The objective of this study was to investigate a clonal relationship among thermotolerant Campylobacter spp. isolates from different stages of the poultry meat supply chain in Argentina. A total of 128 thermotolerant Campylobacter spp. (89 C. jejuni and 39 C. coli) isolates from six poultry meat chains were examined. These isolates were from: a) hens from breeder flocks, b) chickens on the farm (at ages 1 wk and 5 wk), c) chicken carcasses in the slaughterhouse, and d) chicken carcasses in the retail market. Chickens sampled along each food chain were from the same batch. Campylobacter spp. isolates were analyzed using pulsed-field gel electrophoresis to compare different profiles according to the source. Clustering of C. jejuni isolates resulted in 17 profiles, with four predominant genotypes and many small profiles with just a few isolates or unique patterns, showing a very high degree of heterogeneity among the C. jejuni isolates. Some clusters included isolates from different stages within the same chain, which would indicate a spread of strains along the same poultry meat chain. Moreover, twenty-two strains of C. coli clustered in seven groups and the remaining 17 isolates exhibited unique profiles. Evidence for transmission of thermotolerant Campylobacter spp. through the food chain and cross contamination in the slaughterhouses were obtained. This collective evidence should be considered as the scientific basis to implement risk management measures to protect the public health.
El objetivo de este trabajo fue investigar la relación clonal entre aislamientos de Campylobacter spp. termotolerantes obtenidos de diferentes etapas de la cadena cárnica aviar en Argentina. En total se examinaron 128 aislamientos de Campylobacter spp. (89 de Campylobacter jejuni y 39 de Campylobacter coli) obtenidos de 6 cadenas cárnicas muestreadas en los siguientes puntos del circuito productivo: a) gallinas reproductoras; b) pollos en las granjas (de una y 5 semanas de edad); c) carcasas de pollo en frigorífico, y d) carcasas de pollo en puntos de venta final. Las muestras de pollos fueron obtenidas a lo largo de las cadenas cárnicas siguiendo el mismo lote. Los aislamientos de Campylobacter spp. fueron analizados mediante electroforesis de campos pulsados y se compararon los diferentes perfiles. Los aislamientos de C. jejuni se agruparon en 17 perfiles, 4 de ellos predominantes y el resto en perfiles que agruparon pocos aislamientos o patrones únicos, lo que ilustra una gran heterogeneidad. Algunos agrupamientos incluyeron aislamientos obtenidos de diferentes etapas de una misma cadena cárnica, lo cual indicaría una dispersión de cepas a lo largo de las cadenas cárnicas. Por otra parte, 22 aislamientos de C. coli se agruparon en 7 grupos y otros 17 aislamientos presentaron perfiles únicos. Se obtuvieron evidencias de transmisión de Campylobacter spp. termotolerante en la cadena cárnica aviar y contaminación cruzada en frigoríficos. La evidencia reunida debería servir como base científica para implementar estrategias de manejo del riesgo, destinadas a proteger la salud de los consumidores de carne aviar.
Thermotolerant Campylobacter spp., especially C. jejuni and C. coli, causes significant morbidity both in developing and developed countries10,13 and constitutes the most frequent cause of foodborne illness worldwide4,5. Poultry meat is one of the most important sources of human campylobacteriosis. The reduction and elimination of thermotolerant Campylobacter spp. in the food chain, particularly from chicken products, are major strategies in the efforts to control human exposure and to improve the public health11.
In developing countries, information on food-borne disease is scant due to the inadequate data provided by the passive/non-existent surveillance systems. Additionally, outbreak information is frequently unsubstantial because health authorities lack the capabilities or resources for detection of diarrheal diseases18. A study conducted in Argentina6 concluded that thermotolerant Campylobacter spp. was the most important gastrointestinal pathogen in humans whose incidence rate was higher than other common pathogens such as Salmonella spp., Shigella spp., and Escherichia coli.
Recent studies have shown high prevalence of thermotolerant Campylobacter spp. in poultry carcasses in slaughterhouses and the retail market in Argentina19, most of which were resistant to quinolines and erythromycin20. However, there is a lack of research in Argentina conducted with the aim to investigate the epidemiology of campylobacteriosis in the whole food chain from farm to fork. Molecular typing is a useful tool to enhance epidemiological studies13. Pulsed-field gel electrophoresis (PFGE) is considered a gold standard due to its high discrimination potential and this information is essential to develop effective plans to control the disease17.
The objective of this study was to investigate a clonal relationship among thermotolerant Campylobacter spp. isolates from different stages of the poultry meat supply chain in Argentina.
Materials and methodsCollection of Campylobacter isolatesA total of 128 thermotolerant Campylobacter spp. strains were isolated from six different companies (here referred to as poultry meat food chains) in the Santa Fe region of Argentina during the years 2011–2012. The stages sampled in each poultry meat chain were: a) hens from breeder flocks (n=75), b) broilers in flocks (aged <1 wk (n=180) and >5 wk (n=180)), c) chicken carcasses in the slaughterhouse (n=60), and d) chicken carcasses in the retail market (n=60). The chickens sampled along the meat supply chain were from the same batch (defined as a group of chickens from the same flock, sent to the same slaughterhouse at the same time, and sold together in the same retail market). Additionally, samples of litter (n=24), feed (n=24), and drinking water (n=24) were taken from the flocks. At the slaughterhouses, cecal (n=60) and liver (n=60) samples were randomly collected from the evisceration line.
Fecal samples (hens from breeder flocks and broilers in flocks) were randomly collected from the cloaca using sterile cotton swabs, which were placed in capped plastic tubes containing 10ml of Cary-Blair (Britania®) transport medium and transported to the laboratory under refrigeration conditions within 4h. Together with the cloacal samples, samples of chicken feed (500g), drinking water (1l) and litter (500g) were also taken from each flock. Feed, drinking water, and litter samples were taken directly from the feeders. Cecal and liver samples in the slaughterhouses were randomly collected from the evisceration line by one of the researchers and placed into sterile plastic bags. Broiler carcasses were taken from the processing line after chilling, using a clean pair of latex gloves and put into a sterile bag with 200ml of Ringer's solution ¼ strength. Carcasses were rinsed by shaking for 60seconds in each of two directions to ensure that the solution came into contact with all the surfaces; the solution was recovered and transported to the lab in sterile plastic tubes (under refrigeration conditions), within 4h. Chickens were packaged at the processing plant in the slaughterhouse and transported to the retail market where whole chickens from the same flock were randomly sampled, following the same procedure described for the broiler carcasses in the slaughterhouse.
Campylobacter spp. were isolated using the selective media Bolton Broth (OXOID®) and Preston Agar (OXOID®)2. Isolation of Campylobacter spp. was performed by a previously described methodology2,8. All incubations were performed under microaerophilic conditions (5% O2, 10% CO2 and 85% H2) at 42°C. Preliminary identification of thermotolerant Campylobacter spp. isolates was based on colony morphology, microscopic appearance (curved gram-negative bacilli with typical motility), and the following phenotypic characteristics: oxidase and catalase production8. All presumptive Campylobacter spp. isolates were identified to the species level (C. jejuni and C. coli) by multiplex PCR, as proposed by Vandamme et al.15. DNA was extracted using a Wizard genomic DNA purification kit (Promega®) and PCR products were analyzed on 1.5% agarose gels and stained with GelRed (Biotium®).
Positive isolates were subcultured on Columbia blood agar and stored in glycerol broth (15% glycerol and 85% serum broth) at −80°C14.
PFGE-typingAnalysis of C. jejuni (n=89) and C. coli (n=39) isolates by PFGE was performed according to the method described in the PulseNet protocol12 using SmaI (Fermentas®) as restriction endonuclease. Salmonella spp. H9812 was used as reference marker (digested with XbaI Fermentas®)7. PFGE banding patterns were analyzed using BioNumerics version 6.6 (Applied Maths, Belgium). Images of gels were normalized by alignment with the appropriate size standard lanes. Matching and dendrogram of fingerprints were determined by the unweighted pair group method with averages (UPGMA) and performed using the Dice coefficient (position tolerance, 1.0%). The PFGE cluster was based on a 95% similarity cut off.
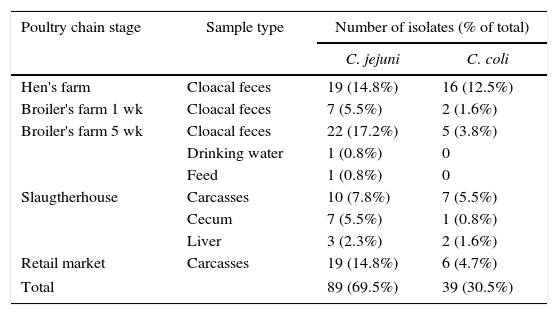
ResultsAmong the 128 isolates available, 89 were C. jejuni and 39 were C. coli. The origin of the isolates is shown in Table 1.
Origin of thermotolerant Campylobacter isolates from different stages of poultry meat supply chain
| Poultry chain stage | Sample type | Number of isolates (% of total) | |
|---|---|---|---|
| C. jejuni | C. coli | ||
| Hen's farm | Cloacal feces | 19 (14.8%) | 16 (12.5%) |
| Broiler's farm 1 wk | Cloacal feces | 7 (5.5%) | 2 (1.6%) |
| Broiler's farm 5 wk | Cloacal feces | 22 (17.2%) | 5 (3.8%) |
| Drinking water | 1 (0.8%) | 0 | |
| Feed | 1 (0.8%) | 0 | |
| Slaugtherhouse | Carcasses | 10 (7.8%) | 7 (5.5%) |
| Cecum | 7 (5.5%) | 1 (0.8%) | |
| Liver | 3 (2.3%) | 2 (1.6%) | |
| Retail market | Carcasses | 19 (14.8%) | 6 (4.7%) |
| Total | 89 (69.5%) | 39 (30.5%) | |
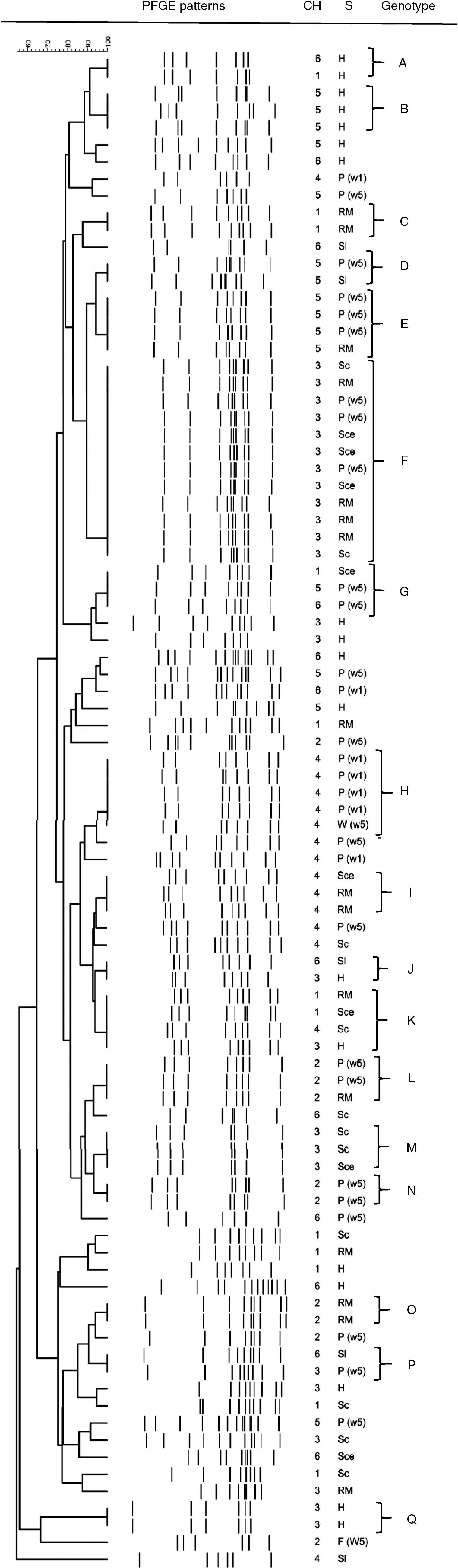
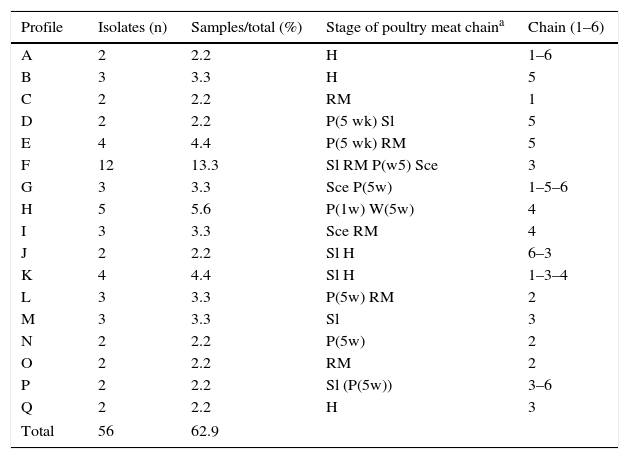
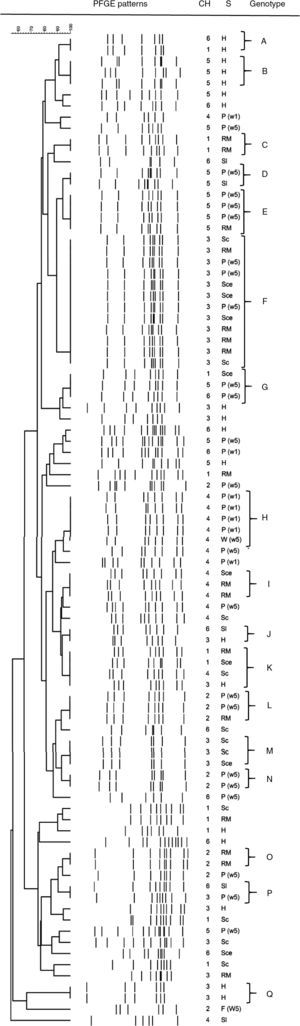
Overall, clustering of C. jejuni isolates resulted in 17 profiles, with four predominant genotypes (clusters E, F, H, and K) shared by four or five isolates, except profile F which was shared by 12 isolates (Table 2). Many small profiles with just a few isolates (two or three) were observed. Additionally, it is important to mention that only 56 of 89 isolates were grouped, the rest presented unique patterns (Fig. 1), showing a very high degree of heterogeneity among the C. jejuni isolates. Seven out of 17 profiles were constituted by isolates from a single stage. Among them, only group A consisted of isolates from breeding hens isolated from different chains. The remaining profiles contained isolates from different stages of the poultry meat chain. Some clusters (E, F, H, I, and L) included isolates from different stages within the same chain, which would indicate a spread of strains along the same poultry meat chain. The same strain of C. jejuni in broiler flock and carcasses in the slaughterhouse and the retail market could be observed. Another remarkable finding was that four out of 17 profiles (G, J, K, and P) were represented by isolates obtained from different stages in different poultry meat chains.
PFGE profiles of C. jejuni isolated at different stages of the poultry meat chain
| Profile | Isolates (n) | Samples/total (%) | Stage of poultry meat chaina | Chain (1–6) |
|---|---|---|---|---|
| A | 2 | 2.2 | H | 1–6 |
| B | 3 | 3.3 | H | 5 |
| C | 2 | 2.2 | RM | 1 |
| D | 2 | 2.2 | P(5 wk) Sl | 5 |
| E | 4 | 4.4 | P(5 wk) RM | 5 |
| F | 12 | 13.3 | Sl RM P(w5) Sce | 3 |
| G | 3 | 3.3 | Sce P(5w) | 1–5–6 |
| H | 5 | 5.6 | P(1w) W(5w) | 4 |
| I | 3 | 3.3 | Sce RM | 4 |
| J | 2 | 2.2 | Sl H | 6–3 |
| K | 4 | 4.4 | Sl H | 1–3–4 |
| L | 3 | 3.3 | P(5w) RM | 2 |
| M | 3 | 3.3 | Sl | 3 |
| N | 2 | 2.2 | P(5w) | 2 |
| O | 2 | 2.2 | RM | 2 |
| P | 2 | 2.2 | Sl (P(5w)) | 3–6 |
| Q | 2 | 2.2 | H | 3 |
| Total | 56 | 62.9 | ||
Dendogram of C. jejuni SmaI PFGE profiles isolated at different stages of the poultry meat supply chain. References: Ch: chain; S: stage of the poultry meat supply chain; H: breeding hens, P(1w): poultry <1 wk, P(5w): poultry >5 wk, W(5w): drinking water in poultry flocks >5 wk, Sl: carcass at slaughterhouse, Sce: ceca at slaughterhouse, RM: carcass at retail market.
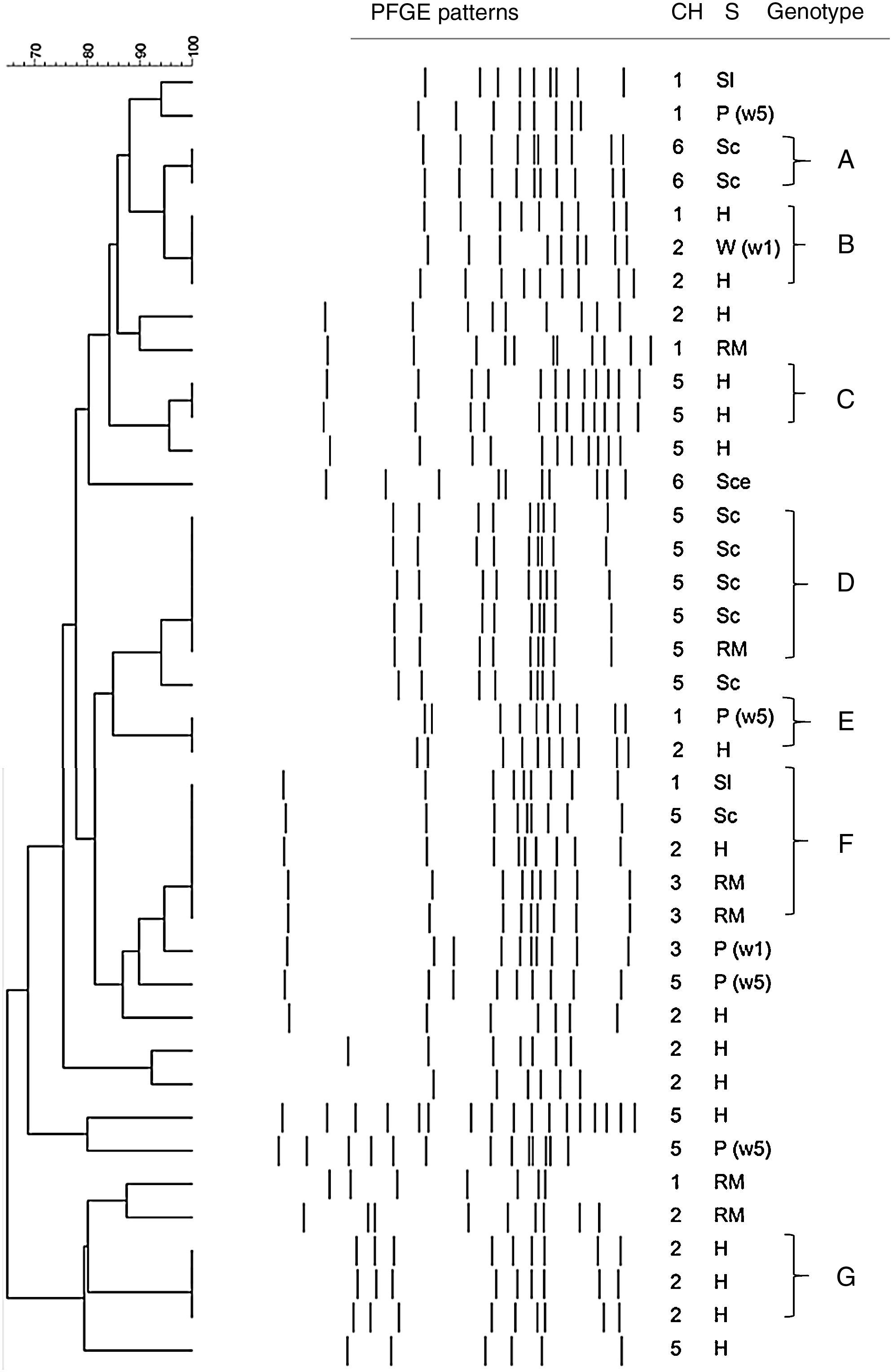
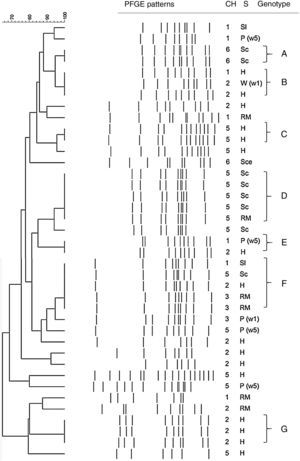
On the one hand, twenty-two strains of C. coli were clustered into seven groups (Table 3) and the remaining 17 isolates presented unique profiles (Fig. 2). Profiles D and F were predominant and each of them had five isolates. The others were small profiles with just a few isolates (two or three). Three (A, C, and G) out of these seven profiles were represented by isolates obtained from the same stage within the same poultry meat chain. Profile B was constituted by isolates from breeding hens and drinking water in the broiler flock from two different chains. On the other hand, profile D included all isolates from the same poultry meat chain but isolated from two different stages (carcasses in the slaughterhouse and the retail market), which supports the hypothesis of thermotolerant Campylobacter spp. transmission through the poultry meat chain. Profiles E and F were constituted by isolates from different stages of the chain and at the same time from different poultry meat chains.
PFGE profiles of C. coli isolated at different stages of the poultry meat chain
| Profile | Isolates (n) | Samples/total (%) | Stage of poultry meat chaina | Chain (1–6) |
|---|---|---|---|---|
| A | 2 | 5.1 | Sce | 6 |
| B | 3 | 7.7 | H W(1w) | 1–2 |
| C | 2 | 5.1 | H | 5 |
| D | 5 | 12.8 | Sce RM | 5 |
| E | 2 | 5.1 | P(5w) H | 1–2 |
| F | 5 | 12.8 | Sl Sce H RM | 1–2–3–5 |
| G | 3 | 7.7 | H | 2 |
| Total | 22 | 56.4 | ||
Dendogram of C. coli SmaI PFGE profiles isolated at different stages of the poultry meat supply chain. References: Ch: chain; S: stage of the poultry meat supply chain; H: breeding hens, P(1w): poultry <1 wk, P(5w): poultry >5 wk, W(5w): drinking water in poultry flocks >5 wk, Sl: carcass at slaughterhouse, Sce: ceca at slaughterhouse, RM: carcass at retail market.
Transmission along the food chain is generally recognized as a major source of human campylobacteriosis4,5,21. This study was conducted with the aim to evaluate the genetic diversity among thermotolerant Campylobacter spp. isolates and then to follow up Campylobacter spp. isolates along the poultry meat chain from hens through the carcass in the retail market. This transmission of strains throughout the poultry meat supply chain was observed in certain isolates and especially in the later stages of the poultry meat chain (broilers in the flocks and carcasses in slaughterhouses and the retail market). This finding supports the hypothesis that certain genotypes may be transmitted along the poultry meat chain as was observed in other countries such as Hungary4, Slovenia and Bosnia and Herzegovina21, and Spain10. These matching Campylobacter spp. profiles were able to survive under environmentally adverse conditions along the poultry production chain. However, not all the isolates showed this behavior because some strains isolated at early stages were not recovered at later stages of the poultry meat chain.
Moreover, the observed matching of isolates from different chains would have various explanations. For example, it may be a reflection of a common source of contamination. Because all farms were part of the same integrated production system and basically applied the same system for sanitization, feeding, animal health procedures between the farms, Campylobacter spp. may have survived in certain niches, fomites or vectors that were present on any of these and transported to other farms and slaughterhouses. In this sense, further studies should be conducted with the aim to establish the role of animate (birds, insects, small mammals, poultry farmer) and inanimate agents (poultry litter, supply air, food, water, trucks) in the maintenance of thermotolernt Campylobacter spp. among different production cycles within a chain and among different chains.
The most common profile seems to support the idea that each stage of the poultry meat chain has stable and particular genotypes. Additionally, the results of our study showed that the flocks exhibited different C. jejuni and C. coli profiles. Several works showed similar quantity of clusters for C. jejuni and many unique profiles for this species9,10. The genetic diversity of both Campylobacter species was also reported in previous studies4,17,21,22 and supported by an acquisition of foreign DNA or random recombination of large DNA segments, may well cause alterations detectable using PFGE16. As a previous work10, genotype diversity of the isolates for flocks increased through the poultry production chain, with the highest diversity being detected at the slaughterhouse level. However, it was interesting that the isolates recovered in the carcasses in the slaughterhouses and the retail market were not identified in previous stages of the same chain. It is possible that Campylobacter spp. can survive in the slaughterhouse environment even after cleaning, as suggested by Melero et al.10. The ability of C. jejuni to form biofilms as protective mechanisms and to survive longer and increase resistance to disinfectants, antimicrobials, and antibiotics was recognized3. Biofilm formation on abiotic surfaces may help Campylobacter spp. survive in the environment and these biofilms are a significant reservoir of antibiotic-resistant Campylobacter spp. even without antibiotic selective pressure1. Thermotolerant Campylobacter spp. may survive in the slaughterhouses and be a contamination source to poultries from different chains. Therefore, future studies of biofilm in slaughterhouses should provide tools for both the Campylobacter spp. epidemiology in order to implement appropriate intervention measures to eliminate thermotolerant Campylobacter spp. from slaughterhouse surfaces.
Previous studies conducted by our group allowed us to conclude that: a) thermotolerant Campylobacter spp. was present in a high proportion in the different stages of the poultry meat chain, especially in broilers in flock and carcasses in the slaughterhouse and the retail market19 and b) a significant proportion of the thermotolerant Campylobacter spp. isolated was resistant to the antibiotics commonly used in human medicine and most of them showed multiresistance patterns20. These findings show that poultry meat is a significant source of Campylobacter spp. contamination and that the consumers in Argentina are exposed to different strains of thermotolerant Campylobacter spp. which constitute a significant public health problem. Further studies should be conducted with the aim to establish the epidemiological link between the strains isolated from the poultry meat chain and human isolates. Furthermore, MLST typing could be useful for comparing our isolates with those circulating in other countries.
ConclusionsThe present work is the first in Argentina to reveal that thermotolerant Campylobacter spp. shows high genetic diversity. Evidences for transmission through the food chain and cross contamination in slaughterhouses were found. This collective evidence should be considered as the scientific basis to implement risk management measures to protect the public health. It is clear that poultry are colonized on the farm and further studies should be conducted to evaluate the importance of different fomites and vectors and therefore, to understand the epidemiology of this food-borne pathogen.
Ethical responsibilitiesProtection of people and animalsThe authors state that the procedures followed conformed to the ethical standards of the responsible human experimentation committee and in agreement with the World Medical Association and the Declaration of Helsinki.
Confidentiality of dataThe authors state that no patient data appears in this article.
Right to privacy and informed consentThe authors state that no patient data appears in this article.
Conflict of interestThe authors declare that they have no “conflicts of interest”.
LSF, LPS, MVZ and MLS are Research Career Member from the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET, Argentina). JEB, DMA, ER, APB, ARS and JAZ are doctoral fellow from CONICET (Argentina). The authors would like to thank Dr. Gerardo Leotta (Instituto de Genética Veterinaria (IGEVET-CONICET/UNLP) Facultad de Ciencias Veterinarias (UNLP) for the use of the Bionumerics software. We also thank Dr. Horacio Terzolo and Alejandra Velilla (National Institute of Agricultural Technology-EEA Balcarce, Argentina) for their assistance and cooperation in the training of our working group in bacteriological techniques of thermotolerant Campylobacter. This study was funded by CONICET (PIP 11220120100481CO) and SENASA (Research, Transfer and Communication – Health, Safety and Quality Agrifood Research Group for training. Collective award from SENASA).