likely the result of the brain's initial, nonspecific processing of visual stimuli. At approximately 146 milliseconds after stimulus presentation, Dehaene observed a notation effect. When subjects processed number words, they showed a significant negative electrical wave on the electrodes that recorded from the left posterior occipital-temporal brain areas. In contrast, when subjects processed Arabic numerals, they showed a similar negative electrical wave on electrodes recording from both the left and right posterior occipital-temporal areas. This suggested that number words are processed primarily on the left side of the brain, but that Arabic numerals are processed on both the left and right sides. To look for a distance effect and the timing and localization of the magnitude comparison stage, Dehaene compared the ERPs for digits close to 5 (4, four and 6, six) with the ERPs for digits far from 5 (1, one and 9, nine). This comparison revealed a parieto-occipto-temporal activation in the right hemisphere that was associated with the distance effect. This effect was greatest approximately 210 milliseconds before the subjects gave their responses.
What is significant here, according to Dehaene, is that the timing and distribution of the electrical currents was similar for both Arabic digits and verbal numerals. This supports the claim, Dehaene argues, that there is a common, abstract, notation independent magnitude representation in the brain that we use for numerical comparison. To make a numerical comparison, we apparently translate both number words and Arabic digits into this abstract magnitude representation.
Finally, Dehaene found a response-side effect that occurred approximately 332 milliseconds after the stimulus or equivalently 140 milliseconds before the key press. This appeared as a substantial negative wave over motor areas in the brain. The motor area in the left hemisphere controls movement of the right side of the body, and the motor area in the right hemisphere controls movement of the left side of the body. Thus, as expected, this negative wave appeared over the left hemisphere for right-hand responses and over the right hemisphere for left-hand responses.
Dehaene's experiment exemplifies how cognitive neuroscientists use cognitive theories and models in brain imaging and recording experiments. Well-designed, interpretable imaging and recording studies demand analyses of cognitive tasks, construction of cognitive models, and use of behavioral data, like reaction times, to validate the models. Experiments like these suggest how neural structures implement cognitive functions, tell us new things about brain organization, and suggest new hypotheses for further experiments. Dehaene's experiment traces the approximate circuitry the brain uses to identify, compare, and respond to number stimuli. The experiment reveals several new things about brain organization that suggest hypotheses for further experiments. First, the experiment points to a bilateral neural system for identifying Arabic digits. This is something that one could not discover by analyzing behavioral data from normal subjects. Nor is it a finding found as a result of the neuropsychological study of patients with brain lesions and injuries that could reliably and unambiguously be supported. In fact, Dehaene suggests, the existence of such a bilateral system could explain some of the puzzling features about the patterns of lost versus retained number skills neuropsychologists see in these patients.
Second, this experiment suggests there is a brain area in the right hemisphere that is used in numerical comparison. This area might be the site of an abstract representation of numerical magnitude, a representation that is independent of our verbal number names and written number symbols. This, too, runs counter to common neuropsychological wisdom. Neuropsychologists commonly hold that the left parieto-occipito-temporal junction, not the right, is the critical site for number processing because damage to this area in the left hemisphere causes acalculia. Dehaene's finding of right hemisphere involvement during the comparison phase suggests that neuropsychologists should look more carefully than they might have in the past at numerical reasoning impairments among patients who have suffered damage to the right posterior brain areas. They might find, for example, patients who are able to read Arabic numerals and perform rote arithmetic calculations, but who are unable to understand numerical quantities, make numerical comparisons, or understand approximate numerical relations. Dehaene's work is just one example of how cognitive neuroscience is advancing our understanding of how brain structures might support cognitive function. Cognitive neuroscientists at numerous institutions are starting to trace the neural circuitry for other cognitive constructs and culturally transmitted skills. Several studies suggest that automatic and controlled processing rely on distinct brain circuits (Raichle et al., 1994).
Other studies show how attention can reorder the sequence in which component cognitive skills are executed in a task: the areas of brain activation remain the same, but the sequence in which the areas become active changes (Posner & Raichle, 1994). We are beginning to understand the different brain systems that underlie language processing and their developmental time course (Neville & Bavelier, 2002). Using our rather detailed cognitive models of reading – particularly word recognition –, PET, fMRI, and ERP studies allow us to trace the neural circuitry for early reading skills and to document the developmental course of this circuitry in children between the ages of 5 to 12 years (Posner, Abdullaev, McCandliss, & Sereno, 1999). However, in most cases, we are still far from understanding how these results might contribute to advances in the clinic, let alone in the classroom. It is not yet clear how we move from results like these across the bridge to educational research and practice. The example does, however, make two things clear. First, there is no way that we could possibly understand how the brain processes numbers by looking at children's classroom or everyday use of numbers or by looking at math curricula. Second, there is no way we could possibly design a math curriculum based on Dehaene's results. It is the cognitive research that creates both of those possibilities. When we do begin to understand how to apply cognitive neuroscience in instructional contexts, it is likely that it will first be of most help in addressing the educational needs of special populations. Cognitive psychology allows us to understand how learning and instruction support the acquisition of culturally transmitted skills like numeracy and literacy.
Cognitive Psychology in combination with brain imaging and electrophysiological recording technologies also allows us to see how learning and instruction alter brain circuitry. It opens the possibility of being able to see and to compare these learning-related changes in normal-versus-special learning populations. Such comparative studies might yield insights into specific learning problems and, more importantly, into alternative, compensatory strategies, representations, and neural circuits that children who learn with greater difficulty than others in the traditional learning settings. might exploit. These insights could in turn help us develop better instructional interventions to address specific learning problems.
Language SkillsSensory information undergoes extensive organization into associative networks necessary for incorporation into texture of cognition. The normal operation of such a system allows for the integration of motor and cognitive function of the kind that one sees in reading and language. Damage to or dysfunction in this system of the kind often found in post-stroke individuals can be exemplified in disconnection syndromes such as alexia without agraphia and a color-naming deficit with no other form of anomia evidenced (Leisman, 1976; Leisman, 2011; Leisman, Braun-Benjamin et al., 2014). This process of integration occurs along a synaptic hierarchy, which includes the primary sensory, up- and downstream unimodal, hetero-modal, paralimbic and limbic zones of the cerebral cortex. Connections from one zone to another are reciprocal and allow higher synaptic levels to exert a feedback (top-down) influence upon earlier levels of processing. Each cortical area provides a nexus for the convergence of afferents and divergence of efferents. The resultant synaptic organization allows each sensory event to initiate multiple cognitive and behavioral outcomes.
Upstream sectors of unimodal association areas encode basic features of sensation such as color, motion, form, and pitch. More complex contents of sensory experience such as objects, faces, word-forms, spatial locations, and sound sequences become encoded within downstream sectors of unimodal areas by groups of coarsely tuned neurons. Hetero-modal, paralimbic and limbic cortices, collectively known as trans-modal areas, occupy the highest synaptic levels of sensory-fugal processing. The unique role of these areas is to bind multiple unimodal and other trans-modal areas into distributed but integrated multimodal representations. Trans-modal areas in the mid-temporal cortex, Wernicke's area, the hippocampal-entorhinal complex and the posterior parietal cortex provide critical gateways for transforming perception into recognition, word-forms into meaning, scenes and events into experiences, and spatial locations into targets for exploration. All cognitive processes arise from analogous associative transformations of similar sets of sensory inputs. The differences in the resultant cognitive operation are determined by the anatomical and physiological properties of the trans-modal node that acts as the critical gateway for the dominant transformation. Interconnected sets of trans-modal nodes provide anatomical and computational epicenters for large-scale neurocognitive networks.
In keeping with the principles of selectively distributed processing, each epicenter of a large-scale network displays a relative specialization for a specific behavioral component of its principal neuropsychological domain. The human brain contains at least five anatomically distinct networks. The network for spatial awareness is based on trans-modal epicenters in the posterior parietal cortex and the frontal eye fields; the language network on epicenters in Wernicke's and Broca's areas; the explicit memory/emotion network on epicenters in the hippocampal-entorhinal complex and the amygdala; the face-object recognition network on epicenters in the mid-temporal and temporopolar cortices; and the working memory-executive function network on epicenters in the lateral prefrontal cortex and perhaps the posterior parietal cortex. Individual sensory modalities give rise to streams of processing directed to trans-modal nodes belonging to each of these networks. The fidelity of sensory channels is actively protected through approximately four synaptic levels of sensory-fugal processing. The modality-specific cortices at these four synaptic levels encode the most veridical representations of experience. Attentional, motivational, and emotional modulations, including those related to working memory, novelty-seeking, and mental imagery, become increasingly more pronounced within downstream components of unimodal areas, where they help to create a highly edited subjective version of the world.
The synaptic architecture of large-scale networks and the manifestations of working memory, novelty-seeking behaviors, and mental imagery collectively help to loosen the rigid stimulus-response bonds that dominate the behavior of lower animal species. This phylogenetic trend has helped to shape the unique properties of human consciousness and to induce the emergence of second order (symbolic) representations related to language. Through the advent of language and the resultant ability to communicate abstract concepts, the critical pacemaker for human cognitive development has shifted from the extremely slow process of structural brain evolution to the much more rapid one of distributed computations where each individual intelligence can become incorporated into an interactive lattice that promotes the trans-generational transfer and accumulation of knowledge.
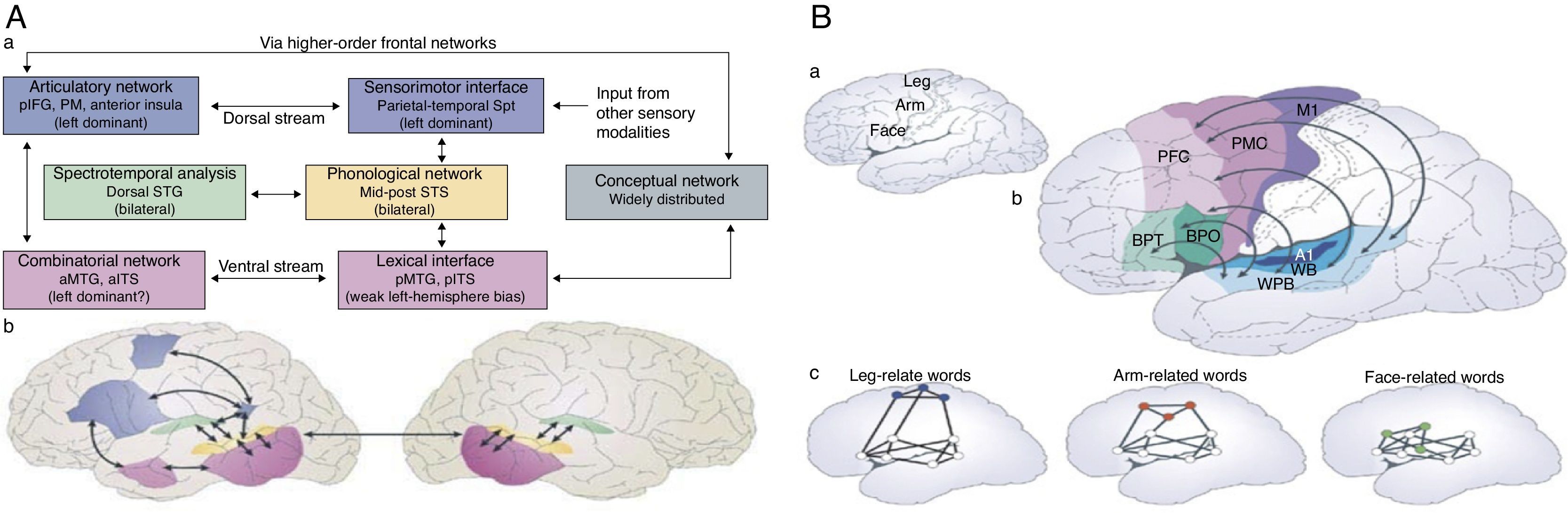
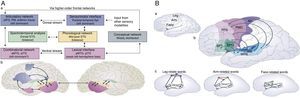
The transfer of knowledge from the environment and the development of skills to interact with that environment is a direct consequence of the ability to organize physical and measurable associational networks. Examples of such networks for language are represented in Figs. 7 (A and B) and in turn represent language embodiment in the networks rather than language ascribed to one particular brain region. Figs. 8 represent the power of Connectography in measuring the efficiencies of practical learning based on graph theory and Connectography.
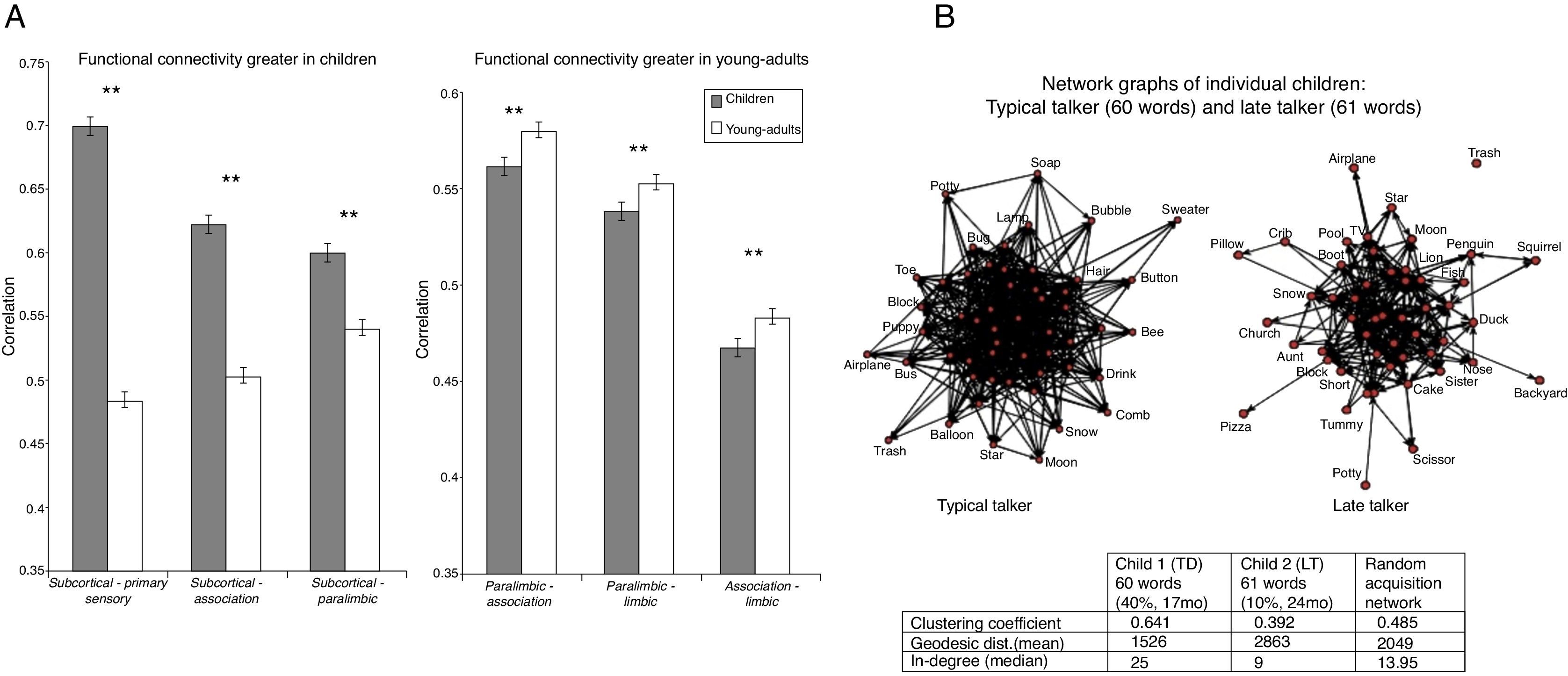
(A) Characterization, Organization & Development of Large-Scale Brain Networks in Children Using Graph-Theoretical Metrics. (B) The graph on the left is a typically developing (TD) child (17 mo, 40%) and the graph on the right is of an at-risk, late-talker (LT) (24 mo., 10%). The network of the TD child includes the 60 words in the child's productive vocabulary and the network of the at-risk LT child includes the 61 words in the child's productive vocabulary. The apparent visual differences in the networks are supported by the differences in the corresponding table, with the typical talker's network showing higher clustering coefficient and higher median in-degree, but lower geodesic distance, than the LT. These differences are consistent at both the individual and population level (cf. Leisman, 2013).
What we can learn from the characterization, organization, and development of large-scale brain networks in children using graph-theoretical metrics (Leisman, Rodriguez-Rojas et al., 2014) is that functional brain networks in children and young-adults show small-world properties. In mathematics, physics, and sociology, a small-world network is a type of mathematical graph in which most nodes are not neighbors of one another, but most nodes can be reached from every other node by a small number of steps. Specifically, a small-world network is defined as a network where the typical distance L between two randomly chosen nodes (the number of steps required) grows proportionally to the logarithm of the number of nodes N in the network (Watts & Strogatz, 1998). Functional connectivity networks of brain from EEG (Leisman, 2011) as well as MEG (Stam, 2004) have also been shown to possess small-world architecture. Large-scale brain networks in 7-9-y-old children show similar small-world, functional organization. Functional brain networks in children show lower levels of hierarchical organization compared to young-adults. Children and young-adults possess different interregional connectivity patterns, stronger subcortical-cortical connectivities in young adults and weaker cortico-cortical connectivities in children. Large-scale brain connectivity involves functional segregation and integration, stronger short-range connections in children, and stronger long-range connections in young-adults. In taking this concept further, we note that represented in Fig. 8(A) and is a representation of functional connectivity along the posterior-anterior and ventral-dorsal axes showing elevated subcortical connectivity and decreased paralimbic connectivity in children, compared to young-adults. This clearly demonstrates that the wiring and connectivities of young children is significantly different that that of teenagers. The change in organization of these connectivities directly speaks to the issue of optimization of pathways and is a direct consequence of training and therefore of education.
In attempting to apply graph theory to an understanding of language acquisition, Fig. 8(B) shows the responses of both typically developing (TD) and of at-risk, late-talkers (LT). There exists a significant and apparent visual difference in the networks with the TD's network showing higher clustering coefficient and higher median in-degree, but lower geodesic distance than the LT's connectivity networks of brain from EEG (Leisman, 2011) as well and MEG (Stam, 2004) have also been shown to possess small-world architecture. Large-scale brain networks in 7-9-y-old children show similar small-world, functional organization. Functional brain networks in children show lower levels of hierarchical organization compared to young-adults. Children and young-adults possess different interregional connectivity patterns, stronger subcortical-cortical connectivities in young adults and weaker cortico-cortical connectivities in children. Large-scale brain connectivity involves functional segregation and integration, stronger short-range connections in children, and stronger long-range connections in young-adults. In taking this concept further, we note that what is shown in Figs. 8(A) and (B) is a representation of functional connectivity along the posterior-anterior and ventral-dorsal axes showing elevated subcortical connectivity and decreased paralimbic connectivity in children, compared to young-adults. This clearly demonstrates that the wiring and connectivities of young children are significantly different from those of teenagers and beyond and the change in organization of these connectivities directly speaks to the issue of optimization of pathways and is a direct consequence of training and therefore of education.
Sensory Perceptual SkillsVisual processingVision is thought to be like all other high level abilities and therefore does not involve a single process. The function of early childhood play in general, and formal education in particular, is to integrate the various subsystems which compute information about the spatial properties of objects, movement, shape, color, etc. One type of visual processing is accomplished by the so-called ventral system because computations take place in the more ventral occipito-temporal and inferior-temporal cortices. This system has also been characterized as the “what system” (Ungerleider & Mishkin, 1982) as opposed to the “where system” functioning in the parietal lobe which focuses on object recognition. The two hemispheres are known to act differently in the way they encode shapes (Leisman, 1976; Melillo & Leisman, 2009).
It has been argued that many different functions of vision could be achieved effectively if the system could encode information at multiple levels of scale (Marr, 1982). In other words, one way to distinguish whether you were seeing an edge or just a change in texture is to determine whether changes in intensity are present at multiple scales. For instance, if they are noticeable with only high resolution, they are most likely texture variations; if they are present at multiple levels, they are probably edges (DeValois & DeValois, 1988). The evidence from research at this time suggests that the two hemispheres focus on different types of features of visual input when forming object representations. The left hemisphere is thought to focus on smaller parts, higher spatial frequencies, or details. The right hemisphere is thought to focus on the global form, lower spatial frequencies or course patterns.
To explain this asymmetry there are two theories that have been proposed (Brown & Kosslyn, 1995). One, a structural theory, proposes that one or more processing subsystems have become specialized in the hemispheres. The allocation theory states that the hemispheres tend to employ different strategies that often produce these results but there are not specific structural differences between hemispheres.
Visual processing can be divided into three phases of low, intermediate, and high levels (Marr, 1982) and the hemispheres can differ in their allocation of resources at any of these levels. While, as in most lateralized functions, each type of processing is found in both hemispheres, to different degrees, the hemispheres differ in the relative efficiency of the individual subsystems for a particular type of processing (structural theory) or in their predominance for using certain strategies (allocation theory) (Brown & Kosslyn, 1995).
The neurodevelopmental skills represented in pre-primary and primary education should be consistent with the normal development of visual processing. At the lowest level, subsystems organize the input so that distinct figures are separated from the ground. This processing takes place in a structure known as the visual buffer (Kosslyn, 1987). Computations in the visual buffer specify edges, regions of common color, and texture, and other characteristics that distinguish one object from its background. It is thought that not all the information in the visual buffer can be considered in detail, therefore, some information is chosen for additional processing. This has been referred to as an attention window that can be focused to a specific size, shape, and location to select a specific area of the visual buffer for more processing (Treisman & Gelade, 1980).
According to structural theories, the right hemisphere may more effectively detect large variations in light intensity over space. The left hemisphere more efficiently detects small variations in light intensity over space. This would suggest that the hemispheres differ in their sensitivity to different spatial frequencies (Sergent & Hellige, 1986).
At the intermediate level of visual processing, stimuli are organized into perceptual groups useful for later object recognition. In this model, the contents of the attention window are sent to a preprocessing subsystem in the occipito-temporal regions. Features such as texture gradients and color are found at this level (Kosslyn, Flynn, Amsterdam, & Wang, 1990). According to the structural theories, the subsystem is focused to detect different kinds of information in the two hemispheres. For instance, a child may wish to look at an overall pattern like a face, whereas in other instances the child may want to look at one of its components such as an eye. The two functions are not compatible with each other. The global function needs to incorporate into a whole the same things that need to be separated out by the local process. Therefore, it may be more efficient to have separate process operate in parallel at the two levels. These concerns should be addressed in the approach that nursery, kindergarten, and primary teachers take in working with perceptual problem solving and with the organization of visually based classroom materials.
High-level vision is concerned with matching input to representations in stored memory. It is thought that an object is reorganized when a match is made. In this same model, the output from the preprocessing subsystem serves as the input to the pattern activation subsystem found in the inferior temporal lobes. It is here that the perceptual input is compared to the stored visual information, and recognition is achieved if a match is made. If the input does not match a previously stored representation well enough, then the new pattern is stored. It is thought that size per se is not likely to be represented at the level of object recognition. It is thought that neurons in the inferior temporal lobe that are sensitive to high level visual properties are insensitive to changes in visual angle (Leisman, 1976; Melillo & Leisman, 2009; Plaut & Farah, 1990). The hemispheres may function differently in their ability for encoding parts in the whole or at different levels of hierarchy in a structural description. A structural description specifies how components are organized to compose a whole. The shape of a person is one example given to illustrate how parts are organized to compose a whole. In this example, a person is represented as a tree diagram, with the body at the top, head, trunk, arms, and legs as branches; upper arms, forearm, and hand as branches from the arm (Marr, 1982).
It is possible according to one theory that one hemisphere could store the (larger) wholes and the other could store the (smaller) parts. Another theory suggests that it is not size that is different but that the hemispheres store by preferred level of hierarchy. The left hemisphere may compute input farther down in a structural hierarchy whereas the right hemisphere may compute parts or wholes on higher or lower levels in hierarchy. Several experiments have been used to verify the functional differences between the two hemispheres. These are useful to review because they emphasize the functional differences in a practical way. In addition, the same techniques that are used to identify functions can be used to diagnose dysfunction, or if one hemisphere is decreased in activation as compared to the other. Additionally, if we know what each hemisphere responds to we can later use this information to concentrate rehabilitation on the performance of one hemisphere.
One of the most common experiments (Heinke & Humphreys, 2003) involves use of letter stimuli that are most commonly used in global precedence studies; the features of the global-level object (e.g., two vertical lines and one horizontal line forming an H) are determined by the positioning of the local elements. Therefore, there is a confounding between size and level of hierarchy; the larger letter is made up of smaller letters. In this case, the term hierarchy refers to objects that are made up only of their constituent parts. For example, a dog's body is hierarchically structured because it is made out of head, trunk, legs, and so on; if these parts are removed, nothing remains. In contrast, patterns on a shirt are not hierarchically related to the shirt, if one removes them, the shirt would remain intact.
In other experiments (Paquet & Merikle, 1988), investigators removed these confounding four letter stimuli. In addition to letter stimuli, picture stimuli could be employed that are not hierarchically arranged removing the possibility of processes that are specialized for reading. In most real world objects, global features provide general information about object identity, whereas local feature can be used to identify specific information. In one experiment (Martin, 1979), stimuli were presented consisting of pictures of garments with smaller pictures on them, the larger pictures were not composed of the smaller ones, and therefore there was not a hierarchic relation between the two. The smaller pictures were also garments, providing the same types of objects at the local and global level without a hierarchic arrangement. In one divided visual feed study of global precedence (Martin, 1979) it was found that the global (larger) was processed faster than the local (smaller) level in both hemispheres when the global and local level letters were different. However, the global level was processed faster following right hemisphere presentation than following left hemisphere presentation when subjects attended to the global level. In addition, there was a greater interference from the global level letter when stimuli were presented originally to the right hemisphere than when the subjects selectively attended to the local level. In this case, the subjects evaluated the stimuli more quickly when they were presented initially to the left hemisphere than to the right hemisphere.
To test whether local-global hemisphere specialization is different for hierarchic letter or non-hierarchic pictures (Lamb, Robertson, & Knight, 1990; Martin, 1979), which would suggest high-level visual processing, subjects were shown two types of stimuli: letters composed of smaller letters and articles of clothing with patterns of smaller articles of clothing printed on them. Results showed that for pictures, subjects did detect targets at the local level faster when stimuli were shown initially to the left hemisphere than the right hemisphere; however, they evaluated targets at global level equally well with both hemispheres. This confirms a local precedence and a trend toward the expected pattern of hemispheric specialization. In comparison, although a global precedence was found for the letter stimuli, the effect was the same when stimuli were presented initially to the right hemisphere or to the left. For letters, subjects responded faster and made fewer errors when the targets were at the global level than when they were at the local level.
In an attempt to explain the attention allocation hypothesis, Kosslyn, Chabris, Marsolek, & Koenig (1992) propose a specific mechanism where they suggest that the right hemisphere preferentially monitors outputs from neurons that have relatively large receptor fields. Also the hemispheres may differ in their ability to monitor the outputs from different size receptive fields even if the same outputs are available in both hemispheres (Kosslyn, Chabris, Marsolek, & Koenig, 1992). Kosslyn et al. (1992) suggest that the bias to encode outputs from neurons with different size receptive fields allows the ventral (object) and dorsal (spatial) systems to be coordinated. It is thought that an individual during movement not only needs to know the precise metric distances of objects (dorsal) but also the specific shapes of objects (ventral).
The two types of processing need to be linked and it is thought that this is best achieved if they are both in the same hemisphere – the right (Kosslyn et al., 1992; Marsolek, Kosslyn, & Squire, 1992). In addition, when an individual attempts to identify objects, they may need to ignore variations in shape among specific examples and may need only to know the type of spatial relations among parts, not the specific positions of parts of a given object. For example, to reorganize the shape of a dog, one would ignore the type of dog and the exact position of limbs. The left hemisphere is thought to have a special role both in generalizing over shapes (Marsolek et al., 1992) and in categorizing spatial relations (Kosslyn et al., 1992). Therefore, differences in receptive field size may act to coordinate the encoding of shapes and spatial relations, and could therefore cause the right hemisphere to specialize in computing metric spatial relations and specific shapes and the left hemisphere to specialize in computing categorical spatial relations and categories of shapes.
So far we have suggested that hemispheres function differently in their effectiveness of focusing attention at scales of different size, and that the underlying mechanism involves sampling outputs from neurons with different size receptive fields. This is similar to a spatial-frequency hypothesis. In fact, the receptive field and spatial-frequency theories predict similar results.
The two concepts are closely related. The smaller its receptive field, the higher the spatial frequency a cell will respond to; on the other hand, the larger the receptive field, the lower the spatial frequency. In this case it is thought therefore that a large receptive field, or a lower spatial frequency, is more of a right hemisphere function, whereas small receptive fields and higher spatial frequency is more of a left hemisphere function with the effects modulated by attentional variables. Normal processing of global aspects is thought to depend on the posterior superior temporal lobe of the right hemisphere. Normal processing of local elements depends on the posterior superior temporal lobe of the left hemisphere (Lamb et al., 1990).
Another important quality of visual information processing is the ability to localize a visual image in space. The observer is required to detect whether one object touches another object, this would be an on and off quality. Another requirement is the discernment of near or far and above or below. There are hemispheric performance differences for all of these tasks. Studies have shown that there is a left hemisphere advantage for the on-off tasks and a right hemisphere advantage for distance judgment tasks (Kosslyn, Sokolov, & Chen, 1989). Hellige and Michimata (1989) tested individuals by having observers indicate whether a dot was within 2cm of the line (a coordinate or distance task called near-far task). For the above-below task, there is a left hemisphere advantage whereas the right hemisphere shows an advantage for the near-far task.
Researchers have examined how these different asymmetries arise during the course of ontogenetic development. Compared to adults, the visual sensory system of newborns is especially limited in its transformation of information carried by high spatial frequencies (DeSchonen & Mathivet, 1989). It has been suggested that the development of various brain areas is more advanced in the right hemisphere than in the left hemisphere at the time of birth and possibly for a short time after. Hellige (1993) postulates a certain critical period for incoming visual input modification, which occurs earlier for the right hemisphere than for the left hemisphere. Once modified by highly degraded visual input, the right hemisphere is not only predisposed to become dominant for processing low spatial frequencies but is also less able than the left to take full advantage of higher frequencies when they finally do appear. If this notion is accurate, then it would also follow that the resulting hemispheric differences in visual processing would influence asymmetry for any task that depends on the relevant aspects of visual information whether the activity requires stimulus identification or stimulus location.
Enrichment Should be Synonym for Early Childhood EducationAlthough studies of the adverse effects of deprivation on brain development are powerful and compelling, they tell us little about the benefits of enrichment. Much of what we know about the impact of early experience on brain architecture comes from animal or human studies of deprivation. As we work to clarify further the patterns of genetic expression required for normal neural structure, we have also recognized that an optimal level of environmental input or “expectable” environment must exist in parallel. Increasing evidence suggests that this “expectable environment” of early development requires not only the variation in light necessary for vision, or the tones heard in a spoken language, but also the emotional support and familiarity of a caregiver (Nelson et al., 2007).
The well-documented negative impacts of deprivation on brain circuitry do not mean that excessive enrichment produces measurable enhancements in brain function. A small number of case reports exist in which neglected children with very little language experience in early childhood were given enriched language exposure in a protective environment (Curtiss, 1977; Itard, 1932). Longitudinal follow-up studies of these children demonstrated that, after several years of language exposure, they were unable to achieve adult-level native language abilities.
Most recently, early intervention to correct a deeply impoverished early environment has been shown to greatly improve cognitive, linguistic, and emotional capabilities in humans (Nelson et al., 2007; Windsor et al., 2007). Activity-dependent mechanisms of network formation may be responsible for such changes when children were placed into a stimulating environment for learning and exploration. With continued research into the modification of sensitive periods, as well as the factors influencing cortical plasticity throughout life, we may remain optimistic about the possibility of recovery from early deprivation. This in turn may provide hope for children who lack the biological framework, or necessary environment required for optimal neural and cognitive growth.
The possibility of cognitive and neural rehabilitation leads to theories of enrichment beyond the norm to a level of enhanced development. Educational and environmental enrichment of preschool children from impoverished economic settings has been shown to improve cognitive measures through early adulthood (Campbell, Pungello, Miller-Johnson, Burchinal, & Ramey, 2001). Cognitive capabilities, however, may follow a pattern similar to the growth curve of the human body; that is, while it is possible to enhance the environment of a child to assume the pattern of the normal curve, it is not possible to exceed the predicted trajectory to a significant extent without causing some potential harm. This is suggested by large studies of children from varying socioeconomic status, which demonstrated an improvement in cognitive performance only in those born to a low socioeconomic class, with no significant difference between those of middle-class and high-income families (Jeffaris, Power, & Hertzman, 2002).
If the possibility for enhancement exists, it is perhaps related to forms of enrichment that lie outside access to unlimited resources – i.e., beyond the expectable environment. Creativity, for example, is a key component to enhanced cognitive functioning, yet we have not been able to define the neural processes or environmental attributes that can enrich this aspect of cognition, nor are there sure-fire ways of boosting creativity among the population at large. Similarly, exposure to art or music or great literature or horseback riding may not confer any evolutionary advantage (i.e., reproductive success), yet these activities may confer some advantage among certain strata of society. Thus, perhaps it would be useful to draw a distinction between enrichment as applied to those experiencing downward deviations from the expectable environment (such as those reared in situations of neglect or deprivation) and enhanced enrichment applied to those reared in typical (expectable) environments. Enrichment may lead to a restoration of typical development whereas enhanced enrichment may lead to exceeding the typical environment. Of course, a challenge here lies in accounting for individual differences, as some individuals have greater potential to benefit from art or music lessons than others.
Individual heterogeneity may be under control of gene x gene, gene x environment, and environment x environment factors. For example, animal studies show that epigenetic mechanisms – whereby environmental factors and experiences early in life can permanently alter the genome of an individual through chemical modification – will impact long-term cognitive and social-emotional functioning (Szyf, McGowan, & Meany, 2008). The field awaits translation of this type of mechanism into human experiences. Finally, how might the field of developmental psychology benefit from advances being made in developmental neuroscience? First, given that our genome contains many fewer genes than we surmised even a decade ago (approximately 20,000), and given advances being made in the field of epigenetics, renewed attention should be paid to the origins and elaboration of complex human behaviors. Second, those working in the field of intervention should take stock in what is now known about neural plasticity; for example, it is quite possible that we could witness a revolution in new treatment approaches based on what we know about the malleability of the human brain. Finally, for the millions of children around the world who begin their lives in adverse circumstances, we should be mindful of what is known about sensitive periods, and act with alacrity to improve the lives of these children before neural circuits become well-established and thus, difficult to modify. To borrow an analogy from economics, by investing early and well in our children's development we increase the rate of return later in life, and in so doing improve not only the lives of individuals but of societies as well.
Resumen ampliadoDesde los tiempos de Camillo Golgi y Santiago Ramón y Cajal, en la década de los 90 del siglo XIX, hasta la moderna neurociencia, se han producido grandes avances en nuestro conocimiento del cerebro. Sin embargo, muy poco de ese conocimiento se ha trasladado al aula, y aún menos a las políticas educativas. Fruto de ese extenso recorrido científico, han surgido una serie de principios básicos de funcionamiento y desarrollo del sistema nervioso que son del mayor interés para la educación. Entre estos principios cabe destacar los siguientes:
- 1.
El cerebro humano se desarrolla desde la concepción hasta el comienzo de la segunda década de vida, empezando por los procesos más básicos. Es decir, se comienza por las funciones y controles vitales y autónomos, siguen los procesos cognitivo-motores sensoriales y perceptivos y culmina con los procesos de integración y toma de decisiones.
- 2.
El cerebro del niño recibe la influencia combinada de la genética y la experiencia.
- 3.
La capacidad del cerebro para modificarse decrece con la edad.
- 4.
Las capacidades cognitivas, emocionales y sociales están inexorablemente unidas a lo largo de toda la vida.
- 5.
Las funciones cognitivas y motoras interactúan en nuestro cerebro como consecuencia directa de nuestra postura bípeda.
- 6.
La presencia de tóxicos daña la arquitectura cerebral, lo que puede conducir a problemas para el aprendizaje, la conducta y la salud mental y física de por vida.
- 7.
El entorno del niño afecta directamente a la sinaptogénesis y permite la optimización neurológica.
Una de las primeras consecuencias de estos principios es que el papel de las escuelas infantiles y de los profesores en esta etapa de la vida resultará crítico para el establecimiento de unos sólidos cimientos funcionales que serán necesarios para el desarrollo ulterior del niño y para la neurobiología del adulto. Se ha constatado que el ambiente del niño tiene un impacto directo en los tiempos y la naturaleza de la expresión génica que afecta directamente a la arquitectura cerebral del niño.
El desarrollo cerebral, cognitivo, sensorial y perceptivo no ocurre simultáneamente sino más bien en diferentes etapas de desarrollo, como se puede apreciar en la figura 1. Lo importante a destacar aquí es que cada una de las capacidades perceptivas, cognitivas y emocionales se fundamenta en el andamiaje proporcionado por las primeras etapas de la vida. Todo esto ocurre porque aunque existan instrucciones genéticas para formar numerosas neuronas y sus conexiones, esto ocurre de manera no direccional, es decir, sin especificación de su forma concreta. Es más bien la reorganización epigenética posterior (la estabilización sináptica, consecuencia del uso y la experiencia) la que determina la forma y configuración de los circuitos neurales del cerebro. En este sentido, la neurociencia ha demostrado la estrecha relación que existe entre el grado de enriquecimiento ambiental y procesos neurobiológicos tan importantes como la bioquímica cerebral, la gliogénesis, la neurogénesis o la arborización dendrítica, entre otros.
De ahí la crucial importancia de la experiencia en las primeras etapas de la vida. Se sabe además que los precursores de lo que posteriormente serán las distintas áreas funcionales del cerebro emergen aproximadamente durante los dos primeros trimestres de la gestación en el humano. Esta organización es la base sobre la que se fundamenta el detallado desarrollo posterior de los circuitos neurales. Por lo tanto, estas etapas del desarrollo serán también fundamentales y cualquier influencia sobre las mismas (p. ej., exposición a tóxicos) puede tener consecuencias de largo alcance.
Es cierto que nuestro cerebro es más plástico que el de muchos otros organismos, manteniendo esta propiedad incluso en el adulto. Sin embargo, el fundamento de la arquitectura cerebral del adulto se establece en edades tempranas y se sabe que la influencia del ambiente durante la infancia es tremendamente significativa para los mismos procesos sensoriales de la percepción. Y es sobre estos procesos sobre los que se fundamenta el funcionamiento de los procesos cognitivos superiores alcanzados en etapas posteriores y utilizados en la adultez. Cada sistema sensorial y cognitivo tiene su propio período sensible y los que se alcanzan más tarde tienen su fundamento en los alcanzados previamente. De ahí que unas mismas condiciones ambientales puedan tener efectos muy distintos dependiendo de la edad del niño de la que hablemos.
Efectivamente, los circuitos neurales de alto nivel, que llevan a cabo operaciones mentales sofisticadas, dependen para su correcto funcionamiento de la calidad de la información que les proporcionan los sistemas de bajo nivel. Los sistemas de bajo nivel cuya arquitectura haya sido moldeada por experiencias saludables en las primeras etapas de la vida proporcionarán a los circuitos de alto nivel información precisa y de alto nivel. Ésta, combinada con una experiencia rica y sofisticada en etapas posteriores de la vida, permitirá que la arquitectura de los circuitos implicados en funciones cognitivas superiores alcance todo su potencial genético. Por lo tanto, el aprendizaje temprano es el fundamento del aprendizaje posterior y es esencial (aunque no suficiente) para un desarrollo óptimo de la arquitectura cerebral. Dicho de otra forma, una experiencia temprana enriquecida debe de ir seguida de más experiencia enriquecida y sofisticada, especialmente cuando los circuitos de orden superior están madurando. La neurociencia ha constatado, en este sentido, que el metabolismo cerebral de la glucosa entre los 3 y los 10 años de edad –período que se corresponde con una etapa de conectividad neural exuberante– es de en torno al doble del de un adulto.
El enriquecimiento ambiental debería ser por tanto sinónimo de educación infantil. Para los millones de niños de todo el mundo que comienzan la vida en circunstancias adversas, deberíamos tener presente lo que se sabe acerca de los períodos sensibles del desarrollo y actuar con diligencia para mejorar su vidas antes de que los circuitos neurales se establezcan y afiancen y, por tanto, sean más difíciles de modificar. Empleando una metáfora en términos económicos, podríamos decir que al invertir bien y pronto en el desarrollo de los niños incrementaremos la cantidad de retorno en etapas posteriores de la vida. De este modo, no sólo mejoraríamos la vida de las personas sino también la de toda la sociedad.
Conflict of InterestThe authors of this article declare no conflict of interest.
Financial SupportThis work was supported in part by the government of Israel through the Kamea Dor-Bet program and by the Children's Autism Help Project-USA.