We studied and compared the prevalence of Campylobacter jejuni and Campylobacter coli in chicken carcasses from conventional and kosher broiler abattoirs and retail stores. The prevalence of thermotolerant Campylobacter-positive carcasses was 94.0 (kosher) and 32.0% (conventional) (p<0.0001), while the prevalence of samples contaminated with C. jejuni, C. coli and simultaneously with both species was 36.0, 2.0 and 56.0% (kosher) and 26.0, 4.0 and 2.0% (conventional) (p<0.0001), respectively. Samples of chicken carcasses (n=25) and food contact surfaces (tables, n=25; knives, n=25) from 25 retails were collected and risk quantification was performed. Retails were categorized as high-risk (n=11), moderate-risk (n=11) and low-risk (n=3). Nineteen (76.0%) carcasses, 20 (80.0%) tables and 18 (72.0%) knives were Campylobacter-positive. Retails and abattoirs proved to be sources of carcass contamination with Campylobacter spp. Carcasses from kosher abattoirs were mostly contaminated with Campylobacter spp., whereas C. coli was the most prevalent species isolated from carcasses in retail stores.
El objetivo del estudio fue determinar y comparar la prevalencia de Campylobacter jejuni y Campylobacter coli en carcasas de pollo obtenidas en frigoríficos por faena convencional y kosher, y en locales de expendio. La prevalencia de Campylobacter spp. termotolerante fue del 94,0 (kosher) y del 32,0% (convencional) (p<0,0001). La prevalencia de muestras contaminadas con C. jejuni, C. coli y con ambas especies fue del 36,0, del 2,0 y del 56,0% (Kosher) y del 26,0, del 4,0 y del 2,0% (convencional) (p<0,0001), respectivamente. Se tomaron muestras de carcasas (n=25) y superficies (tablas, n=25; cuchilla, n=25) en 25 locales. Los locales fueron categorizados como de riesgo alto (n=11), moderado (n=11) y bajo (n=3). Diecinueve (76,0%) carcasas, 20 (80,0%) tablas y 18 (72,0%) cuchillas fueron positivas para Campylobacter spp. Frigoríficos y locales fueron fuente de contaminación de carcasas con Campylobacter spp. La prevalencia de Campylobacter spp. fue mayor en carcasas kosher. Campylobactercoli fue la especie más prevalente en carcasas de locales.
Consumption of contaminated food with Campylobacter jejuni subsp. jejuni and Campylobactercoli is among the four main causes of diarrheal disease worldwide4. Thermotolerant Campylobacter species are among the most relevant microbiological hazards associated with poultry meat consumption13,15. Although the prevalence of Campylobacter has been studied in conventional broiler abattoirs15, the impact of kosher slaughter procedures on broiler chicken has not been described yet.
The aims of this study were to: (a) determine and compare the presence of C. jejuni and C. coli at conventional and kosher broiler abattoirs, and (b) determine the presence of C. jejuni and C. coli in retail stores.
The study was conducted between November 2011 and April 2013 in broiler abattoirs and retail stores from Buenos Aires province, Argentina. Chicken carcass samples were obtained from two broiler abattoirs (n=50 each) that applied conventional and kosher slaughter processes authorized by the National Service of Agrifood Health and Quality of Argentina. Chicken carcasses were collected and sampled using sterile sponges (Whirl-Pak speci-sponge, Nasco, USA) soaked in 10ml 3M™ Campylobacter enrichment broth (3M, MN, USA) containing 3M™ Campylobacter supplement for Tecra™ kits (3M, NSW, Australia), according to Baker et al.2, with slight modifications, while all carcasses were sponged. At the same time, 25 retail stores were randomly sampled and risk quantification was performed according to Leotta et al.8. The study was conducted in full agreement with the local sanitary authorities. The checklist used for risk quantification included five groups of variables (total value, 100): (1) situation and conditions of the building (10.0), (2) equipment and tools (15.0), (3) handlers (25.0), (4) raw materials and products for sale (20.0), and (5) production flow (30.0). Risk assessment on a 1–100 scale was quantified as high-risk (1–40), moderate-risk (41–70), or low-risk (71–100). Samples were taken from chicken carcasses (n=25) and food contact surfaces (tables, n=25; knives, n=25). One chicken carcass from each retail store was sponged as already described for abattoir carcasses. All the carcasses were provided by conventional abattoirs. In addition, three areas of 20×20cm each from tables and the entire surface of knife blades were sponged.
Each abattoir and retail sponge were incubated with 150ml Campylobacter 3M broth+supplement under microaerophilic conditions (5% O2, 10% CO2 and balance N2) for 24h at 42°C. After incubation, DNA was extracted using the Short Prep II kit (Biotecon, Postdam, Germany). Real-time (RT) PCR was performed using Foodproof® Campylobacter 5′Nuclease (Biotecon) in a thermal cycler (Mx3005P, General Electric, Massachusetts, USA). Positive samples were placed onto Karmalí agar (Oxoid, Thermo Scientific, UK) containing Campylobacter supplement (Oxoid). Plates were incubated as mentioned above for sponges. All the isolated strains were identified and characterized using the API Campy system (bioMérieux, Marcy l’Etoile, France) and RT-PCR (Biotecon) as previously described.
The association between Campylobacter-positive chicken carcass samples and slaughter process (conventional vs. kosher) was determined using the Chi-square test. The Fisher's exact test was used to evaluate the association of Campylobacter-positive chicken carcass and food contact surface samples with retail store risk categorization. The Chi-square test was performed to evaluate statistical differences in the percentage of C. jejuni-positive and C. coli-positive carcass and food contact surface samples. All statistical analyses were performed with XLSTAT 2018.4, with a significance of p<0.05.
The prevalence of Campylobacter-positive carcass samples was 94.0 (47/50) and 32.0% (16/50) in kosher and conventional abattoirs, respectively (p<0.0001). The discrimination of results according to Campylobacter species showed 13/50 (26.0%) C. jejuni-positive and two C. coli-positive carcasses, while only one was positive for both species in the conventional abattoir. In the kosher abattoir, 18 carcasses were positive for C. jejuni, one for C. coli and 28 for both. The proportion of samples contaminated simultaneously with C. jejuni and C. coli during kosher and conventional slaughter was 56.0 (28/50) and 2.0% (1/50), respectively (p<0.0001).
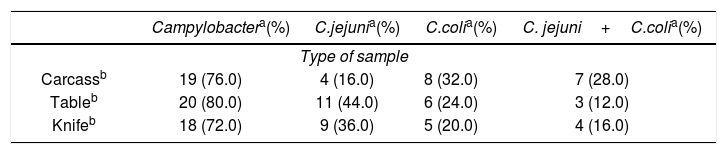
Risk categorization of retail stores resulted in 11/25 (44.0%) high-risk, 11/25 (44.0%) moderate-risk and 3/25 (12.0%) low-risk stores. The prevalence of Campylobacter spp. in each type of sample from the retail stores is shown in Table 1. From the total number of samples (n=25 each), 19 (76.0%) chicken carcass, 20 (80.0%) table surface and 18 (72.0%) knife surface samples were Campylobacter-positive, distributed as follows: one (9.1%) food contact surface and 10 (90.9%) food contact surface and chicken carcass samples in high-risk retail stores; four (33.4%) food contact surface and eight (66.6%) food contact surface and chicken carcass samples in moderate-risk retail stores; one (50.0%) food contact surface and one (50.0%) chicken carcass sample in low-risk retail stores. We found no association between the proportion of samples positive for Campylobacter spp. in chicken carcasses (p=0.180), food contact surfaces (at least one positive; p=0.487) and tables (p=0.661) with the risk assigned to retail stores. However, such proportion was higher in knife samples from high-risk retail stores (p=0.020).
Prevalence of Campylobacter spp., Campylobacter jejuni, C. coli and C. jejuni+C. coli in retail stores.
Undercooked meat and meat products, mainly chicken meat, are most frequently associated with human campylobacteriosis13. All stages – from primary broiler production to the consumer – play an important role in the transmission of this pathogen13. In our study, the prevalence of Campylobacter spp. in chicken carcasses obtained by conventional slaughter (32.0%) was similar to that reported in China (34.1%)9 and Argentina (33.3%)15, but lower than that found in France (96.9%)1. In agreement with previous studies in Argentinian conventional abattoirs, C. jejuni was the most prevalent species isolated from the carcasses15. While there are no studies on the presence of Campylobacter spp. in kosher abattoirs, we observed significant differences in the proportion of Campylobacter-positive samples obtained by conventional (32.0%) and kosher (94.0%) slaughter processes, and of samples contaminated simultaneously with C. coli and C. jejuni. It is important to note some differences between kosher and conventional slaughter. Kosher slaughter must be performed individually by a trained expert called shochet. Shochets use a sharp blade called chalef to sever the trachea, esophagus, carotid arteries, jugular veins and vagus nerve. After slaughter, the chicken is placed on a conveyor system and left to hang so that the blood drains from the body. Chicken defeathering must be done using cold water. According to Baker et al.2, the absence of heat during scalding undoubtedly contributes to the higher C. jejuni count in kosher slaughter. The chicken is then soaked, salted and rinsed to take away any remaining visible blood3. By contrast, conventional abattoirs apply procedures and measures aimed at reducing the risk of microbial contamination of the product, such as desensitization and automated slaughter, defeathering with hot water (explicitly prohibited in kosher slaughter), automatic gutting, self-cleaning and self-sanitizing, and strict temperature and chlorine control of the chiller equipment11.
Giombelli and Gloria7 have shown 100% Campylobacter spp. prevalence in broilers from Brazil. The significantly different prevalence between conventional and kosher abattoirs reported in the present study is associated with the slaughter process. The particular characteristics of each method, the slaughter technique used and the risk-mitigation strategies applied to avoid the contamination of meat and the environment with pathogenic bacteria will determine food safety. Therefore, if contamination decreases in the abattoirs, it would be possible to prevent it from reaching the retail stores.
In this study, the prevalence of Campylobacter spp. in retail store chicken carcasses (76.0%) agrees with previously reported data in Argentina (83.0%)15. However, it was higher than that found in China (31.3%)9 and in another study conducted in Argentina6. While C.coli was most frequently isolated from broiler carcasses, C. jejuni was isolated from food contact surfaces. The higher presence of C.coli regarding C. jejuni in chicken carcasses from the retail stores detected in this work differs from data of previous studies from Argentina6,15, USA14 and Europe10. However, C. coli was the most prevalent species isolated from chicken liver in Argentina15 and Chile5. Although laboratory methods could facilitate the isolation of one species over the other, differences in prevalence could be due to the risk categorization of retail stores. The retail environment can be the source of food contamination with pathogenic bacteria, and the absence of standardized sanitation operating procedures would worsen and perpetuate this situation8,12.
Based on our results, retail stores and abattoirs were sources of chicken carcass contamination with Campylobacter spp. Differences in the proportion of chicken carcasses contaminated with Campylobacter spp. and simultaneously with C. jejuni and C. coli in kosher vs. conventional abattoirs were significant. C. coli was the species most frequently isolated from chicken carcasses in retail stores from Argentina. More studies are needed to determine the risk of acquiring a foodborne disease due to kosher chicken consumption. The application of corrective actions on variables associated with the presence of Campylobacter spp. in abattoirs, particularly kosher ones, and good manufacturing practices in retail stores could improve the prevention of thermotolerant Campylobacter-caused disease.
Funding sourcesThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestNone declared.
The authors thank A. Di Maggio for manuscript correction and editing.