Female sex workers (FSWs) have been considered a key population for sexually transmitted infections (STIs); therefore, they are periodically screened as a requirement to obtain a work card. However, there is insufficient epidemiological data on STIs among FSWs in Mexico. The detection of Trichomonas vaginalis is limited to microscopic studies and the molecular screening of Human papillomavirus (HPV) is only done to women 35 years of age and older. The objective of this study was to determine the prevalence of T. vaginalis and HPV infections in FSWs in the city of Orizaba, Veracruz, Mexico. Samples from 105 FSWs were obtained by cervical swab and analyzed. The identification of T. vaginalis and HPV was performed by molecular methods. HPV DNA was identified in 5.71% of the samples with the presence of HPV16, HPV18, and HPV58. A percentage of 25.7% samples were positive for T. vaginalis for optical microscopy and 23.8% for PCR. The results of the study indicate the need to incorporate more sensitive methods for the timely diagnosis of STIs as well as comprehensive health promotion programs directed to the most vulnerable groups among FSWs.
Las mujeres trabajadoras sexuales (MTS) han sido consideradas una población clave para las infecciones de transmisión sexual (ITS), por ello son examinadas periódicamente como requisito para obtener una tarjeta de trabajo. Sin embargo, no existen datos epidemiológicos suficientes sobre las ITS en las MTS en México. La detección de Trichomonas vaginalis se limita a los estudios microscópicos, y el cribado molecular del virus del papiloma humano (Human papillomavirus: HPV) solo se realiza en las mujeres de 35 años o mayores. El objetivo de este estudio fue determinar la prevalencia de T. vaginalis e infecciones por HPV en las MTS de la ciudad de Orizaba, Veracruz, México. Se analizaron 105 muestras de las MTS, obtenidas mediante frotis cervical. La identificación de T. vaginalis y HPV se realizó por métodos moleculares. El ADN del HPV se identificó en el 5,71% de las muestras, con la presencia de HPV16, HPV18 y HPV58. El 25,7% de las MTS fueron positivas para T. vaginalis por microscopia óptica el 23,8% por PCR. Los resultados del estudio indican la necesidad de incorporar métodos más sensibles para el diagnóstico oportuno de ITS y programas integrales de promoción de la salud en los grupos más vulnerables, entre las MTS.
Sexually transmitted infections (STIs) are a group of conditions, which as its name suggests, are predominantly transmitted by sexual contact. Some of these infections are considered reportable in most countries and often continue to be considerably high especially among sexually active young people of reproductive age18. However, several STIs such as Trichomonas vaginalis also have high prevalence in middle-aged women41. Infections with T. vaginalis and Human papillomavirus (HPV) are the most common STIs. The latter has contributed to a variety of adverse outcomes for both sexes, including its partnership in the acquisition of Human immunodeficiency virus (HIV) and, in some cases, it has had a role in the development of cervical neoplasia, among other obstetrical outcomes28. However, the presence of T. vaginalis frequently leads to false positive results in Pap tests. On the other hand, the persistent infection with several HPV types, known as high-risk or oncogenic viruses, is the most important risk factor for developing cervical cancer6,29. In most countries there are certain groups of people who are especially vulnerable to STIs. Overall, the prevalence of STIs tends to be higher in urban areas than in rural areas, among single people and young adults17. The problem of STIs is poorly understood in Mexico, and there are only reports in some areas of the country9,27. STIs in key populations such as female sex workers (FSWs) have been mostly analyzed in Mexico–US border cities2,32,34. In Mexico, trichomoniasis and HPV infection are diseases that are subject to epidemiological surveillance and mandatory notification to health authorities. In 2016, the Health Secretariat reported 46808 new cases for trichomoniasis in women with a higher incidence in 25 to 29-year-old women and a national incidence rate of 90.36/100000 female population. The state of Veracruz was ranked in the first national place with 6829 new cases; while 28454 new cases of HPV infections were reported in 2016 with a higher incidence in 45 to 49-year-old women and a national incidence rate of 45.43/100000 women1. The national strategy of cervical cancer primary screening is performed by the Pap test and only 35–64-year-old women are screened for HPV DNA by the hybrid capture method. In this study, we aimed to determine the prevalence of T. vaginalis and HPV in FSWs in the city of Orizaba, Veracruz, using molecular detection techniques starting from cervical swabs.
Materials and methodsStudyThe cross-sectional study was conducted on FSWs receiving attention in the clinical laboratories of Health Jurisdiction VII from Orizaba, Veracruz, between January 19th, 2011 and November 10th, 2011. Those who provided written informed consent were enrolled in this study. Information on socio-demographic data (age, country of birth, marital status) reproductive health (number of pregnancies, children, termination of pregnancies, type of contraceptives (hormonal or not) and barrier methods used, smoking habits, time in commercial sex work, reasons for attending the clinic, past history of STIs and/or genitourinary infections were obtained in private, using structured questionnaires applied by one investigator. Criteria for inclusion in this study were the following: belonging to the clinical population and patient's willingness to participate. Exclusion criteria were patient refusal and inability to give informed consent. Full gynecological examinations were conducted. Vaginal fluid specimens were collected using sterile cotton swabs before speculum insertion T. vaginalis detection, and cervical and endocervical samples were collected using cytobrushes for the Pap smear and HPV detection, physiological solution was only used to facilitate the introduction of the speculum. Samples were received in the laboratory of Health Jurisdiction VII, stored at 4°C and transported to the laboratory of immunology and molecular biology for further processing. This study was authorized by the Bioethics Committee of the Facultad de Ciencias Químicas (FCQ-CBE-11-033).
Optical microscopy assaysA thin layer spread on slides was made using a vaginal swab. After making the smears, they were dried and fixed with methanol for 3min. Smears from each vaginal sample were processed for the Giemsa staining technique, were dyed for 20min and at the end of the staining were washed with distilled water and finally dried at room temperature for further microscopic observation. Samples were considered positive when at least one trophozoite of T. vaginalis was observed. On the other hand, Pap smears were conducted in the laboratories of Health Jurisdiction VII and interpreted using Bethesda 2001 classification38.
Obtaining DNAA vaginal swab and a cervical brush of each sample were immersed in 1ml of phosphate buffer [PBS (137mM NaCl, 2.7mM KCl, 4.3mM Na2HPO4, 1.4mM KH2PO4, pH 7.4)] and repelleted at 2000×g for 10min. The supernatant was discarded, and the pellet was frozen at −20°C. DNA was extracted as previously described26, thawed samples were resuspended in 600μl of lysis buffer (1M Tris, 0.5M EDTA, 10% glucose, and lysozyme 2mg/ml), heated at 80°C for 5min, and then cooled to room temperature. The samples were RNase-treated (Promega, Madison, Wis.) (0.5mg/ml) for 1h at 37°C. Proteins were precipitated with 0.2N NaOH, 1% sodium dodecyl sulfate, 5M potassium acetate (pH 4.8) for 5min on ice and then centrifuged for 3min at 2000×g. DNA was precipitated with 600μl of isopropanol and then centrifuged for 3min at 2000×g, and then the DNA pellet was washed with 600μl of 70% ethanol and centrifuged for 3min at 2000×g. The DNA pellet was dried, resuspended in 50μl of 10mM Tris (pH 7.4), 1mM EDTA (pH 8.0), and heated at 65°C for 1h. The presence of genomic DNA was confirmed in each sample by electrophoresis prior to PCR amplification.
PCR for T. vaginalis and HPVT. vaginalis-specific primers TV3 (5′-ATTGTCGAACATTGGTCTTACCCTC-3′) and TV7 (5′-TCTGTGCCGTCTTCAAGTATGC-3′)23, and HPV-specific primers MY09/MY01131, were used for PCR amplification. The PCR mixture consisted of 5μl of 10× PCR buffer, 4μl of deoxynucleoside triphosphates (2.5mM each), 0.5μl of each primer pair (10pmol/μl), 0.5μl of Taq DNA polymerase (Promega) (5U/ml), 10μl of sample (5–10ng/ml), and 29.5μl of distilled water. Positive and negative controls were included in all PCR runs. The positive control consisted of DNA from ATCC T. vaginalis isolate 30 184 and HPV 43 clone 2B, ATCC 40 339. Negative controls included DNA from Trypanosoma cruzi MHOM/MX/1994/INC-1 strain, PCR mix with primers but no DNA, and human genomic DNA. PCR amplification consisted of 30 cycles of 1min at 90°C, 30s at 60°C, and 2min at 72°C for T. vaginalis, and 40 cycles of 60s at 95°C, 60s at 55°C, and 60s at 72°C for HPV. After amplification, there was an additional extension step at 72°C for 7min, and then the samples were cooled to 4°C. Five microliters of amplified product were electrophoresed on 1.8% agarose, 0.5mg/ml ethidium bromide gel, viewed on a UV light box and photographed. Samples containing a 300bp fragment were considered positive for T. vaginalis, and samples containing a 450bp fragment were considered positive for HPV.
HPV typing by E6 nested multiplex PCRTyping was performed according to reports by Sotlar et al.39. Briefly, from DNA HPV positive samples, there was a first PCR reaction using consensus primers (GP-E6-3F, GP-E7-5B, GP-E7-6B). The PCR mixture consisted of 5μl of 10× PCR buffer, 4μl of deoxynucleoside triphosphates (2.5mM each), 0.5μl of each primer pair (15pmol/μl), 0.5μl of Taq DNA polymerase (Promega) (5U/ml), 10μl of sample (5–10ng/ml), and 29.5μl of distilled water. PCR amplification consisted of 40 cycles of 1min at 94°C, 60s at 40°C, and 1min at 72°C. After amplification, there was an additional extension step at 72°C for 10min. With the product of the first PCR reaction, a second reaction was performed, using the same reaction mixture but with specific primers for each HPV type, HPV16 (5′-CACAGTTATGCACAGAGCTGC-3′ and 5′-CATATATTCATGCAATGTAGGTGTA-3′), HPV18 (5′-CACTTCACTGCAAGACATAGA-3′ and 5′-GTTGTGAAATCGTCGTTTTTCA-3), and HPV58 (5′-GTAAAGTGTGCTTACGATTGC-3′ and 5′-GTTGTTACAGGTTACACTTGT-3′). PCR amplification consisted of an initial denaturation step at 94°C for 4min, 35 cycles of 30s at 94°C, 30s at 56°C, and 45s at 72°C. After amplification, there was an additional extension step at 72°C for 4min. Five microliters of amplified product were electrophoresed on 1.8% agarose, 0.5mg/ml ethidium bromide gel, viewed on an UV light box, and photographed. Samples containing a 457bp, 322pb and/or 224pb fragment were considered positive for HPV16, HPV18 and HPV58 respectively. Negative controls were included in all PCR runs.
Statistical methodsFrequency distribution of demographic data, characteristics of the population, sexual history and clinical manifestations were analyzed. The relationship between selected risk factors and the prevalence of trichomoniasis and HPV infection were compared using χ2 or the Fisher exact test when appropriate. Ninety-five percent confidence intervals were calculated to evaluate statistically significant differences between the collection methods. The relationship between age and prevalence rate was assessed by the Chi-square test and regression analysis. The GraphPad Prism 6 statistics software was used for the statistical analysis.
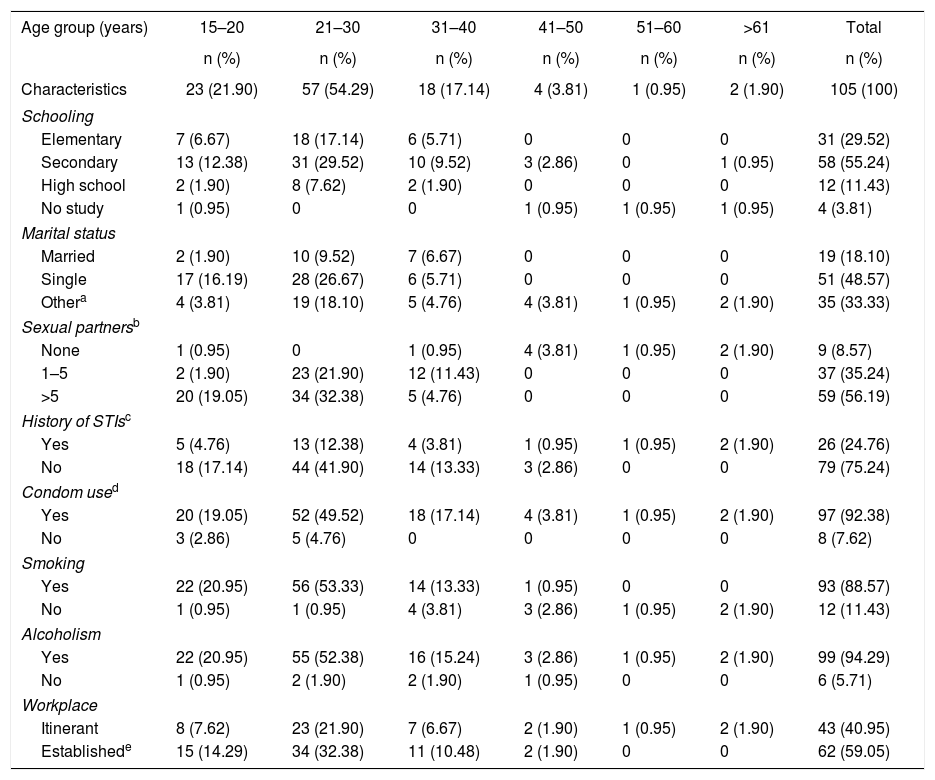
ResultsSociodemographic characteristicsThe 105 samples were divided into 6 age groups as shown in Table 1, age of the participants ranged from 15 to 64 years old, the group between 21 and 30 years had the largest number of samples with 57 (54.29%); the education level showed that of the total studied population, 29.52% (CI 95% 27.4–32) have completed elementary school, 55.23% (CI 95% 52.33–58.13) have completed secondary education, 11.42% (CI 95% 9.67–13.17) have completed high school education, and 3.80% (CI 95% 3.3–4.3) had no education. In relation to marital status, 48.57% (CI 95% 45.38–51.76) were single, 18.10% (CI 95% 16.16–20.02) were married, and 33.3% (CI 95% 31.11–35.49) reported living together, being divorced or widowed.
Socioeconomic characteristics and sexual behavior of FSWs
| Age group (years) | 15–20 | 21–30 | 31–40 | 41–50 | 51–60 | >61 | Total |
|---|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Characteristics | 23 (21.90) | 57 (54.29) | 18 (17.14) | 4 (3.81) | 1 (0.95) | 2 (1.90) | 105 (100) |
| Schooling | |||||||
| Elementary | 7 (6.67) | 18 (17.14) | 6 (5.71) | 0 | 0 | 0 | 31 (29.52) |
| Secondary | 13 (12.38) | 31 (29.52) | 10 (9.52) | 3 (2.86) | 0 | 1 (0.95) | 58 (55.24) |
| High school | 2 (1.90) | 8 (7.62) | 2 (1.90) | 0 | 0 | 0 | 12 (11.43) |
| No study | 1 (0.95) | 0 | 0 | 1 (0.95) | 1 (0.95) | 1 (0.95) | 4 (3.81) |
| Marital status | |||||||
| Married | 2 (1.90) | 10 (9.52) | 7 (6.67) | 0 | 0 | 0 | 19 (18.10) |
| Single | 17 (16.19) | 28 (26.67) | 6 (5.71) | 0 | 0 | 0 | 51 (48.57) |
| Othera | 4 (3.81) | 19 (18.10) | 5 (4.76) | 4 (3.81) | 1 (0.95) | 2 (1.90) | 35 (33.33) |
| Sexual partnersb | |||||||
| None | 1 (0.95) | 0 | 1 (0.95) | 4 (3.81) | 1 (0.95) | 2 (1.90) | 9 (8.57) |
| 1–5 | 2 (1.90) | 23 (21.90) | 12 (11.43) | 0 | 0 | 0 | 37 (35.24) |
| >5 | 20 (19.05) | 34 (32.38) | 5 (4.76) | 0 | 0 | 0 | 59 (56.19) |
| History of STIsc | |||||||
| Yes | 5 (4.76) | 13 (12.38) | 4 (3.81) | 1 (0.95) | 1 (0.95) | 2 (1.90) | 26 (24.76) |
| No | 18 (17.14) | 44 (41.90) | 14 (13.33) | 3 (2.86) | 0 | 0 | 79 (75.24) |
| Condom used | |||||||
| Yes | 20 (19.05) | 52 (49.52) | 18 (17.14) | 4 (3.81) | 1 (0.95) | 2 (1.90) | 97 (92.38) |
| No | 3 (2.86) | 5 (4.76) | 0 | 0 | 0 | 0 | 8 (7.62) |
| Smoking | |||||||
| Yes | 22 (20.95) | 56 (53.33) | 14 (13.33) | 1 (0.95) | 0 | 0 | 93 (88.57) |
| No | 1 (0.95) | 1 (0.95) | 4 (3.81) | 3 (2.86) | 1 (0.95) | 2 (1.90) | 12 (11.43) |
| Alcoholism | |||||||
| Yes | 22 (20.95) | 55 (52.38) | 16 (15.24) | 3 (2.86) | 1 (0.95) | 2 (1.90) | 99 (94.29) |
| No | 1 (0.95) | 2 (1.90) | 2 (1.90) | 1 (0.95) | 0 | 0 | 6 (5.71) |
| Workplace | |||||||
| Itinerant | 8 (7.62) | 23 (21.90) | 7 (6.67) | 2 (1.90) | 1 (0.95) | 2 (1.90) | 43 (40.95) |
| Establishede | 15 (14.29) | 34 (32.38) | 11 (10.48) | 2 (1.90) | 0 | 0 | 62 (59.05) |
When asked about sexual intercourse during their last week of work, 56.19% (CI 95% 52.59–59.80) reported having intercourse more than five times a week, 35.24% (CI 95% 32.18–38.25) said they had intercourse between 1 and 5 times, 8.57% (CI 95% 7.67–9.47) had no intercourse at all. In order to keep a historical record about the presence of STIs in this study group, they were questioned whether they had ever suffered at least one STI in their lives or if they had related symptoms; 75.23% (CI 95% 71.51–78.95) reported not having had an STI while the remaining 26 participants (24.76%, CI 95% 23.02–26.5) reported having suffered at least one STI; however, they did not reveal its causal agent. Similarly, they were asked whether they regularly used condoms during intercourse, 7.61% (8/105, CI 95% 6.12–9.1) demonstrated inconsistent condom use. In relation to smoking and alcoholism, 88.57% (93/105, CI 95% 84.15–92.99) of FSWs were smokers, and 99 of them (94.28%, CI 95% 90.2–98.36) consumed alcohol while performing their activities. Finally, in relation to the work site where they offered their services, 40.95% FSWs (43/105, CI 95% 38.45–43.42) answered that they did so on the street, and the remaining 62 (59.05%, CI 95% 55.77–62.31), reported offering their services in established places such as bars, nightclubs, strip clubs, and massage parlors.
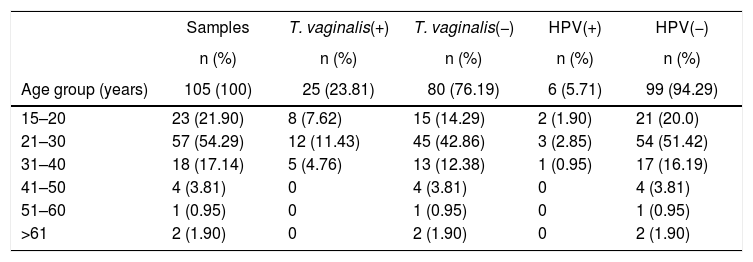
Prevalence and variables associated with the presence of risk T. vaginalis and HPV infectionsAll 105 samples were used to identify the presence of T. vaginalis and HPV by PCR, a parallel sample of the exudate was used to search for trophozoites of T. vaginalis by light microscopy, 25.7% (27/105, CI 95% 23.56–27.85) of positive samples were obtained by microscopic analysis for the presence of trophozoites of T. vaginalis (data not shown), moreover, the samples were analyzed by the Pap test for screening of cervical cancer precursor lesions, finding 7.61% (8/105, CI 95% 6.25–8.97) of samples with cytological abnormalities such as atypical squamous cells of undetermined significance (ASC-US) (25.0%, 2/8), low-grade squamous intraepithelial lesions (LSIL) (62.5%, 5/8) and high-grade squamous intraepithelial lesions (HSIL) (12.5%, 1/8).
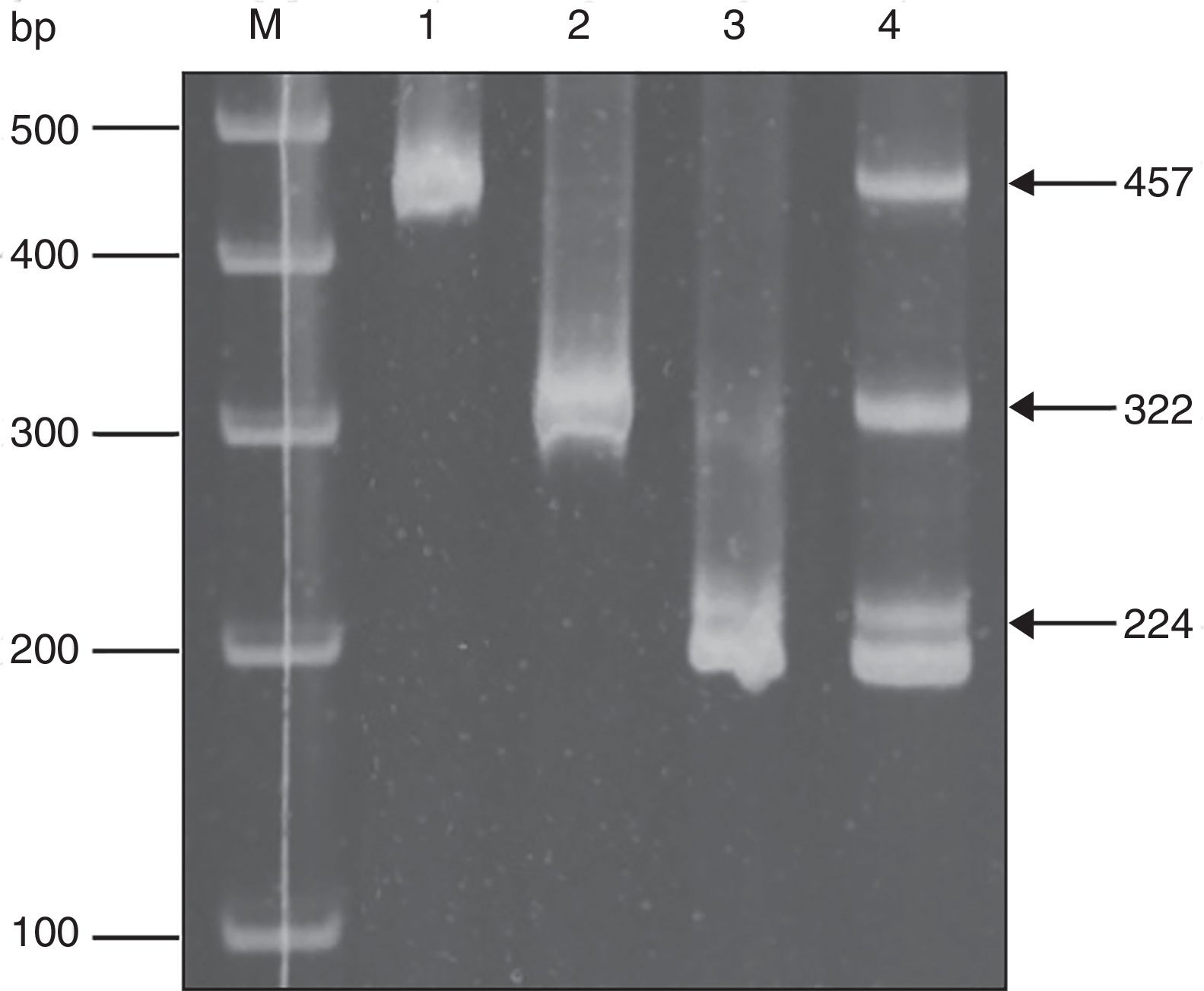
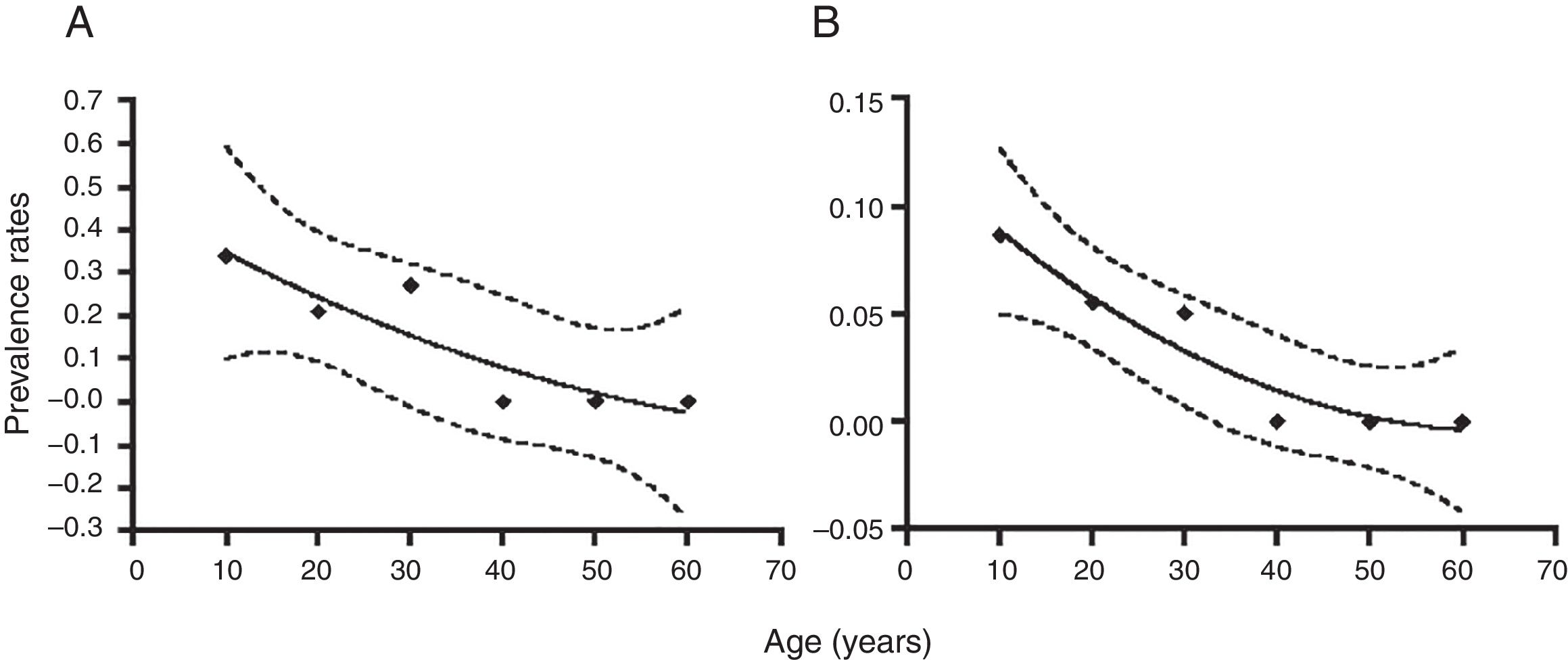
Molecular diagnosis of T. vaginalis showed an overall prevalence of 23.8% (25/105, CI 95% 21.82–25.78), as shown in Table 2, all positive samples showed a 300bp amplicon, identifying a mean age of 24.7 years for women positive for T. vaginalis. When we analyzed the presence of HPV genomic material in the 105 samples studied, 5.71% (6/105, CI 95% 4.7–6.72) samples resulted positive for amplification of a 450bp fragment specific for HPV, as shown in Table 2, with an average age of 27.6 years for women who tested positive for the presence of HPV. Additionally, we performed the genotyping of HPV-positive samples and we observed the presence of high-risk types; identifying HPV16 (16.6%, 1/6), HPV18 and HPV58 (33.3%, 2/6), and HPV16, HPV18 and HPV58 (50.0%, 3/6) as shown in Figure 1. In relation to the diagnosis of T. vaginalis, 25 samples had positive results for two tests; the agreement between the optical microscopy and the molecular diagnostic was very good, (optical microscopy versus PCR κ=0.949±0.036, CI 95% 0.879–1.019). Further stratification of FSWs from the municipality of Orizaba showed a significant difference in infection rate (T. vaginalis) according to age (χ2=64.27, degrees of freedom=5, p=0.0001), and HPV (χ2=21.85, degrees of freedom=5, p=0.0006). Prevalence rate was significantly correlated with age (r2=0.8178, p=0.0159, by second-order polynomial regression) for T. vaginalis infection, and (r2=0.922, p=0.0076 by second-order polynomial regression) for HPV infection, as shown in Figure 2. On the other hand, the presence of infection by T. vaginalis was significantly associated with educational level (basic education versus higher education) (3% versus 21%, p=0.0138, CI 95% 0.0875–0.8496, by the Fisher's exact test), but not for HPV infection (p=0.0765, by the Fisher's exact test), for both infections there was neither association with marital status of participants (p=0.274 for T. vaginalis infection, p=0.146 for HPV infection, by Fisher's exact tests), nor with the number of clients per week (p=0.074 for T. vaginalis infection, p=0.40 for HPV infection, by Fisher's exact tests). However, there was a significant association between the presence of infection and participants who reported having a history of STIs (p<0.0001, CI 95% 3.68–16.58, RR=7.81, for T. vaginalis infection, and p=0.025, CI 95% 1.28–33.77, RR=6.58, for HPV infection, by Fisher's exact tests). It was also noted that there was no significant association between condom use (T. vaginalis p=0.391, HPV p=0.386, by the Fisher's exact test), alcohol consumption (T. vaginalis p=1.0, HPV p=0.303, by the Fisher's exact test), and smoking (T. vaginalis p=0.726, HPV p=0.138, by the Fisher's exact test) with the presence of infection. Finally, we observed a significant association between the place of work (street versus established place) and the presence of T. vaginalis infection (p=0.0023, CI 95% 1.45–6.45, RR=3.064, by Fisher's exact test) but not for HPV infection (p=0.224, by the Fisher's exact test).
DNA detection of T. vaginalis and HPV by age
| Samples | T. vaginalis(+) | T. vaginalis(−) | HPV(+) | HPV(−) | |
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | n (%) | |
| Age group (years) | 105 (100) | 25 (23.81) | 80 (76.19) | 6 (5.71) | 99 (94.29) |
| 15–20 | 23 (21.90) | 8 (7.62) | 15 (14.29) | 2 (1.90) | 21 (20.0) |
| 21–30 | 57 (54.29) | 12 (11.43) | 45 (42.86) | 3 (2.85) | 54 (51.42) |
| 31–40 | 18 (17.14) | 5 (4.76) | 13 (12.38) | 1 (0.95) | 17 (16.19) |
| 41–50 | 4 (3.81) | 0 | 4 (3.81) | 0 | 4 (3.81) |
| 51–60 | 1 (0.95) | 0 | 1 (0.95) | 0 | 1 (0.95) |
| >61 | 2 (1.90) | 0 | 2 (1.90) | 0 | 2 (1.90) |
HPV typing by multiplex PCR. The result of a sample with multiple infections is shown. (M) Size markers, (1) HPV16 positive control, (2) HPV18 positive control, (3) HPV58 positive control, (4) sample with a co-infection with HPV16, HPV18 and HPV58. Arrows indicate the fragment size.
Distribution of infection by T. vaginalis, and HPV in FSWs by age groups. The population was stratified according to the indicated age groups and infection rates are shown as mean±95% confidence interval. Numbers on top of each point refer to the number of samples for each group. Prevalence rates were significantly associated with age (r2=0.8178, p=0.0159, by second-order polynomial regression), (A) T. vaginalis infection, and (r2=0.922, p=0.0076 by second-order polynomial regression), (B) HPV infection, as indicated by the regression line and its 95% confidence area (dotted lines).
Positive FSWs to T. vaginalis, HPV and/or abnormal cytology were referred to gynecological care according to national health guidelines.
DiscussionThis study identified the characteristics in the FSWs of the central area of Veracruz, Mexico whose labor-economic activity exposes them to acquiring STIs. Some sociodemographic characteristics of FSWs correspond to those found in other studies in Mexico and some countries in the region, which show that sex work is performed by young women between the ages of 15 and 40, some of them being minors, with low school level, single or in free union and heavy smokers and alcohol consumers24,36.
The region of the study is composed of municipalities with high social and economic inequality, some of them classified as having the lowest index of human development in Mexico. These conditions of poverty and the lack of legislation that guarantee the protection of sexual work, place the FSWs of Veracruz in a vulnerable position30. However, this same regional context excludes them from other phenomena present in border cities, mainly in cities of the US-Mexico border, such as the use of injectable drugs by FSWs and their clients, which increases the risk of acquiring and transmitting HIV, sex tourism and violence associated with the migratory context11,12,42.
FSWs have historically been a key population in the HIV epidemic and strategies have been implemented to reduce the bridge of HIV transmission in sex work. Of the total HIV cases in Mexico, 20% occur in women with an incidence rate of 2.01/100000 women1. In 2014, the prevalence of HIV among FSWs was 0.67%, which contrasts with that reported in male sex workers, which was 24.1%, with FSWs being the only key population that has decreased their HIV incidence due to the promotion of condom use16,20. In our study, we found a higher percentage of condom use compared to FSWs in Mexico's border cities like Tijuana and Ciudad Juárez44 and Central American countries40. The use of condoms by sex workers shows special patterns according to the regional context, such as free access to condoms, differential condom uses with clients and non-commercial sexual partners, and the negotiation of sexual practices not protected by money4. Therefore, further studies on the perception and coverage of condom use in the FSWs of Veracruz, Mexico are needed.
Trichomoniasis and HPV infection are notifiable diseases in Mexico, however, epidemiological data reported by the Health Secretariat does not represent the real magnitude of these STIs because there is no total coverage of the public health services and because many STIs are asymptomatic. In addition, there is no official data of these STIs in FSWs; therefore, the information available is limited to academic studies conducted in a few cities in Mexico. In our study, we found a prevalence of T. vaginalis of 23.81% in FSWs, which is lower than that shown in cities in the Mexico–US Border Region, which was 35% in the same population type43. In Latin America countries, such as El Salvador, Guatemala, Honduras, Nicaragua and Panama, prevalence was 11%40, while in Peru and Argentina the prevalence of T. vaginalis in FSWs was 9% and 7%, respectively3,8,35. These data show a marked regionalization of T. vaginalis transmission through sex work.
On the other hand, the prevalence of HPV in FSWs was substantially lower than that reported in studies conducted in other cities in Mexico22,33. In HPV positive samples, we identified the presence of HPV16 and HPV18 high risk types, associated with 70% of cervical cancers worldwide5,15,19, and HPV58 high risk type identified with high frequency in studies conducted in women of Mexico, Latin America and China7,13,25. These results suggest that strategies for the prevention of HPV transmission and cervical cancer, including HPV vaccination, condom promotion and primary cervical cancer screening, have been successful in reducing the prevalence of HPV. However, more HPV screening studies including FSWs who work clandestinely or without a registration card are needed to obtain complete evidence.
Mexico's national immunization program includes the application of the vaccine against the HPV16 and HPV-18 high risk types in 11-year-old girls; however, it is necessary to consider the implementation of the second generation HPV vaccine that protects against high-risk HPVs associated with approximately 90% of cervical cancer that includes HPV5810,21, as well as the incorporation of HPV genotyping as part of the strategy of evaluation and control of preventive schemes based on prophylactic vaccines14.
Sex work in Mexico is regulated by each municipal government that requires the periodic screening of some STIs to issue registration licenses to FSWs, however, diagnostic studies are performed in private laboratories that FSWs pay at high cost and often cause extortion and abuse by health authorities, leading to some of the sexual work being carried out clandestinely and without access to STI prevention strategies37.
In conclusion, the results obtained in this study show the need to develop comprehensive health promotion programs for sex workers that include preventive education, campaigns for the timely detection of STIs using sensitive diagnostic methods and the creation of public policies that recognize and protect the Human Rights of FSWs.
Conflict of interestThe authors declare that there is no conflict of interests.
Appreciate the support provided by Jurisdicción Sanitaria VII for obtaining samples and David A. Madrigal Serrut for paper revision.