In Argentina, hemolytic uremic syndrome (HUS) caused by EHEC has the highest incidence in the world. EHEC infection has an endemo-epidemic behavior, causing 20–30% of acute bloody diarrhea syndrome in children under 5 years old. In the period 2016–2020, 272 new cases per year were notified to the National Health Surveillance System. Multiple factors are responsible for HUS incidence in Argentina including person-to-person transmission. In order to detect possible EHEC carriers, we carried out a preliminary study of the frequency of kindergarten teachers with anti-LPS antibodies against the most prevalent EHEC serotypes in Argentina. We analyzed 61 kindergarten teachers from 26 institutions from José C. Paz district, located in the suburban area of Buenos Aires province, Argentina. Fifty-one percent of the plasma samples had antibodies against O157, O145, O121 and O103 LPS: 6.4% of the positive samples had IgM isotype (n=2), 61.3% IgG isotype (n=19) and 32.3% IgM and IgG (n=10). Given that antibodies against LPS antigens are usually short-lived specific IgM detection may indicate a recent infection. In addition, the high percentage of positive samples may indicate a frequent exposure to EHEC strains in the cohort studied, as well as the existence of a large non-symptomatic population of adults carrying pathogenic strains that could contribute to the endemic behavior through person-to-person transmission. The improvement of continuous educational programs in kindergarten institutions could be a mandatory measure to reduce HUS cases not only in Argentina but also globally.
En Argentina, el síndrome urémico hemolítico causado por Escherichia coli enterohemorrágica (EHEC) tiene la más alta incidencia del mundo. Las infecciones por EHEC tienen un comportamiento endemoepidémico y causan del 20 al 30% de los síndromes de diarrea sanguinolenta en niños menores de 5 años. En el período 2016-2020, se notificaron 272 nuevos casos por año al Sistema de Vigilancia de Salud Nacional. Múltiples factores son responsables de la alta incidencia de SUH en Argentina, incluyendo la transmisión persona-persona. Con el objetivo de detectar posibles portadores asintomáticos de EHEC, realizamos un estudio preliminar de la frecuencia de anticuerpos antilipopolisacáridos contra los serotipos de EHEC más prevalentes en Argentina. El estudio se realizó con muestras de plasma obtenidas de 61 maestras y maestros de jardines de infantes de 26 instituciones del distrito de José C. Paz, localizado en el área suburbana de la provincia de Buenos Aires, Argentina. El 51% de las muestras presentaron anticuerpos contra los serotipos de lipopolisacáridos O157, O145, O121 y O103; el 6,4% de las muestras positivas tuvieron el isotipo IgM (n=2), el 61,3% el isotipo IgG (n=19) y el 32,3% los isotipos IgM e IgG (n=10). Dado que los anticuerpos antilipopolisacáridos presentan usualmente una duración corta, la detección de IgM específica podría indicar una infección reciente. Además, el alto porcentaje de muestras positivas hallado podría indicar una exposición frecuente a las cepas de EHEC en la cohorte estudiada. Asimismo, la gran población de adultos portadores asintomáticos de estas cepas patógenas podría contribuir al comportamiento endémico, a través de la transmisión persona-persona. El perfeccionamiento de programas educacionales continuos en jardines de infantes podría constituir una medida importante para reducir los casos de síndrome urémico hemolítico, no solo en Argentina, sino también en el mundo.
Enterohemorrhagic Escherichia coli (EHEC) infections may cause a wide spectrum of clinical manifestations, such as non-bloody diarrhea, hemorrhagic colitis, and hemolytic uremic syndrome (HUS), the most serious complication. HUS is characterized by microangiopathic hemolytic anemia, thrombocytopenia and variable degrees of kidney injury, which result from Shiga toxin (Stx) activity and may have fatal consequences or long-term morbidity. Children under 5 years of age are the most affected and Argentina has the highest incidence in the world, with an endemo-epidemic behavior. A median of 272 new cases per year was notified to the National Health Surveillance System in the period 2016–2020, with an annual incidence of 7.3 cases per 100,000 children under 5 years of age4. Infection is commonly associated with the ingestion of contaminated meat, vegetables or water, but also with person-to-person contact5,32. In this sense, attending child-care or kindergarten institutions has been reported as risk factors for HUS, probably as a consequence of the person-to-person infection route10,19,22. This fact suggests that it may be important to search for asymptomatic carriers, especially those in contact with children under 5 years old.
Although the detection of Stx-encoding genes by polymerase chain reaction (PCR) in feces is currently the gold standard to detect EHEC infections13,30,34,41, the low infection inoculum that causes disease in children and the short period of EHEC-shedding often makes it hard to establish EHEC infections only by genetic diagnosis12,43. It has been previously reported that infections by EHEC can be reliably detected by using lipopolysaccharide (LPS)-based antibody tests3,18,25,26,42, which are sensitive and specific. At present, we know that the predominant EHEC serotype associated with serious disease in many countries is O157:H71. However, non-O157 serotypes have also been detected in HUS patients at a lower frequency23. It has been recently reported that 73.6% of HUS cases in Argentina were associated with EHEC O157 infection, followed by EHEC O145 (16.8%), O121 (5.4%), and other serotypes (4.2%)1.
Given the epidemiological importance of person-to-person transmission and the close contact of kindergarten teachers with children under 5 years old, the objective of the present study was to determine the frequency of kindergarten teachers with anti-LPS antibodies in plasma against the most prevalent EHEC serotypes in Argentina: O157, O145, O121 and O103. In addition, an informative talk was given to kindergarten teachers by LUSUH (Lucha contra el Síndrome Urémico Hemolítico), a non-governmental association that promotes the implementation of good practices to reduce EHEC transmission, including information on food preparation and appropriate hygiene practices to prevent food-borne diarrhea diseases, particularly associated with EHEC.
Materials and methodsTwo hundred and fifty teachers from twenty-six public kindergartens from José C. Paz district, located in the suburban area of Buenos Aires province, Argentina, participated in the informative talks provided by LUSUH. Talks included information about adequate food handling and hygiene practices to prevent food-borne diarrhea diseases, particularly associated with EHEC. Kindergarten teachers were also provided with educational boxes created by the LUSUH Association (Bulletin No. 15, www.lusuh.org.ar) containing puppets and scripts in order to teach children about three topics: washing hands, washing fruits and vegetables, and proper cooking of meat. In addition, warning signs to place in kitchens and bathrooms were also given to be taken into consideration.
Then, 61 kindergarten teachers (4 males, 57 females) gave blood voluntarily in order to analyze the presence of anti-LPS antibodies in plasma against the most prevalent EHEC serotypes. The Ethics Committee of the University of Buenos Aires approved this study. All volunteers were enrolled in this study after a written informed consent was obtained. Blood samples were obtained by pricking the index finger with a needle and collecting the blood into heparinized capillary tubes and plasma was immediately separated and stored at −20°C until use.
Samples were evaluated for the presence of immunoglobulin isotype M and G (IgM and IgG) against O157, O145, O121 and O103. We used the commercial ELISA kit CHEMLIS® E. coli Combi Glyco-iELISA (Chemtest Argentina), following the manufacturer's indications.
Results were expressed as the reactivity ratio, which was calculated as follows: (absorbance at 450nm (A450) of the test sample/mean A450 of the positive control)×100. The positive-control serum was included in each assay run.
In this work, for methodology convenience, plasma samples were used. Many studies suggest that plasma and serum samples can be used indistinctly for antibody detection27,35 although a validation of the usage of plasma for ELISA kit CHEMLIS® E. coli Combi Glyco-iELISA (Chemtest Argentina) would be necessary for the clinical practice.
Graphs and statistical analyses were done by using the Graph-Pad Prism software (version 5.01 for Windows; San Diego, CA). Frequency values were compared with the Chi-square test. p values <0.05 were considered significant.
ResultsA total of 61 samples were evaluated by Glyco-iELISA for the presence of immunoglobulin isotype M and G (IgM and IgG) against the most prevalent LPS serotypes from EHEC in Argentina: O157, O145, O121 and O103. Fifty-one percent of the samples (n=31) showed antibodies against at least one of the serotypes evaluated (Fig. 1A). When classifying positive samples according to the isotype of immunoglobulin detected, IgM or/and IgG, we observed that 6.4% of the positive samples had IgM isotype (n=2), 61.3% IgG isotype (n=19) and 32.3% IgM and IgG isotypes (n=10) (Fig. 1B). Thus, approximately 39% of the positive samples had IgM isotype. Given that this type of immunoglobulin accounts for the first specific or adaptive immune response, we could suggest that those kindergarten teachers had been exposed at least recently to EHEC LPS, if not at the moment of sampling.
Reactivity against at least one LPS serotype. (A) Samples were grouped according to the IgM and/or IgG antibody detection (IgM+ and/or IgG+) or no antibody detection (IgM− and IgG−) against at least one LPS serotype. (B) Positive samples were grouped according to the isotype of immunoglobulin detected against at least one LPS serotype into: IgM antibody detection (IgM+), IgG antibody detection (IgG+), and IgG and IgM antibodies detection (IgG+ and IgM+).
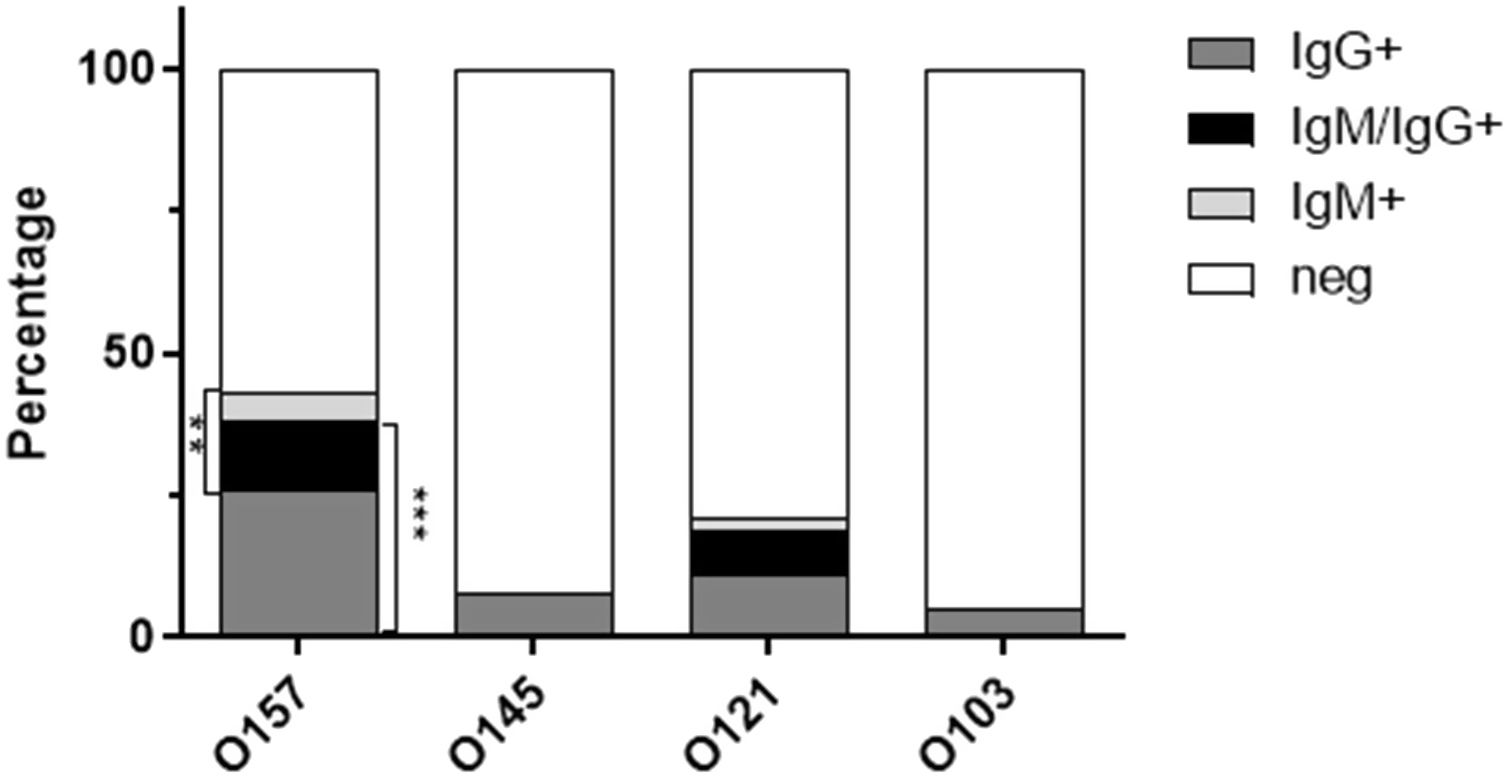
As shown in Figure 2, the presence of antibodies against the analyzed EHEC serotypes was significantly different (p<0.0001, Chi-square test). Antibodies against LPS O157 were the most prevalent, in 43% of samples (n=26), followed by LPS O121 (21%, n=13), LPS O145 (8%, n=5) and LPS O103 (5%, n=3). When classifying positive samples according to the Ig isotype for each LPS serotype (Fig. 3), we observed that the abundance of IgM positive samples was significantly different among EHEC serotypes (p<0.001, Chi-square test), 17% of samples (n=10) had IgM antibodies against LPS O157, 10% (n=6) against LPS O121 and 0% against LPS O145 and O103. In the same way, the abundance of IgG positive samples was significantly different among EHEC serotypes (Fig. 3) (p<0.0001, Chi-square test), 38% of samples (n=23) had IgG antibodies against LPS O157, 19% (n=11) against LPS O121, 8% (n=5) against LPS O145 and 5% (n=3) against LPS O103.
Percentage of reactive samples according to the Ig isotype for each LPS serotype. Positive samples were classified according to the Ig isotype (IgG, IgM, IgM/IgG) reactive with the indicated LPS serotype. **p<0.001 IgM+ vs IgM− for each serotype; ***p<0.0001 IgG+ vs IgG− for each serotype.
Finally, we observed that 30% (n=18) of the samples reacted against only one serotype whereas 21% (n=13) of the samples reacted against more than one LPS serotype: 5% was positive for LPS O157 and O145, 11% for LPS O157 and O121, 2% for LPS O157, O121 and O145 and 3% for LPS O157, O121 and O103 (Fig. 4). Table 1 shows each sample that reacted with more than one LPS serotype segregated by the Ig isotype detected.
Samples that reacted with more than one LPS serotype and the immunoglobulin isotype detected.
| Sample # | O157 | O145 | O121 | O103 | ||||
|---|---|---|---|---|---|---|---|---|
| IgM | IgG | IgM | IgG | IgM | IgG | IgM | IgG | |
| 4 | neg | 70 | neg | neg | 54 | 71 | neg | neg |
| 5 | neg | 65 | neg | neg | neg | 47 | neg | neg |
| 11 | 68 | 73 | neg | neg | 56 | 79 | neg | neg |
| 13 | 50 | 81 | neg | neg | neg | 61 | neg | neg |
| 16 | neg | 64 | neg | 46 | neg | neg | neg | neg |
| 20 | neg | 81 | neg | 69 | neg | 63 | neg | neg |
| 21 | 43 | 63 | neg | 41 | neg | neg | neg | neg |
| 25 | 45 | 63 | neg | neg | 44 | 51 | neg | neg |
| 26 | 50 | neg | neg | neg | 44 | neg | neg | 92 |
| 32 | neg | 70 | neg | 53 | neg | neg | neg | neg |
| 33 | 50 | neg | neg | neg | 44 | 44 | neg | neg |
| 46 | 66 | 86 | neg | neg | neg | 53 | neg | 90 |
| 54 | neg | 61 | neg | neg | neg | 49 | neg | neg |
neg: negative.
Despite 40 years of research, relatively little is known about the factors responsible for the high incidence of HUS associated with EHEC21. Not only person-to-person transmission of EHEC strains but also the detection of household contacts2,21,38,45 of HUS patients with EHEC isolates have been documented broadly and associated with outbreaks or sporadic HUS cases5,10,22,32,39,40. Even if it is often hard to determine the infection source, or if HUS patients were infected by the same contaminated food than close contacts, the detection of asymptomatic EHEC carriers may help to establish diffuse outbreaks undetected by the surveillance system and monitor EHEC circulation and dissemination. Taking into account that attending kindergarten or childcare institutions has been determined as a risk factor to develop HUS in children, in the present study we evaluated 61 kindergarten teachers from 26 institutions from José C. Paz district, located in the suburban area of Buenos Aires province, Argentina. We observed that 51% of the samples had antibodies against the most prevalent EHEC serotypes, O157, O145, O121 and O103. The high percentage of positive samples may be related to the endemic behavior of post diarrheal HUS in Argentina, and agrees with the presence of anti-Stx2 antibodies in healthy children previously found in Argentina17. However, we have previously reported that only 11% of kindergarten teachers from a different cohort from Buenos Aires city and suburban areas had anti-LPS O157 antibodies, while 83% of them had anti-Stx2 antibodies15. The higher prevalence of anti-EHEC LPS antibodies observed in the public kindergartens from José C. Paz district may suggest an improvement in diagnostic technology and/or a higher risk of EHEC infections in this district compared to Buenos Aires city and suburban areas. In this regard, José C. Paz district has severe deficiencies in housing, urbanization and basic public services, with more than 44% of deficient housing. Moreover, 5.88% of the population lives in critical overcrowding conditions (more than 3 people per room), only 17.3% of the population has network water and 6% has sewage11. Twelve percent of households have unsatisfied basic needs (NBI), a percentage that is higher than the average for other suburban areas of Buenos Aires. Although the infant mortality rate showed a downward trend between 2005 and 2014, it continues to be higher than that of other suburban areas. In addition, it concentrates the highest percentages of the population with low educational levels: 1 out of 5 inhabitants over 20 years of age did not complete primary school. We hypothesize that these sociodemographic factors may have an impact on appropriate hygiene practices and food preparation, thus explaining the differences observed in the percentage of kindergarten teachers from the two cohorts with anti-EHEC LPS antibodies in plasma.
Although the current gold standard to confirm EHEC infection involves the detection of EHEC in feces by culture or Stx-encoding genes by PCR in fecal culture13,30,34, the drawback is that a negative result does not often rule out EHEC infection. In this sense, it has been recently published that EHEC infection is confirmed in only 65% of HUS cases in Argentina24. This is fundamentally due to the short period of excretion, since EHEC is shed rapidly during the prodromal phase, which has often ceased before HUS. In addition, these pathogens have an unusually low infectious dose; about 10–100 bacteria can cause disease9,37. It was shown that the detection of serum antibodies against EHEC LPS by ELISA may increase the confirmation of EHEC etiology of HUS. Greatorex and Thorne showed that combining the serologies for antibodies to LPS O157 with fecal culture produced evidence of infection by these organisms in 82% of HUS patients20. In a 10-year study, Chart and Cheasty demonstrated that in 97.5% of patients evidence of infection with EHEC O157 was provided solely by serology, since only in 17 out of 676 children a fecal isolate of E. coli O157 was obtained7. More recently, it has been demonstrated that serological evidence of EHEC infection improved diagnosis from 54% to 77% in HUS patients in The Netherlands42, and from 38.7% to 88.9% in Argentina18. Although we did not evaluate feces from kindergarten teachers for the presence of EHEC, the low percentage of EHEC isolates in HUS patients, in which the bacterial load may be higher than in asymptomatic adults, led us to suppose that a negative result in feces culture from asymptomatic adults may not be indicative of absence of EHEC carriage. In this sense, a study of sera from members of rural communities in England showed that healthy people with occupational exposure to cattle had antibodies to LPS O157 and/or to Stx14. A Canadian study showed that 6.3% of apparently healthy people associated with cattle had fecal EHEC including O157 and serum antibodies against LPS O157, and suggested that occupational exposure to cattle harboring EHEC may lead to subclinical infections in humans44. An independent study showed that people associated with dairy farming were more likely to have serum antibodies to Stx and LPS O157 than urban residents31. In agreement with these previous studies, our results may indicate a frequent exposure to EHEC strains in the cohort studied.
On the other hand, previous studies have demonstrated that an acute infection with E. coli O157 results in an IgM-class antibody response and/or an IgA-class response but not IgG14. Moreover, the presence of high anti-LPS antibody titers was demonstrated to be concomitant with the onset of the symptoms8. In this sense, the IgM isotype is the first immunoglobulin isotype detected when an adaptive immune response arises. Evans et al. suggested that the identification of IgG-class antibodies in volunteer's sera may indicate that people with occupational exposure had come into contact with LPS O157 on more than one occasion14. Furthermore, they detected IgG antibodies in farm workers’ sera up to two years after enrolling. LPS are known T-independent antigens, thus they do not elicit T cell help for B cells with the induction of polysaccharide-specific IgM-to-IgG switching and B cell and T cell memory responses. Therefore, antibodies against LPS antigens are usually short-lived and suggest a recent infection. However, Chart et al. have found antibodies against LPS O157 up to 75 days post-infection in sera obtained from HUS patients8. In this regard, it was shown that capsule polysaccharide antigens bound to carrier proteins are able to activate CD4+ T cells to help carbohydrate-specific B cells produce long-lasting IgG antibodies through both cognate and cytokine-mediated interactions36. Maybe, in the context of an infection with the whole EHEC bacteria, LPS antigens are bound to other components of the cell membrane and presented on the surface of antigen-presenting cells in the context of major histocompatibility complex class II (MHC II) molecules to CD4+ T cells, thus explaining the presence of specific IgG antibodies against specific LPS antigens. In our study, we detected that 29 out of 61 (47%) kindergarten teachers had IgG antibodies against at least one LPS serotype, which not only may indicate repeated EHEC exposure but also may be in agreement with the high circulation of EHEC strains in Argentina. Additionally, 12 out of 61 samples (20%) showed IgM antibodies against at least one LPS serotype, thus we could suggest that those kindergarten teachers had been exposed at least recently to EHEC LPS, if not at the moment of sampling. Nevertheless, further microbiological and epidemiological research is necessary to support this assumption.
In the present study, anti-O157 antibodies were the most abundant, found in 43% of the samples, followed by anti-O121 in 21%, -O145 in 8% and -O103 in 5%. Although O157 has been shown to be the most prevalent serotype among HUS patients in Argentina, representing nearly 75% of them28,29, O145 was demonstrated to be the second most prevalent among HUS patients accounting for 16.8% of HUS cases, followed by O121 in 5.4%, and other serotypes in 4.2%1. Even though non-O157 strains, such as O145, O121, O26, O103, O104, O111, O55, are increasingly detected1, their isolation remains a challenge and may underestimate the number of HUS cases associated with non-O157 strains16. Most non-O157 EHEC ferment sorbitol and could be missed when using sorbitol MacConkey agar for EHEC isolation. Similarly, the immunomagnetic separation technique using beads coated with antibodies against LPS O157 fails to identify non-O157 EHEC. Therefore, the differences observed in the prevalence of strains isolated from HUS patients and the prevalence of serotypes observed in the serum antibodies from kindergarten teachers in the present study may be related to that difficulty in the diagnosis of non-O157 strains. Moreover, the prevalence of strain serotypes in a resistant population, such as adults, may be different from that observed in HUS patients. In this sense, to our knowledge, there is no other study where the prevalence of EHEC serotypes in diarrhea non-associated with HUS or in adults has been determined in Argentina. On the other hand, the high percentage of positive samples against LPS O157 is worrying, since the O157 serotype is the most associated with HUS in children.
Finally, we observed that 21% (n=13) of the samples reacted against more than one LPS serotype: 5% was positive for LPS O157 and O145, 11% for LPS O157 and O121, 2% for LPS O157, O145 and O121 and 3% for LPS O157, O121 and O103. The carriage of more than one EHEC serotype was already reported25,27,33. Indeed, the isolation of different EHEC serotypes from the same patient, including E. coli O157, has been reported25,27,32. Even more, the simultaneous presence of anti-O157 and -O145 antibodies26, and anti-O26 and -O1037 were also reported, thus indicating a possible coinfection. Due to the fact that the Glyco-iELISA test used in the present study was developed by using bacterial engineered O-polysaccharide–protein conjugates26, which demonstrated no cross-reactivity6, we ruled out cross-reactions. Even if the Glyco-iELISA test used in the present study appears to be a highly sensitive and specific assay, the isolation of EHEC strains in feces from kindergarten teachers would have provided proof of coinfection. In conclusion, in the present study we demonstrated a high prevalence of anti-EHEC antibodies in kindergarten teachers from Jose C. Paz, Buenos Aires province, and propose that more studies, including clinical microbiology and epidemiology analyses, may help to confirm if they are asymptomatic carriers who could contribute to EHEC transmission.
Ethical approvalThe study was approved by the Ethics Committee of the University of Buenos Aires.
Informed consentA written informed consent was received from kindergarten teachers.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank the school authorities and the educational staff for their excellent collaborative attitude and kindergarten teachers for providing blood samples.
We also thank Alberto Fernandez (Universidad Nacional de José C. Paz, Buenos Aires, Argentina) who participated in the organization of the teacher meeting.
We are grateful to Mohamed Karmali from Canada for the critical reading of the manuscript.
This work was supported by the National Scientific and Technical Research Council (CONICET-Proyecto de Vulnerabilidad Social 2019-OCA Houssay), Argentina.