The consumption of soybean isoflavones (IS) is associated with several beneficial properties on human health. Some lactic acid bacteria possess β-glucosidase enzyme, that allows to obtain the active form of IS (aglycone). The solid state fermentation (SSF) has received great attention in the last years in order to obtain several valuable compounds. SSF, using soybean as substrate and Lactobacillus rhamnosus CRL 981 as starter, was studied in the present work. Sucrose was added into soybean paste to study the effect on the behavior of the selected strain. The development of L. rhamnosus CRL 981 through pH and recount measures, sugar intake, organic acid production, β-glucosidase activity and IS conversion were analyzed. No significant differences in growth and acidity were observed between soybean pastes with and without sucrose added, but the production of lactic acid was higher in the latter paste. The β-glucosidase activity was detected in both pastes and the complete hydrolysis of IS at 12h of fermentation was observed. Also, this strain was able to increase the free amino acids in soybean paste. SSF, using soybean as substrate and L. rhamnosus CRL 981 as starter culture, is an alternative process to obtain a soybean product bio-enriched in active IS with attractive nutritional characteristics.
El consumo de isoflavonas de soja (IS) está asociado a diversos beneficios para la salud humana. Ciertas bacterias lácticas poseen la enzima β-glucosidasa, que permite obtener la forma bioactiva (agliconas) de las IS. La fermentación en sustrato sólido (FSS) ha recibido gran atención en los últimos años debido a sus numerosas ventajas, y permite la obtención de productos con valor agregado. En el presente trabajo se estudió la FSS utilizando soja como sustrato y Lactobacillus rhamnosus CRL981 como cultivo iniciador. Con el fin de estudiar el efecto de una fuente de carbono externa sobre el comportamiento de la cepa seleccionada, se adicionó sacarosa a la pasta de soja. Se evaluó el crecimiento de L. rhamnosus CRL 981 a través de medidas de pH y recuento en placa. Además, se analizó el consumo de azúcares, producción de ácidos orgánicos, actividad β-glucosidasa y conversión de IS. No se observaron diferencias significativas en el crecimiento y acidez entre las pastas de soja sin adición de sacarosa y con ella, sin embargo, la producción de ácido láctico fue mayor en esta última. La actividad de β-glucosidasa se detectó en ambas pastas y se observó la hidrólisis completa de IS a las 12h de fermentación. Además, esta cepa fue capaz de aumentar los aminoácidos libres en la pasta de soja. La FSS, utilizando soja como sustrato y L. rhamnosus CRL 981 como cultivo iniciador, es un proceso alternativo para obtener un producto de soja bioenriquecido en IS bioactivas con características nutricionales atractivas.
Solid state fermentation (SSF) is a process in which microorganisms grow on solid materials without the presence of free liquid25. The soybean is an economic and high nutritional substrate to use in SSF for several applications such as enzyme production, to obtain food with added value and antioxidant compounds10,15. Among the components of soybeans there are a serie of phytochemicals called isoflavones (IS) whose consumption has been associated with several beneficial properties for human health. The IS are reported to play a role in the prevention of cardiovascular disease27, hormone dependent cancers35, metabolic syndrome17, and relieving postmenopausal symptoms6. IS-aglycones are the responsible for the mentioned biological effects but they are in low amount in the soybeans. IS-aglycones can be obtained from hydrolysis of isoflavone glucosides by the enzyme β-glucosidase32 present in several microorganisms, such as lactic acid bacteria (LAB) and bifidobacteria. They can play an important role in altering the profile of IS during soymilk fermentation increasing the amounts of biologically active IS, resulting an interesting alternative to obtain novel functional foods12,16,19. It is common to apply submerged fermentation (SmF) to study the behavior of these microorganisms, however, the active compounds are generally obtained in low concentrations12,21. An interesting alternative to enhance the yield could be the SSF.
Our group has previously studied SSF, using soybean as substrate and lactic cultures as starters, and the main fermentation parameters were optimized28. Lactobacillus rhamnosus CRL 981 showed the best growth on this substrate29. This strain was also studied in soymilk where biological properties were demonstrated in vitro and in vivo20,21. The aim of this study was to analyze the content of isoflavone aglycones produced during SSF process using L. rhamnosus CRL 981 as starter and soybean paste as substrate. In addition, the kinetics parameters of fermentation were analyzed to characterize the soybean product.
Materials and methodsMicroorganisms and growth conditionsL. rhamnosus CRL 981 was obtained from the culture collection (CRL) of the Centro de Referencia para Lactobacilos (CERELA). This microorganism was selected by its ability to grow on soy substrate and produce the enzyme β-glucosidase. Before experimental use, the culture was propagated (2%, v/v) twice in MRS medium (Laboratorios Britania S.A., Argentina) and incubated at 37°C during 18h. The soybean pastes were inoculated at 4% (v/w)29 and incubated at 37°C. Samples were taken at different times (0, 3, 9, 12 and 24h).
Solid-state fermentationFor each assay, SSF employed 150g of soy paste (wet weight), which was prepared from soybeans and distilled water in a ratio 1:1 to achieve 80% of moisture according Rodríguez de Olmos et al.29. The soybean paste was also prepared with the addition of 1% sucrose (w/w).
Moisture determinationInitial moisture of the soy pastes (after sterilization) was expressed as wet basis moisture content, experimentally determined by the method 950.46.B AOAC.
Microbial counts and pH measurementsCell viability was determined by the plate dilution method using MRS agar. One gram of soy paste was serially diluted in sterile physiology solution and the dilutions were plated in duplicate. The plates were incubated at 37°C for 48–72h. The results were expressed as colony forming units per gram of soy paste (CFU/g).
Changes in pH were monitored during fermentation of soy paste using a pH meter (SARTORIUS PT-10, Germany).
β-Glucosidase activityβ-Glucosidase activity in the fermented soy pastes and controls was evaluated according to Rodríguez de Olmos et al.28.
Analysis of metabolitesSugars and organic acids determinationSugar content (sucrose, fructose and glucose) and organic acids (lactic, acetic, and formic) production was analyzed during fermentation by high performance liquid chromatography (HPLC).
For sugars determination, the samples were deproteinized according to Ortiz ME et al.23. HPLC analysis was performed by the REZEX RPM-Monossaccharide Pb2+ column (300×7.8mm, Phenomenex, Torrance, CA, USA) at 85°C with a flow rate of 0.6ml/min using HPLC grade water as the mobile phase. A Smartline Pump 100 (Knauer GmbH, Berlin, Germany) was used with WellChrom K-2301 refractive index detector coupled to a Smartline 3800 Plus automatic injector and a ZC90 column heater (Zeltec, Buenos Aires, Argentina).
For organic acids, deproteinization of the samples was made with trichloroacetic acid (TCA). One gram of fermented paste was mixed with 3ml of 12.25% TCA, homogenized in Vortex and incubated at 4°C for 30min. It was then centrifuged at 8000×g for 10min. The supernatant was recovered and stored at −20°C until its determination by HPLC, according to Marazza et al.19.
For the quantification of sugars and organic acids, calibration curves for each compound were performed using pure standards at different concentrations.
Quantification of ISFor isoflavones analysis, the soy paste samples were freeze-dried and stored at −20°C until used. The extraction of isoflavones, from fermented and non-fermented soy paste and their quantification by reversed-phase-HPLC (RP-HPLC) were carried out according to Marazza et al.19. Standards of glucosides (daidzin, and genistin) and aglycones (daidzein and genistein) were obtained from Fluka and Sigma, respectively.
Free amino acids in soy pastesThe method of o-phthaldialdehyde (OPA) was used to determinate the amount of free amino acids in soy pastes fermented and controls9. The α-amino groups released by hydrolysis of proteins reactive with OPA in presence of 2-mercaptoethanol to form a compound that strongly absorbs at 340nm. The proteolytic activity was arbitrarily expressed as μmol of glutamic acid (Glu) released by gram of soy paste, using a standard curve of glutamic acid (BDH Chemicals Ltd., Poole, UK). This amino acid was used because it is a higher proportion relative to other amino acids in soy protein.
Statistical analysisAll assays were carried out in triplicate, and results were expressed as mean values with standard deviations. Data were compared by one-way analysis of variance (ANOVA) followed by Tukey test. The statistical analyses were performed with the Minitab-15 software (Minitab Inc., State College, PA, USA) and differences were considered significant at p<0.05.
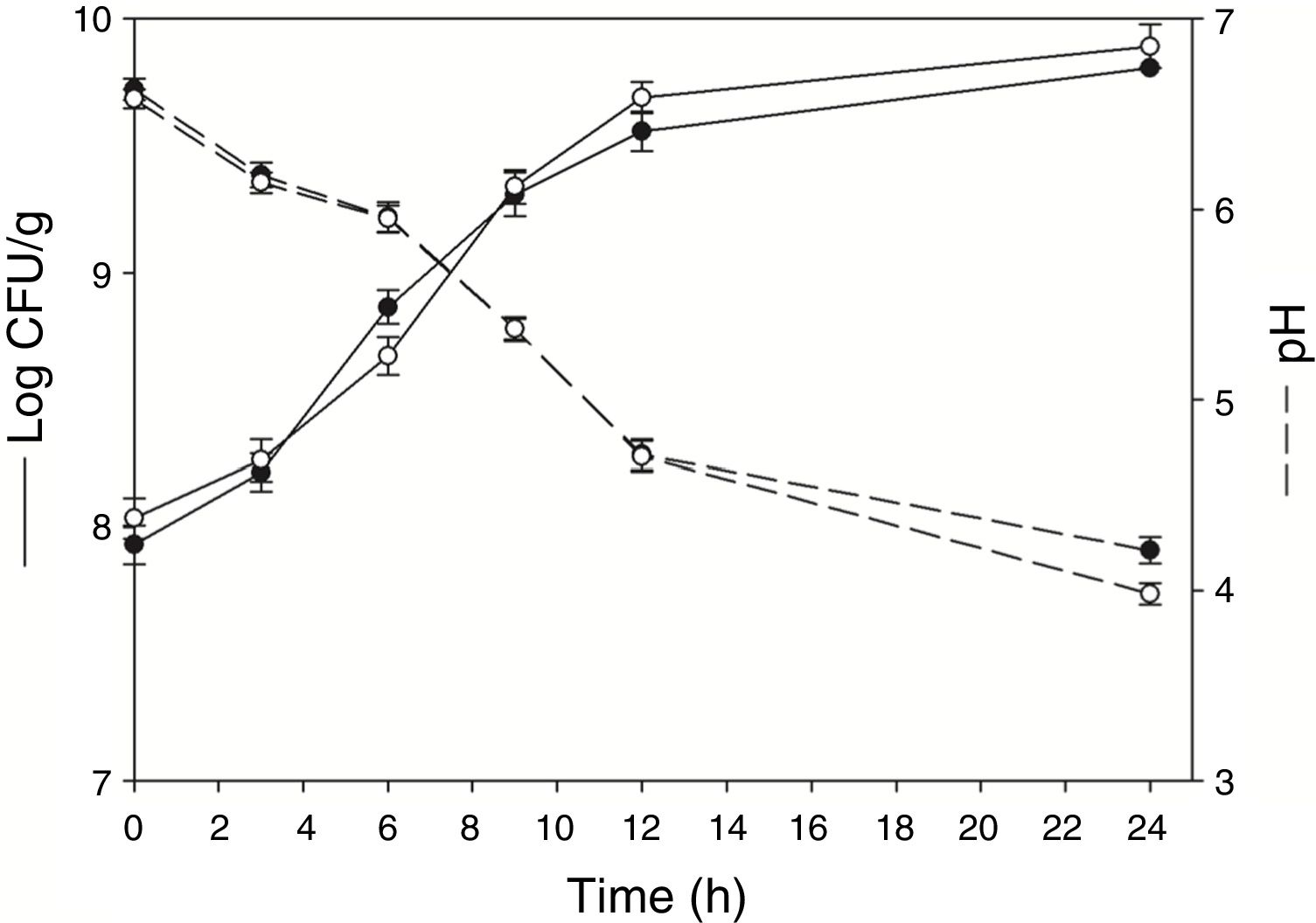
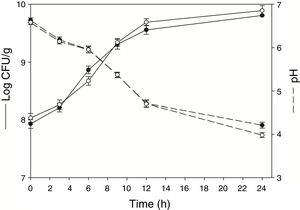
ResultsGrowth pattern of L. rhamnosus CRL 981 in soybean paste (with and without sucrose added)The moisture of both soybean paste was around 80% (without and with sucrose). Figure 1 shows the development of L. rhamnosus CRL 981 in absence and presence of additional sucrose, the initial cell counts were 7.93±0.07 and 8.03±0.08log CFU/g, respectively. Not significant differences (p˃0.05) were evidenced regarding growth of the strain on substrate, reaching 9.80±0.08 and 9.89±0.10log CFU/g at 24h, respectively. The exponential growth phase began at 3h and finished at 9h of fermentation, with a specific growth rate of 0.42 and 0.41h, respectively. However, the final pH was significantly lower (p˂0.05) in the soybean paste with sucrose added (3.98±0.06) in contrast to the other paste (4.21±0.07).
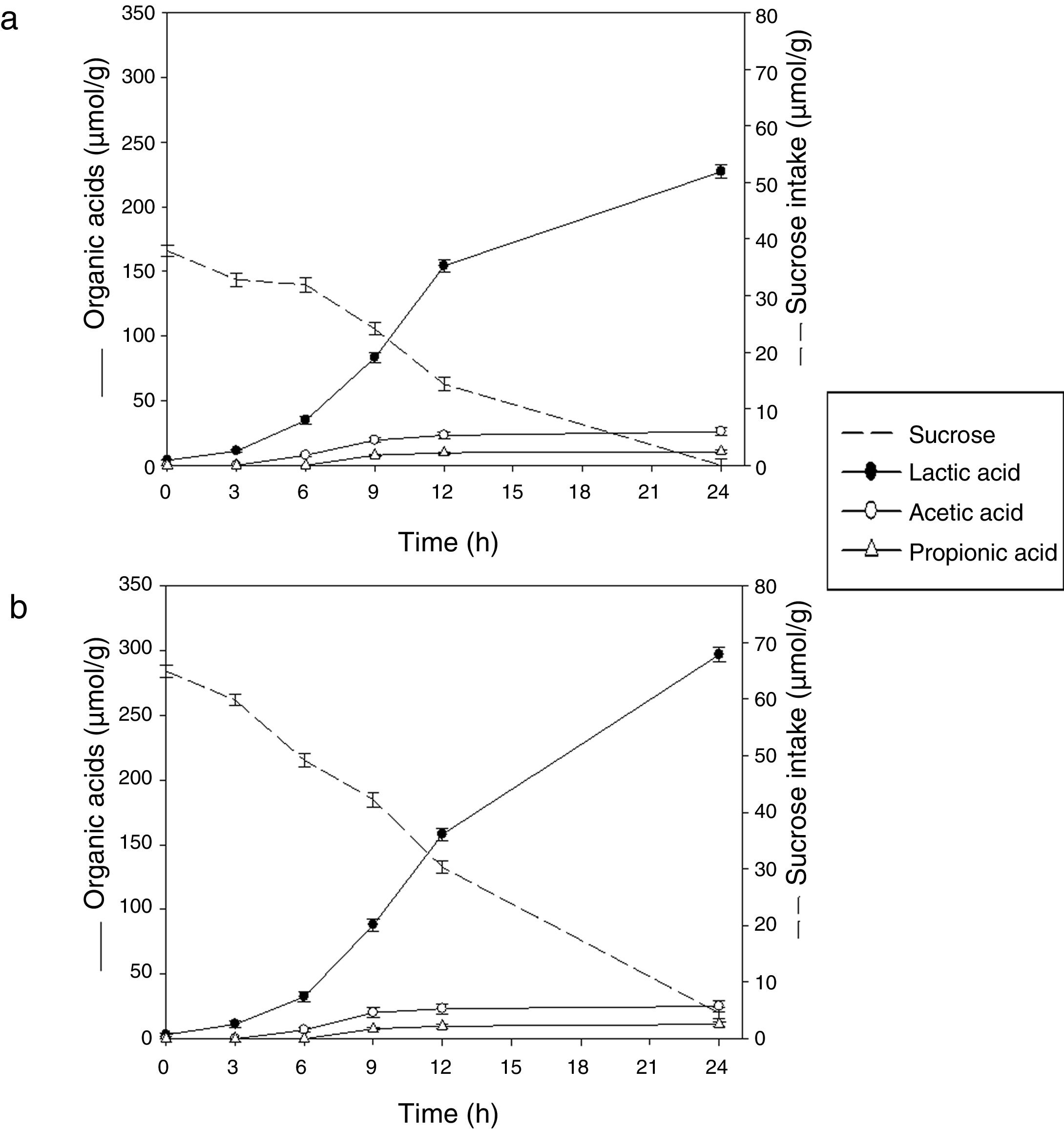
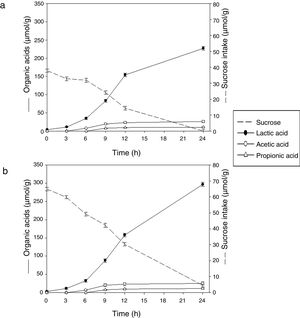
Sugar consumption and end products formationSucrose was preferentially hydrolyzed by the bacterium. The initial sucrose concentration in soybean pastes was 37.00±1.00μmol/g and 65.00±1.10μmol/g (without and with sucrose added, respectively). The substrate consumption started at 3h of fermentation, coinciding with the exponential phase of growth and it was consumed almost completely at 24h in both conditions (Fig. 2). The monosaccharides were not found in any sample, assuming that were consumed by the microorganism during fermentation.
Under the conditions used in this study, L. rhamnosus CRL 981 produced principally lactic acid (227.19±5.20μmol/g of paste) and lower amounts of acetic and propionic acids in soybean paste at 24h (26.06±2.90μmol/g of paste and 10.56±0.80μmol/g of paste, respectively). Similar results were found in the soybean paste supplemented with sucrose up to 12h of fermentation. However, the final concentration of lactic acid was higher in the later paste (35%), because of there was still enough sucrose at 12h (30.32±1.15μmol/g of paste), reaching around 300±5.40μmol/g of lactic acid at 24h. This result is in concordance with the lower pH value found in this sample. Despite the increased production of acid, the growth of the strain did not continue. In both cases, the large amount of produced lactic acid at 24h of fermentation was corresponded with the marked decrease in pH values.
In our work, the acetic and propionic acids production was also evidenced in lower amounts. Acetic began to arise at 6h of fermentation and propionic at 9h. The production of acetic was approximately twice that propionic. No differences were observed in the paste added with sucrose (p>0.05). Given the low production of acetic and propionic acid versus lactic acid, it can be assumed that the fermentation was homolactic. Thus, theoretically it should produce four moles of lactic acid for each mole of sucrose. Making the mass balance at the end of fermentation, the results obtained are according to the expecting values.
Evaluation of β-glucosidase activity and bioconversion of isoflavonesThe extraction and determination of β-glucoside activity were performed in fermented soybean pastes by L. rhamnosus CRL 981, with and without sucrose supplementation. Although yellow coloration was observed due to the presence of p-nitrophenol, product of the synthetic substrate used, its quantification was not possible due to interference by the blank (data no shown). However, it was possible qualitatively to observe evidence of the enzyme at 6h of fermentation in soybean paste without sucrose and at 3h in the paste with sucrose.
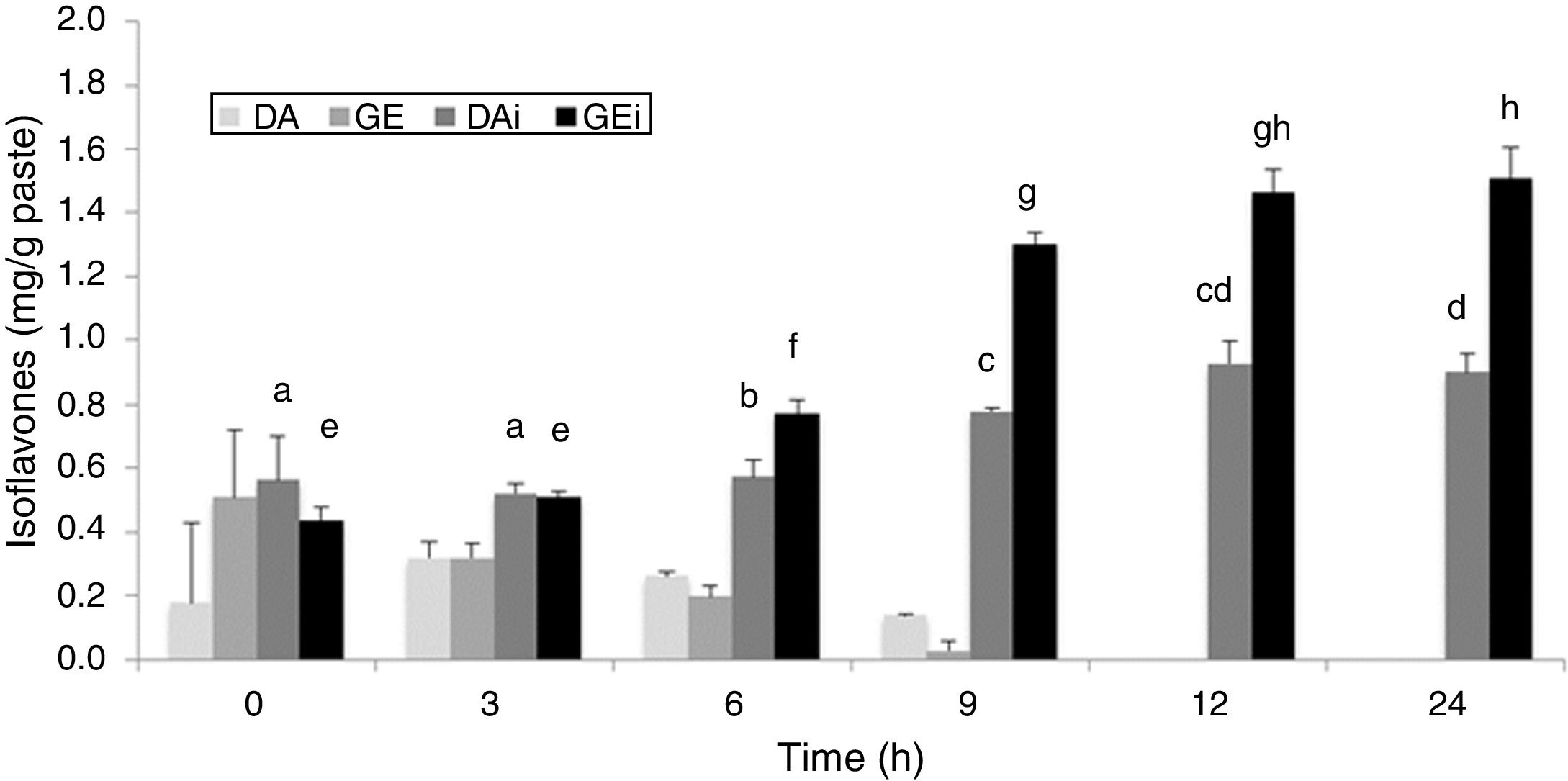
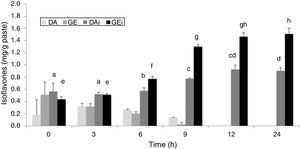
The identification and quantification of the main isoflavones – genistin (GE), daidzin (DA), genistein (GEi) and daidzein (DAi) – were performed in both soybean pastes at different times of fermentation. No significant differences were observed between both soybean pastes (p˃0.05). Figure 3 shows the isoflavones concentration in fermented soybean paste without sucrose added. The initial concentration of total IS was around 2.00±0.23mg/g of paste in both pastes. The proportion of two forms (IS glycoside and IS aglycone) was similar, showing a greater amount of GE. DA and GE concentrations decreased according the fermentation progressed with 100% of hydrolysis at 12h of fermentation and they were not detected at the end of fermentation. Significant differences were detected in the IS aglycones amount (p<0.05) from 6h of fermentation, with the highest value at 12h, without increasing at 24h. The values for IS aglycone of both fermented soybean paste were 0.94±0.06mg/g for DAi and 1.58±0.09mg/g for GEi at the end of the process.
IS glucosides (Daidzin: DA and Genistin: GE) and IS aglycones (Daidzein: DAi and Genistein: GEi) in fermented soybean paste (without additional sugar) by L. rhamnosus CRL 981 during 24h. Different letters (a–d and e–h) on the tops of columns indicate significant (p<0.05) differences among each IS aglycone in the different fermentation times.
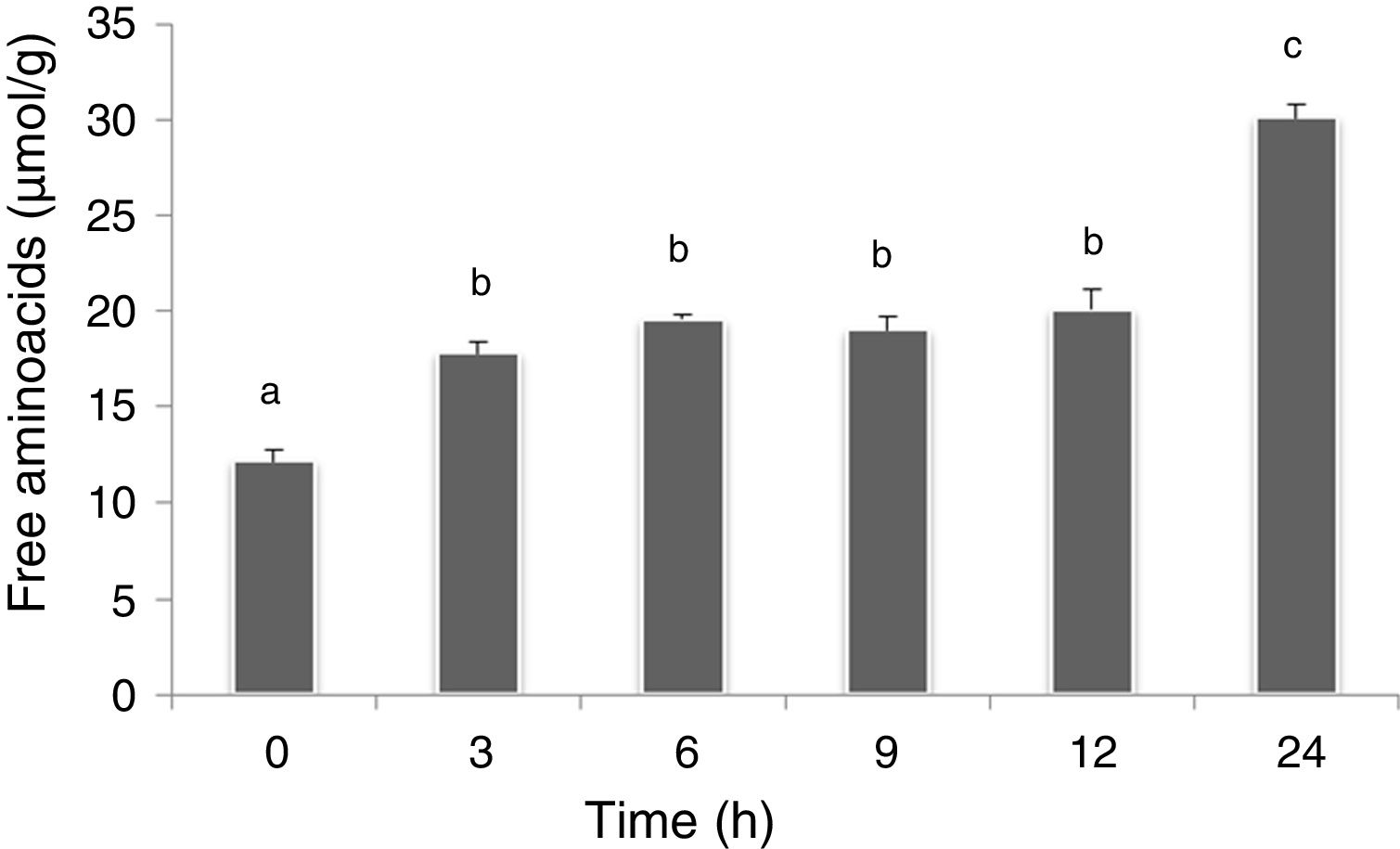
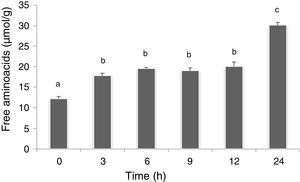
In this work, the free amino acids were determined in soybean paste as indirect way to evaluate the proteolytic activity of L. rhamnosus CRL 981. No significant differences (p>0.05) were found between both soybean pastes (with and without sucrose added). Figure 4 shows free amino acids in soybean paste without sucrose added at different times of fermentation. The amount of free amino acids of the control paste (without fermentation) was 12.2±1.00μmolGlu/g. A significant increasing (5μmolGlu/g) was observed at 3h (p<0.05), which was maintained up to 12h, and the highest value was obtained at 24h (30.1±1.30μmolGlu/g).
DiscussionInterest in soy-based products has significantly grown in the last years due to their reported nutritional and health-promoting benefits. The fermentation with lactic cultures is an alternative widely used to improve the nutritional and functional value of the soy-based products. A lot of studies had used LAB to increase the isoflavone aglycones amount but in the most of these cases the submerged fermentation was applied as biotechnological strategy12,16,19,24,33. In previous studies2,14,19, the growth characteristics and the end-products formation by different LAB in soymilk were reported. However, the active compounds were found in low concentration, so an alternative to this problem would be to study other technology, as SSF. On the other hand, the SSF is an attractive alternative to costly SmF processes for specific applications relative to the food industry26.
L. rhamnosus CRL 981 was previously selected as the best starter to use in solid state fermentation using soybean as substrate without additional added sugar. Also, some fermentation parameters were optimized: moisture (80%), temperature (37°C), inoculum amount (4%, v/w)29. Although the soybean substrate has carbon sources such as sucrose (2.50–8.20%), stachyose (1.40–4.10%), and raffinose (0.10–1.00%)8,22, the used strain is not able to use oligosaccharides19. Because of that, the effect of supplementation of additional sucrose was evaluated regarding growth and pH, kinetics parameters, β-glucosidase activity and content of isoflavone aglycones produced during the fermentation using L. rhamnosus CRL 981. Marazza et al.19 studied the effects of this strain in aqueous extract of soybean (EAS) added with sucrose 1% (w/v). L. rhamnosus CRL 981 showed improved development on soybean paste in contrast with EAS. Viable counts increased from 7.38log CFU/ml to 8.85log CFU/ml after 12h of incubation in soy milk, showing decrease of viability at 24h of fermentation. In soybean paste added with sucrose, the viable count increased from 8.03 to 9.69log CFU/g after 12h of incubation without loss of viability, and the acidification at 24h of incubation was higher in soybean paste. In soymilk the pH reduction caused protein coagulation and the appearance of this soymilk changed after fermentation. In contrast, the reduction of pH did not change the general appearance of the soybean paste. Other studies of SSF using lactic bacteria and soybean have obtained similar values of growth, demonstrating their capacity to be applied in this type of systems3,7. For example, Bartkiene et al.4 performed the SSF technique using LAB and soybeans as substrate, where the pH values were lower than 4.40 after 24h of fermentation. However, the cell counting was lower 7.28log CFU/g, which could be explained by the lower final moisture content of substrate (45%). Also, Chen et al.7 used soy meal as substrate for a SSF with Lactobacillus paracasei BCRC 14023 with similar cell counting (9.69log CFU/g), but higher pH values (4.99) were obtained.
Regarding the sugars consumption and organic acids production, the monosaccharides were not found in any sample at 24h of fermentation, assuming that were consumed by the microorganism during its growth. In addition, lactic acid was the main end product in both conditions. These results are in concordance with the results obtained by Marazza et al.19 in soymilk using the same strain and Garro et al.14, who used a strain of L. casei in soymilk.
Although it was not possible to measure β-glucosidase activity, the presence of this enzyme could be corroborated by the HPLC determination of isoflavones. The enzyme β-glucosidase plays a key role in the hydrolysis of the β-glucoside bond in the glycosylated isoflavones. This hydrolysis releases the IS aglycones which have been related to different biological properties13. There are several studies that demonstrate the activity of this enzyme in different strains of lactic bacteria and bifidobacteria using soy products with the consequent increase of IS aglycones11,13,16,19,33,34. Thereby, the quantification of IS can be utilized as indirect measure to confirm the presence of the enzyme during fermentation. The obtained results were similar with findings of Marazza et al.19 using the same strain in soymilk, which was able to increase bioactive isoflavones to achieve 100% of bioconversion at 12h. Besides, Landete et al. and Otieno et al.18,24 have demonstrated that the isoflavone glucosides content was also reduced in favor of the appearance of IS aglycones by fermentation using Bifidobacterium animalis ssp. lactis Bb12 and L. plantarum, respectively.
The fermented soymilk obtained by Marazza et al.34 and Otieno et al.18 had around 46mg/l of bioactive isoflavones, that means that is necessary intake more than one liter of this product to arrive 50mg, what is the daily requirement recommended5. This situation is difficult or improbable to be applied in Argentina where the consumers do not select the soybean product. An alternative is offer soy solid product with more IS aglycones, representing a less amount of bio-enriched product to be intake. With the soybean pastes obtained in the present work, 50mg of IS aglycones could be ensure with only 22g of the fermented product.
On the other hand, Marazza et al.21 found that aglycones have a higher antioxidant activity than isoflavone glucosides. Therefore, the high aglycone levels found in our soy paste fermented with L. rhamnosus CRL 981 could have the same activity and may be beneficial to design new solid and semisolid functional foods. More studies are necessary to confirm the functional activity of our preliminary soybean paste obtained.
Because of soybean has a high protein content, it resulted interesting to analyze the proteolytic activity of L. rhamnosus CRL 981. In other studies, this activity by LAB was evidenced by the increase of amino acids and free peptides from soybean proteins1,3,4,31. The observed increase of free amino acids at 24h of fermentation could be explained in part by the coagulation of proteins due to the low pH mainly at first hours of fermentation, but could also be due to the proteolytic activity of L. rhamnosus CRL 981. These results are similar with the finding of Aguirre et al.1 and Rodríguez de Olmos et al.28 who observed an increase of free amino acids due to proteolytic activity of a strain of L. paracasei in soymilk and soy paste, respectively. The proteolysis of soy protein is interesting to obtain soy products with nutritional and functional characteristics such as a more digestible protein, less protein for allergenic consumers and/or to increase the bioactive peptides from soy protein. Soy protein possesses numerous bioactive peptides, which are inactive within the parent protein sequences but can be released through proteolytic hydrolysis during fermentation and gastrointestinal digestion30.
ConclusionsIn the present work a semisolid soybean product fermented by L. rhamnosus CRL 981 was obtained with high content of isoflavone aglycones: daidzein and genistein. This strain was able to increase these bioactive isoflavones with 100% of bioconversion at 12h on fermented soy paste. On the other hand, L. rhamnosus CRL 981 had a good development in soybean paste, using the sucrose present in the substrate to growth and it has shown the ability to enhance the free amino acids from the soy protein, demonstrating interesting nutritional characteristics. The amount of sucrose present in the soybean substrate was enough to improve the properties evaluated, so it would not be necessary to add more of this to the soybean paste.
L. rhamnosus CRL 981 could be used as a starter culture to produce differentiated aglycone-rich functional soy products with more digestible protein. SSF technique represents an interesting alternative to obtain new soy-based products with higher product yield.
Conflict of interestThe authors declare that they have no conflicts of interest.
This study was partly supported by grants from Consejo Nacional de Investigaciones Científicas y Tecnológicas (CONICET/PIP), Agencia Nacional de Promoción Científica y Tecnológica (ANPyCT-FONCYT/PICTs 1773/1949), and Consejo de Ciencia y Técnica de la Universidad Nacional de Tucumán (CIUNT), Argentina.