Stenotrophomonas maltophilia is a nosocomial pathogen of increasing importance. S. maltophilia K279a genome encodes a diffusible signal factor (DSF) dependent quorum sensing (QS) system that was first identified in Xanthomonas campestris pv. campestris. DSF from X. campestris is a homologue of farnesoic acid, a Candida albicans QS signal which inhibits the yeast-to-hyphal shift. Here we describe the antagonistic effects of S. maltophilia on C. albicans on filamentation as well as on its planktonic and biofilm modes of growth. To determine the role of the DSF-mediated quorum sensing system in these effects, C. albicans ATCC 10231 and C. albicans tup1 mutant, locked in the filamentous form, were grown with K279a or with its rpfF deletion mutant (DSF–). A significant reduction in viable counts of C. albicans was observed in planktonic cocultures with K279a as well as in mixed biofilms. Furthermore, no viable cells of C. albicans tup1 were recovered from K279a mixed biofilms. Fungal viability was also assessed by labeling biofilms with SYTO 9 and propidium iodide. Confocal images showed that K279a can kill hyphae and also yeast cells. Light microscopic analysis showed that K279a severely affects hyphae integrity. On the other hand, the presence of K279a rpfF did not affect fungal morphology or viability. In conclusion, we report for the first time that S. maltophilia interferes with two key virulence factors of C. albicans, the yeast-to-hyphal transition and biofilm formation. DSF could be directly responsible for these effects or may induce the gene expression involved in antifungal activity.
Stenotrophomonas maltophilia es un patógeno nosocomial de importancia creciente. El genoma de S. maltophilia K279a codifica un factor de señalización difusible (DSF), autoinductor de “quorum sensing” (QS), identificado previamente en Xanthomonas campestris pv. campestris. El DSF de X. campestris es homólogo del ácido farnesoico, señal de QS de Candida albicans, que inhibe la transición levadura-hifa. En este trabajo se describe el efecto antagónico de S. maltophilia sobre la filamentación y el crecimiento planctónico y en biofilms de C. albicans. Para determinar la participación del sistema de QS mediado por el DSF en dichos efectos, C. albicans ATCC 10231 y la mutante C. albicans tup1, que solo crece en forma filamentosa, fueron cultivadas en presencia de K279a o de su mutante K279a rpfF (DSF–). Se observó una reducción significativa del número de viables de C. albicans en cultivos planctónicos y biofilms desarrollados en presencia de K279a. Es de señalar que no se recuperaron células viables de C. albicans tup1 a partir de biofilms mixtos en presencia de K279a. Las imágenes de microscopía confocal revelaron que K279a produce la muerte de hifas y levaduras en biofilms mixtos teñidos con ioduro de propidio y SYTO 9. El análisis por microscopía óptica mostró que K279a afecta la integridad de las hifas. En cambio, la presencia de K279a rpfF no afectó la morfología ni la viabilidad fúngica. En conclusión, informamos por primera vez que S. maltophilia interfiere con dos factores de virulencia de C. albicans, la transición levadura-hifa y la formación de biofilms. Estos efectos pueden ser mediados por el DSF en forma directa o a través de la inducción de genes involucrados en la actividad antifúngica.
Stenotrophomonas maltophilia (formerly Pseudomonas maltophilia and Xanthomonas maltophilia) is a widespread environmental, multidrug resistant bacterium. It has become a nosocomial pathogen of increasing importance; in fact, it is the third most common nosocomial non-fermenting gram-negative bacterium7. Infection occurs principally in immunocompromised subjects, and in patients exposed to invasive devices and/or broad spectrum antibiotics5,21. S. maltophilia has also emerged as one of the most common bacteria isolated from the airway of cystic fibrosis (CF) patients28,34, nevertheless, there is controversy about the role of this opportunistic pathogen as causative agent of CF.
An important virulence factor of S. maltophilia is the capacity to form biofilms, communities of microbial cells that grow on biotic or abiotic surfaces embedded within exopolymeric material16,24,27. Microorganisms growing in biofilms exhibit phenotypic characteristics that are distinct from those of their planktonic counterparts, including increased resistance to host immune defences and antimicrobial compounds9. Microorganisms communicate using chemical signals (autoinducers) to sense cell density and coordinate their physiological processes, including biofilm formation, in a process known as quorum sensing (QS). QS also plays an important role in interspecies communication, including interactions between prokaryotes and eukaryotes30,33.
The genome of S. maltophilia K279a encodes a diffusible signal factor (DSF) dependent QS system that was first identified in Xanthomonas campestris pv. campestris11,17. DSF synthesis is completely dependent on rpfF, which is part of the rpf operon (for regulation of pathogenicity factors)2. The cross-kingdom antagonistic activity of DSF (cis-11-methyl-2-dodecenoic acid) from X. campestris has been reported on Candida albicans germ tube formation35.
C. albicans, one of the most important opportunistic human fungal pathogen, causes various diseases ranging from superficial mucosal infections to life-threatening systemic infections6. The ability of C. albicans to undergo morphogenic shift from yeast to filamentous morphology is central to its pathogenesis and biofilm formation29,32. This morphological transition is induced in vivo and in vitro. Host factors or environmental factors, such as mammalian serum and high temperatures (37°C), are required for C. albicans morphogenesis. Two related QS compounds, farnesoic acid and farnesol inhibit the yeast-to-hyphal shift. C. albicans ATCC10231 produces farnesoic acid while a number of C. albicans clinical isolates produce farnesol15,23. Furthermore, it has been reported that farnesol addition can block C. albicans biofilm formation29. The DSF family of QS signals has been implicated in the interkingdom signaling between bacteria and C. albicans30,33. DSF from X. campestris is a structural and functional homologue of farnesoic acid, and it has been suggested that the bacterial QS signal is recognized by the receptor of farnesoic acid, leading to an arrest in filamentation35. Furthermore, cis-2-dodecenoic acid (BDSF) from Burkholderia cenocepacia inhibits C. albicans germ tube formation either as a pure compound or in coculture, and BDSF also blocks C. albicans biofilm formation by interfering with the morphological switch3,36.
In the present work, we report the antagonistic effects of S. maltophilia K279a on C. albicans filamentation as well as on its planktonic and biofilm modes of growth. To determine the role of the DSF-mediated quorum sensing system in these effects, C. albicans was grown with S. maltophilia K279a or its rpfF deletion mutant (DSF–). C. albicans ATCC 10231 was used as a wild type strain while C. albicans tup1 mutant, locked in the filamentous form, was used to investigate the interactions between bacterial strains and C. albicans filaments.
Materials and methodsStrains and culture conditionsS. maltophilia K279a, whose genome is fully sequenced (http://www.sanger.ac.uk/Projects/S_maltophilia/)1,7, and its derivative DSF-deficient mutant K279a rpfF11 were a kind gift from Dr. M. Dow (BIOMERIT Research Centre, Ireland). C. albicans ATCC10231 was used in this study as a wild type strain. The mutant C. albicans tup14, unable to form yeasts, was a gift from Dr. R. Kolter (Harvard Medical School, Boston, USA). Bacterial strains were routinely cultured at 37°C on trypteine soy agar (TSA, Britania, Argentina) while C. albicans strains were grown on Sabouraud dextrose agar (SDA, Britania). Unless otherwise stated, all experiments were performed with microorganisms grown in trypteine soy broth (TSB, Britania) supplemented with 0.2% (w/v) dextrose (TSB-d), incubated at 200 rpm on a gyratory water bath shaker (Model G75, New Brunswick Scientific Co. Edison N J, USA), at either 30°C or 37°C, depending on the experiment. We had previously confirmed that TSB-d enhanced the yeast-to-hyphae transition and supported C. albicans biofilm formation even though it was slightly less effective than RPMI 1640, a medium generally chosen for filamentation and biofilm assays26. TSB-d was chosen because this medium supported S. maltophilia growth (data not shown) better than RPMI 1640.The viability of C. albicans was determined by plating on SDA or SDA supplemented with 100μg/ml ciprofloxacin (ICN, Basingstoke, UK) to suppress the growth of S. maltophilia. When required, fetal bovine serum (FBS,Bioser, Argentina) was added to TSB-d at a final concentration of 5%. When appropriate, 4.7mM DSF (Santa Cruz Biotechnology, Santa Cruz, CA, USA) stock solutions were made in ethanol and then diluted in TSB-d to the required concentration (20–200μM). An equal volume of ethanol was used in all appropriate controls.
Inhibition of C. albicans germ tube formation by S. maltophiliaAnalysis of C. albicans morphology was performed as described previously with modifications3. Briefly, C. albicans ATCC 10231 was grown as yeast cultures overnight at 30°C in TSB-d. S. maltophilia strains were grown under the same conditions but at 37°C. Overnight C. albicans cultures were diluted in 2ml of fresh TSB-d to contain approximately 105 CFU/ml. Coculture experiments were performed by adding K279a or K279a rpfF cultures at a final concentration of 106 CFU/ml (ratio bacteria-fungi of 10:1). After 15h of incubation at 30°C, FBS was added and incubation proceeded for 3h at 37°C for induction of germ tube formation. Then, cells were Gram stained and percentages of germinal tube production were examined by light microscopy (Olympus BX50, 400X magnification), counting about 400 C. albicans cells per sample.
Inhibition of C. albicans filamentation by DSFOvernight cultures of C. albicans ATCC 10231 in TSB-d at 30°C were diluted 10-fold in fresh TSB-d supplemented with FBS and DSF (0–200μM). After 3h incubation at 37°C, percentages of germ tube formation were examined as described above.
Survival of C. albicans in the presence of S. maltophilia in planktonic culturesC. albicans survival assays were performed as described previously with modifications13. Briefly, fungal (C. albicans ATCC10231 or C. albicans tup1) and bacterial strains (K279a or the rpfF mutant) were grown in TSB-d at 37°C. Overnight cultures were diluted in 2ml of fresh TSB-d and standardized as described above. Fungal cultures used as controls and cocultures with bacterial strains (ratio bacteria-fungi of 10:1) were grown for 48 and 72h at 37°C. After incubation, the number of viable fungal cells, in the presence or absence of S. maltophilia strains, was estimated by plating 10-fold serial dilutions of these suspensions. After 24h of incubation at 37°C, colony-forming units were counted and converted to log10 CFU/ml. Furthermore, cells were Gram stained and examined by light microscopy (400× magnification). Microphotography was done with an Olympus DP70 digital camera. The same experiment was also performed with C. albicans ATCC10231 and bacterial strains but cultures were incubated at 30°C, a condition that favors yeast growth form.
C. albicans biofilm formation in the presence of S. maltophiliaBiofilms were prepared using a static microtiter plate model as described previously25. C. albicans ATCC 10231 or C. albicans tup1 overnight cultures grown in TSB-d at 37 °C were standardized to contain approximately 106 CFU/ml, and for mixed biofilms, bacterial cultures were added in the ratio of 1:1. For each test condition, 4 wells of a sterile flat-bottom 96-well polystyrene microtiter plate (TPP, Trasandingen, Switzerland) were filled with 200μl of the standardized inocula. Uninoculated medium controls were included. After static incubation for 72h at 37°C, the culture medium was removed from each well and plates were washed twice with sterile PBS to remove non-adherent cells. The surfaces of the wells were scraped with sterile cotton swabs (4 wells per condition). Swabs transferred into tubes containing 2ml of PBS were sonicated (ELMA Transonic TI-H-5, Germany) for 3min and vortexed vigorously to aid the dissolution of microrganism clumps. The viability of C. albicans biofilms, in the presence or absence of S. maltophilia strains, was determined by plate counts as described above. The limit of detection in our experimental conditions was 40 CFU/ml. A fluorescence-based technique that distinguishes live cells from dead ones based on the presence of an intact cytoplasmic membrane was also used. Biofilms used for confocal scanning laser microscopy (CSLM) were developed on 8-well chamber slides (Nunc Lab-Tek, Rochester, NY, USA) containing a borosilicate glass base. Chambers were filled with 400μl of the standardized inocula. After 72h of static incubation at 37°C, the wells were rinsed with sterile normal saline [NS; 0.9% (w/v) NaCl] and refilled with NS containing 2.5μM SYTO 9 (Molecular Probes, Grand Island, NY, USA) and 15μM propidium iodide (PI, Sigma, St. Louis, Missouri, USA). Live SYTO 9-stained cells and dead propidium iodide-stained cells were visualized with a confocal laser-scanning microscope (Carl Zeiss LSM510-Axiovert 100M) after 20min incubation in the dark. Dual-colour images were acquired by sequentially scanning with a 488nm argon laser and emitted fluorescence was recorded within the range 505–530nm or 530–600nm in order to visualize SYTO 9 or PI fluorescence, respectively. Images were reconstructed using the ZEN 2009 light edition. CSLM was performed twice independently. Each time, two wells were used per condition. Lastly, biofilms used for light microscopy were formed on Thermanox cover slips placed in 12 well microtiter plates under the same conditions. After incubation, cover slips were removed, washed with PBS, Gram stained and evaluated by light microscopy at 400× magnification.
Statistical analysisAll assays were carried out at least in triplicate and repeated three times, except CLSM. Results were analyzed by two-way analysis of variance (ANOVA), followed by Bonferroni´s multiple-comparison test (GraphPad Prism version 5.00 for Windows, Graph Pad Software, San Diego, CA, USA). Differences were considered significant at a p value of <0.05.
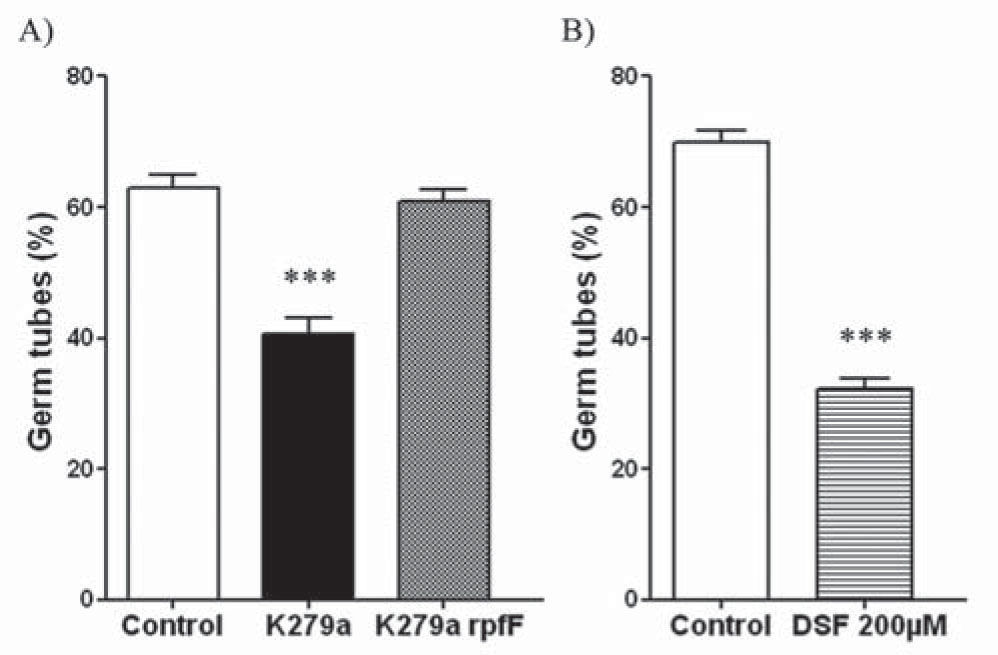
Results and discussionInhibition of C. albicans germ tube formationThe microscopic analysis showed that approximately 61% of C. albicans ATCC 10231 cells counted formed germ tubes when cocultured with K279a rpfF, a similar percentage to that observed in the control (63%). On the other hand, the coculture with K279a significantly decreased germ tube formation to approximately 41% (p <0.001) (Fig. 1A). Thus, the inhibitory effect of S. maltophilia was dependent on the production of DSF, which could be recognized by the receptor of farnesoic acid leading to an arrest in filamentation as it was suggested for DSF from X. campestris35. Furthermore, the inhibitory activity of commercial DSF against C. albicans ATCC 10231 germ tube formation was also evaluated in the presence of FBS. After induction at 37°C for 3h, the microscopic analysis showed that more than 70% of the cells formed germ tubes in the control. DSF at a final concentration of 200μM caused a marked reduction in germ tube formation of approximately 32% (p < 0.001) (Fig. 1B). However, at lower concentrations, DSF did not significantly inhibited germ tube formation under these conditions (data not shown).
Inhibition of C. albicans germ tube formation. A) Effect of S. maltophilia on C. albicans ATCC 10231 germ tube formation. B) Effect of commercial DSF on C. albicans ATCC 10231 germ tube formation. A and B) cells were Gram stained and percentages of germinal tube production were examined by light microscopy, counting about 400 C. albicans cells per sample. The mean and standard error from three repeats are presented. ***Significant difference (p <0.001) respect to the control.
Cross-kingdom antagonistic activity of the DSF family has been reported in C.albicans filamentation30. BDSF from B. cenocepacia is the most potent inhibitor of C. albicans germ tube formation, followed by DSF from X. campestris3,35. Our results highlighted the role of DSF from S. maltophilia, directly or via the induction of genes involved in antifungal activity, in the inhibition of filamentation. Other autoin-ducers, including homoserine lactones (HSL) such as 3-oxo-C12-HSL produced by Pseudomonas aeruginosa, are able to inhibit the Candida yeast-to-hypha transition14. However, it is not known if all these molecules exert their action through the same mechanism. Farnesol can affect more than one regulatory system in different ways. It was demonstrated that farnesol action involves the trans-criptional repressor Tup1 that negatively regulates the yeast-to-hypha transition19. Recently, it has been shown that farnesol and 3-oxo-C12-HSL directly inhibit the adenylyl cyclase Cyr1p, while dodecanol prevents cAMP-dependent hyphal development through a process dependent upon the transcriptional repressor SFL112. Furthermore, B. cenocepacia and possibly BDSF inhibit C. albicans hyphal development through an SFL1-dependent signaling pathway similar to that of dodecanol, which is not a physiologically relevant QS molecule of C. albicans12. Further studies are required to uncover the molecular mechanisms of DSF and BDSF in C. albicans morphogenesis.
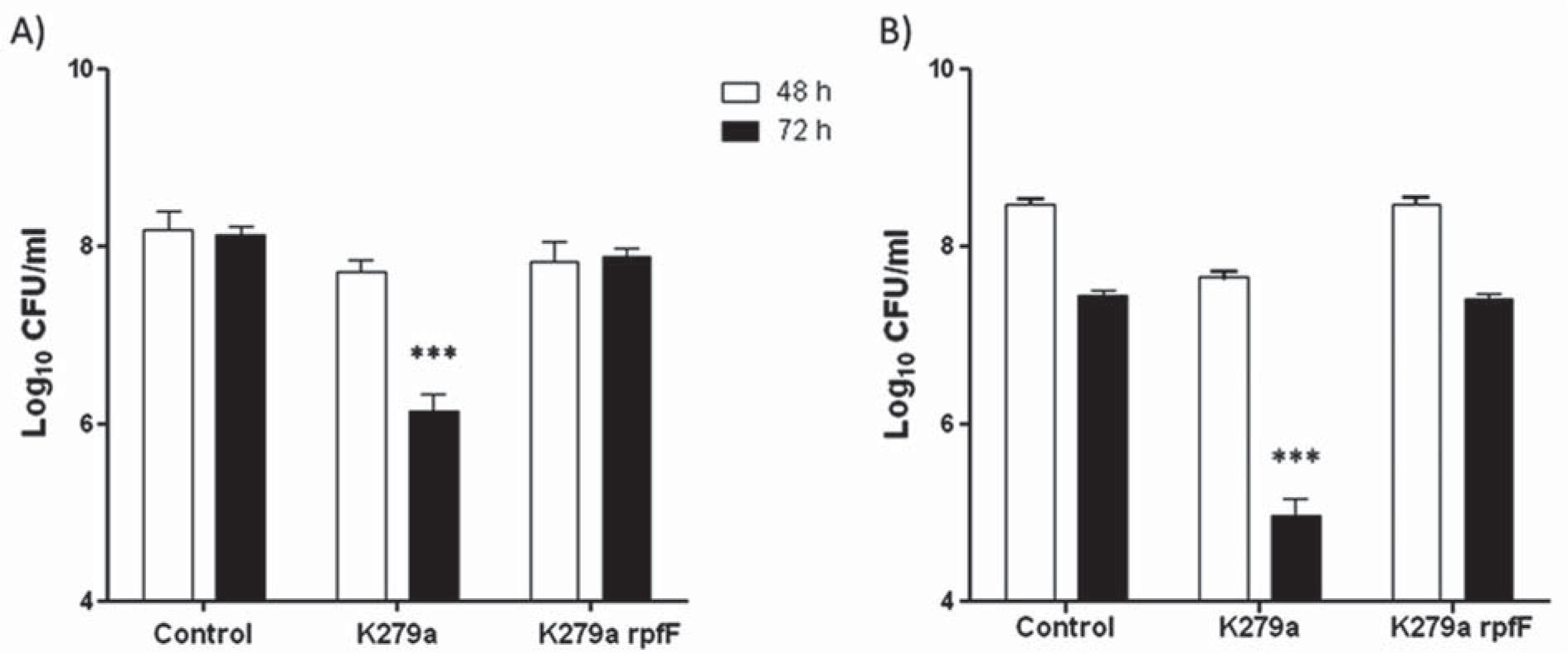
Survival of C. albicans in the presence of S. maltophilia in planktonic culturesFigure 2 shows C. albicans ATCC 10231 and C. albicans tup1 survival assays performed at 37°C. There were not statistically significant differences between the number of viable fungal cells in C. albicans ATCC 10231 cultures and cocultures with K279a rpfF, either after 48h or 72h of incubation (Fig. 2A). On the other hand, cocultures with K279a showed a different behavior. After 48h of incubation, the number of viable fungal cells was similar to that of the control (7.70±0.24 log10 CFU/ml and 8.18±0.39 log10 CFU/ml, respectively). However, after 72h the number of viable cells declined significantly by 2 logs (p <0.001) (Fig. 2A). Survival assays were also performed under a condition that favors yeast growth by incubation of C. albicans ATCC 10231 and bacterial strains at 30°C. Interestingly, under this condition K279a did not affect C. albicans viability. After 72h of incubation the number of viable fungal cells was similar to that of the control (8.08±0.44 log10 CFU/ml and 8.25±0.27 log10 CFU/ml, respectively).
Survival of C. albicans in the presence of S. maltophilia. The viability of C. albicans ATCC 10231 (A), and the constitutively filamentous C. albicans tup1 mutant (B) was measured in the presence or absence of S. maltophilia strains. Cultures were grown in TSB-d for 48 h and 72 h at 37°C and viable counts were performed on SDA or SDA supplemented with ciprofloxacin. Each bar represents mean ± SD log10 CFU/ml from three independent assays. ***Significant difference (p <0.001) with respect to the control at the same time of incubation.
It was observed that the number of viable cells of C. albicans wild type control was similar after 48h and 72h of incubation (Fig. 2A), but for C. albicans tup1 control, a decreased of 1 log was observed after 72h (8.47±0.11 log10 CFU/mland 7.46±0.09 log10 CFU/ml, respectively) (Fig. 2B). Cocultures of the tup1 mutant and K279a rpfF showed a similar behavior to that observed in the tup1 control. Moreover, C. albicans tup1 in association with K279a showed a significant reduction in the number of viable fungal cells by 2.5 logs after 72h of incubation (p <0.001) (Fig. 2B).
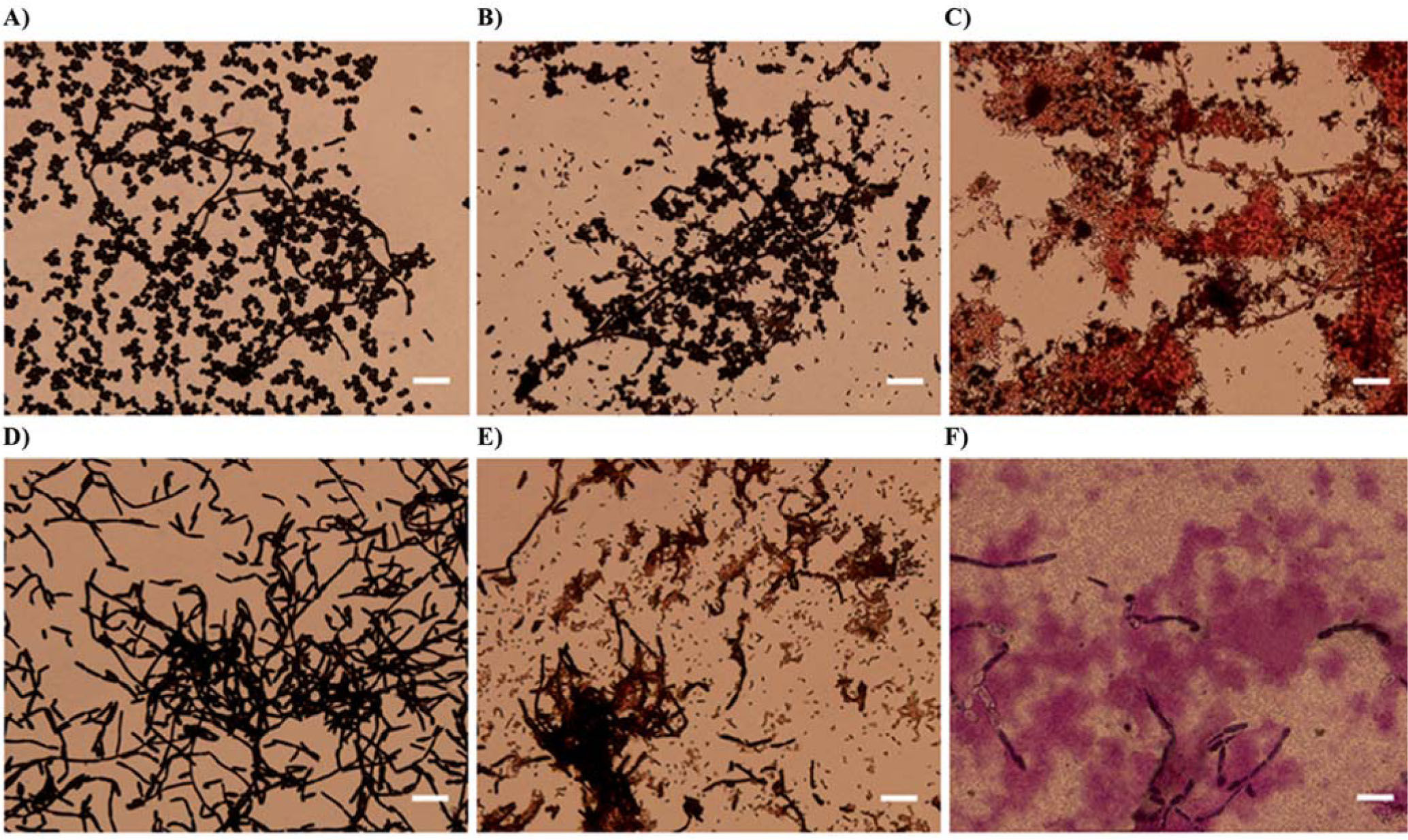
The effect of S. maltophilia strains on C. albicans was also evaluated by light microscopy. The morphology of the Gram stained cells after 72h of incubation is illustrated in Figure 3. C. albicans wild type cultures presented yeast and hyphae-cells (Fig. 3A). Cocultures with K279a rpfF showed bacteria associated to fungal cells (Fig. 3B).On the other hand, in the presence of K279a, hyphae-cells but not yeast-cells, showed a damaged aspect (Fig. 3C). Microscopic observation of C. albicans tup1 control and coculture with K279a rpfF revealed unaffected hyphae structures (Figs. 3D and 3E). However, damaged hyphae-cells with discolored areas and apparently altered walls were observed in the presence of K279a (Fig. 3F).The described effects of K279a were less evident at 48h than at 72h of incubation at 37°C (data not shown).
Microscopic analysis of planktonic cultures of C. albicans in the presence of S. maltophilia. C. albicans ATCC 10231 (upper panel) and C. albicans tup1 (lower panel) were grown alone or in the presence of bacterial strains in TSB-d for 72 h at 37°C. (A) and (D) controls, (B) and (E) cocultures with K279a rpfF, and (C) and (F) cocultures with K279a. Cells were Gram stained and examined by light microscopy (400× magnification). Scale bars correspond to 10μm.
The above results show that the antagonistic effect of S. maltophilia K279a on C. albicans survival was dependent on DSF production. It is worth mentioning that K279a did not affect C. albicans ATCC 10231 viability in coculture experiments performed at 30°C, a condition that favors yeast growth form. We have previously observed that S. maltophilia K279a cultures incubated at 30°C or 37°C for 72h achieved similar final culture densities and presented DSF activity (data not shown).
In fact, the microscopic analysis showed that K279a affects C. albicans hyphae-cells, probably by the action of virulence factors whose synthesis is DSF -dependent. Fouhy et al.11 reported that DSF signaling regulates protease production, a virulence factor of S. maltophilia. Further-more, these authors showed in a nematode model that K279a rpfF reduced killing activity compared to the wildtype K279a. In our study, the prolonged time (72h) needed to detect the detrimental effects of K279a in C. albicans viability and the association observed microscopically between bacterial and fungal cells suggest that the adherence of bacteria to fungal cells could facilitate S. maltophilia virulence factor action.
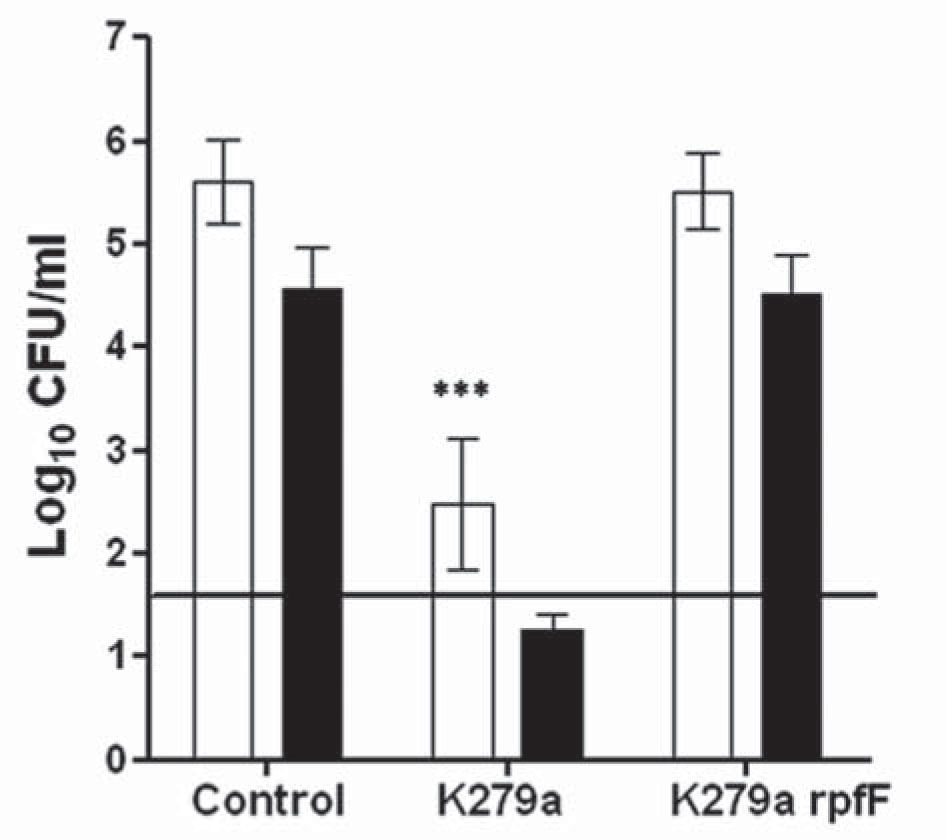
C. albicans biofilm formation in the presence of S. maltophiliaC. albicans biofilms were first studied using a static microtiter plate model in the presence or absence ofS. maltophilia strains. After 72h of incubation, fungal biofilm formation was evaluated by viable counts (Fig. 4). Biofilms of C. albicans ATCC 10231 and C. albicans tup1 formed in the presence of K279a rpfF showed a number of fungal cells similar to that observed in the respective controls. Furthermore, in the presence of K279a the count of C. albicans ATCC 10231 cells decreased by 3 logs with respect to the control (p <0.001) (Fig. 4).The effect of K279a on mutant tup1 biofilms was more severe, and viable counts were under the limit of detection (Fig. 4).
Evaluation of the viability of C. albicans 72 h biofilms in the presence of S. maltophilia. The viability of C. albicans ATCC 10231 (white bars) or C. albicans tup1 (black bars) biofilms formed in microtiter plates was determined by plate counts performed on SDA or SDA supplemented with ciprofloxacin.The limit of detection was 40 CFU/ml and is represented by a horizontal black line. Each bar represents mean ± SD log10 CFU/ml from three independent assays. ***Significant difference (p <0.001) with respect to the control.
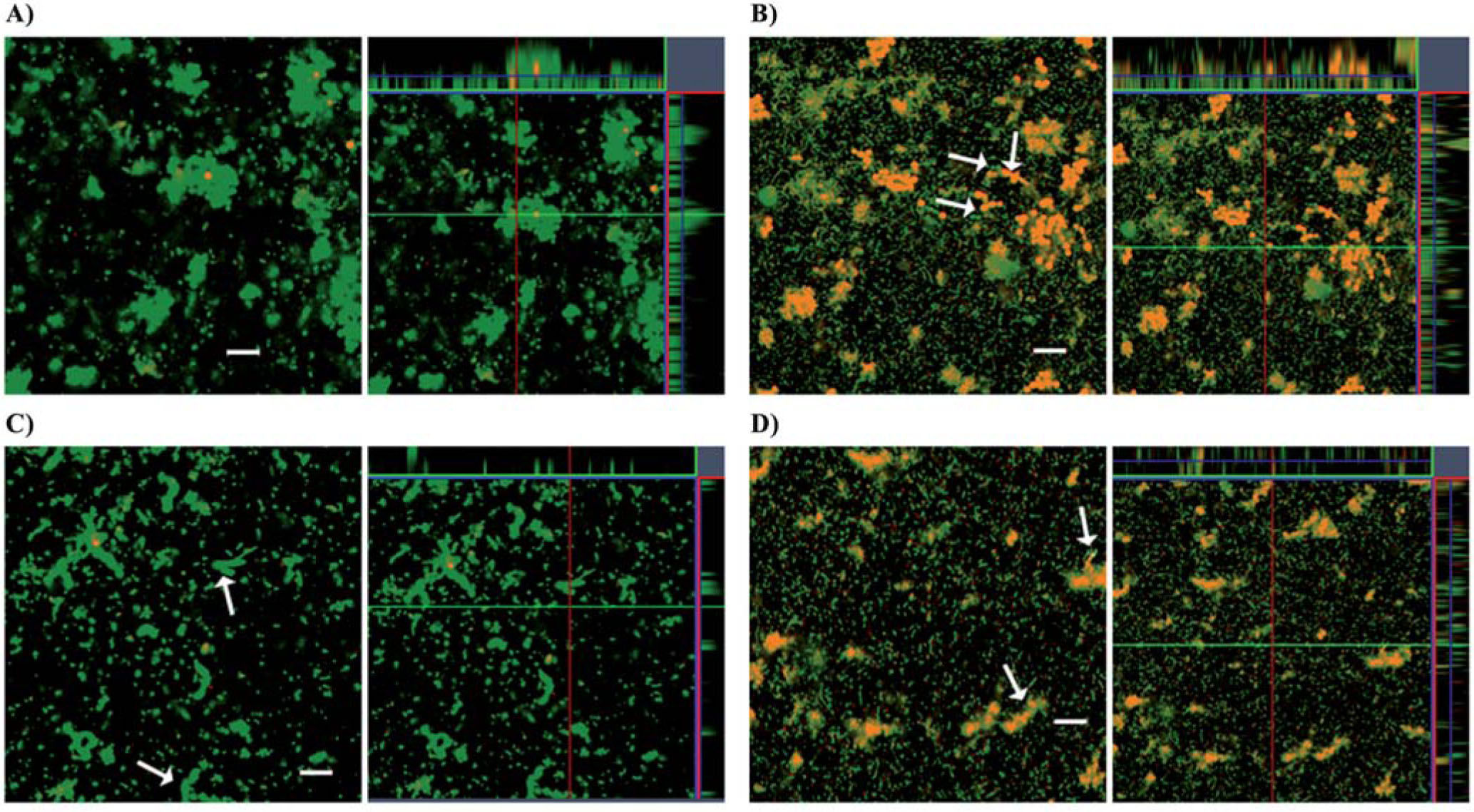
Fungal viability was also assessed by simultaneous labeling biofilms developed on glass chamber slides with the green-fluorescent SYTO9 dye and the red-fluorescent nucleic acid stain PI. Confocal images acquired from C. albicans ATCC 10231 or C. albicans tup1 biofilms grown in mixed cultures with K279a rpfF showed that most of the fungal cells were alive (Figs. 5A and 5C). In contrast, fungal cell death occurred in C. albicans wild type or mutant biofilms developed in the presence of K279a. Furthermore, amorphous red PI-stained material, which may represent DNA containing debris from lyzed cells, was also observed (Figs. 5B and 5D). The images showed that K279a can kill hyphae and also yeasts since C. albicans wild type biofilms mainly included yeast cells under this study conditions.
Confocal scanning laser microscopy images of C. albicans biofilms in the presence of S. maltophilia. C. albicans ATCC 10231 (upper panel) and C. albicans tup1 (lower panel) biofilms were formed on 8-well chamber slides in the presence of bacterial strains. (A) and (C) cocultures with K279a rpfF, and (B) and (D) cocultures with K279a. After 72 h of incubation at 37°C biofilms were stained for cell viability. Live SYTO 9-stained cells (green) and dead propidium iodide-stained cells (red-orange) were visualized with a CSLM (400× magnification). For each micrograph, the middle panel represents the x-y plane, and the adjacent top and side panels represent the x-z and y-z planes, respectively. Scale bars correspond to 20μm. Arrows show fungal dead cells. The results are representative of two independent experiments.
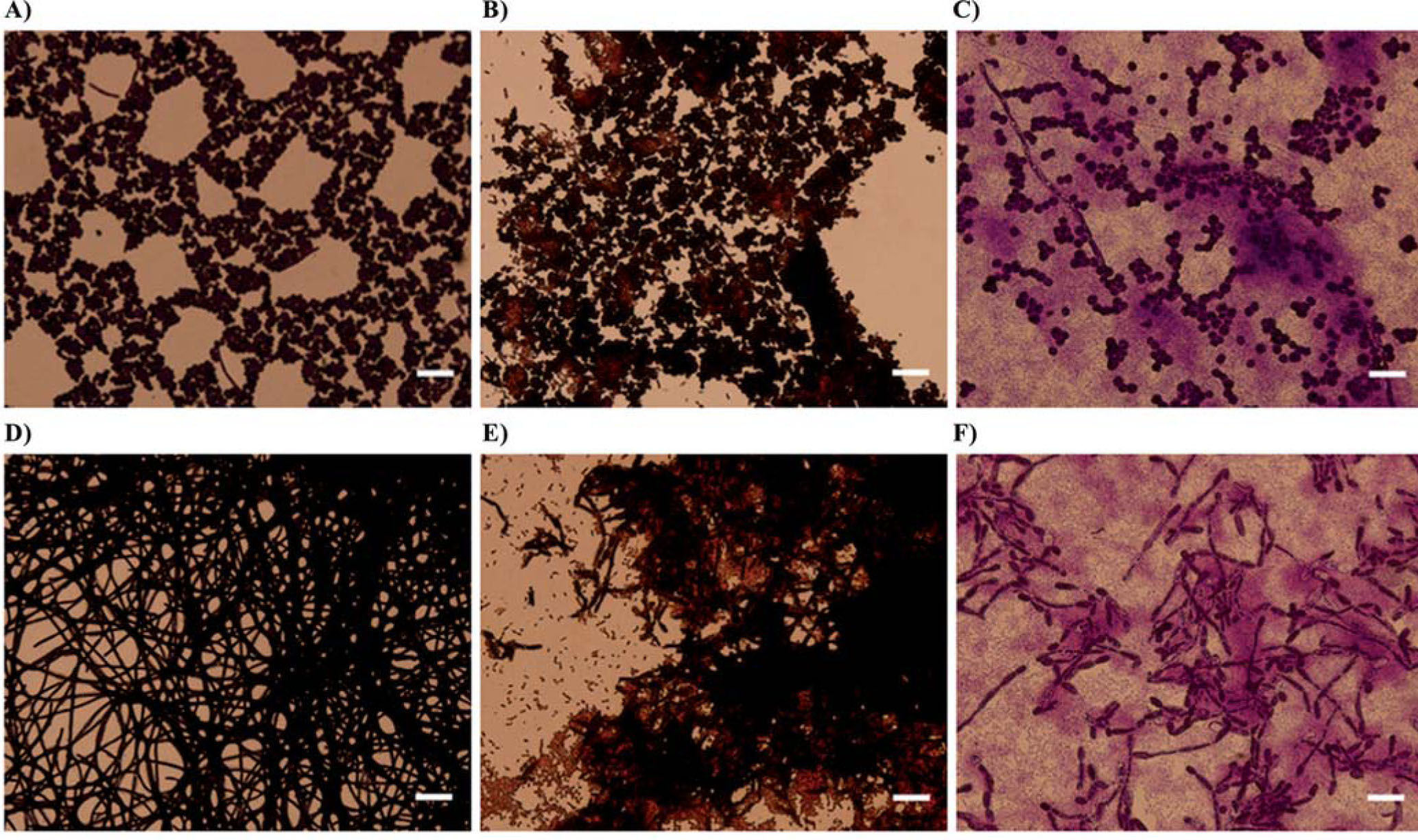
To further study the effect of S. maltophilia on fungal sessile cultures, biofilms formed on Thermanox cover slips were Gram stained and evaluated by light microscopy. Control biofilms of C. albicans wild type mainly included yeast cells forming microcolonies and three-dimensional structures and few hyphae while biofilms of the tup1 mutant showed a dense hyphal network (Figs. 6A and 6D). Similar patterns were observed in the presence of K279a rpfF, where bacteria grew associated to fungal cells (Fig. 6B and 6E). Once more, mixed cultures of C. albicans ATCC 10231 or tup1 with K279a exhibited biofilms with structurally affected hyphal cells (Figs. 6C and 6F).
Microscopic analysis of C. albicans biofilms in the presence of S. maltophilia. C. albicans ATCC 10231 (upper panel) and C. albicans tup1 (lower panel) biofilms were formed on Thermanox cover slips in the absence or presence of bacterial strains. (A) and (D) controls, (B) and (E) cocultures with K279a rpfF, and (C) and (F) cocultures with K279a. After 72 hincubation at 37°C, biofilms were Gram stained and examined by light microscopy (400× magnification). Scale bars correspond to 10μm.
The results obtained by the three different approaches show that S. maltophilia interferes with the biofilm mode of growth of C. albicans and severely affects not only hyphal integrity but also kills yeast cells. DSF could be directly responsible for these effects or may induce virulence gene expression. Bacterial virulence factors might be involved in bacterial-fungal interaction. Hogan and Kolter13 reported that P. aeruginosa forms a dense biofilm on C. albicans filaments and kills the fungus, while neither of them binds to nor kills the yeast form of C. albicans. Hyphal death occurred only after the onset of biofilm formation, and P. aeruginosa virulence factors, including pili and secreted molecules, are important in the killing of C. albicans filaments13. In S. maltophilia, RpfF is essential for the synthesis of DSF, and rpfF mutation leads to a coordinate reduction in the synthesis of virulence factors such as extracellular enzymes, biofilm structure and motility11,31. S. maltophilia formed biofilms on C. albicans cells and this contact could favor the effect of virulence factors, regulated by DSF, in the killing of C. albicans filaments but also yeast cells. S. maltophilia possess traits associated with biocontrol mechanisms including, among others, extracellular enzymes such as proteases and chitinases10,18,37. We have previously mentioned that DSF regulates protease production in this species. StmPr1, the major extracellular protease of clinical strain K279a, has been implicated as a virulence determinant and the environmental strain S. maltophilia W81 can protect sugar beet against Pythium-mediated damping-off disease through the production of an extracellular protease7,10.
Minkwitz and Berg22 characterized 50 S. maltophilia isolates from clinical and environmental sources by their in vitro antagonism against the human pathogenic fungus C. albicans and the plant pathogens Verticillium dahliae, Sclerotinia sclerotiorum and Rhizoctonia solani. Eight of the 25 clinical isolates (32%) and 21% of the environmental isolates displayed remarkable activity against C. albicans. Antifungal activity against plant pathogens was more common among the environmental isolates, although not exclusive to them. All isolates, clinical and environmental, produced proteases and all but two of the environmental isolates produced chitinases.
The genome of K279a (NC_010943) contains the gene chiA encoding for chitinase A (Gene ID 6393430), a well-known antifungal trait exhibited by several isolates from the species S. maltophilia7. A rhizosphere strain ofS. maltophilia, MUJ, is strongly antagonistic towards fungal phytopathogens. Its purified chitinase inhibited the growth of fungi belonging to the genera Fusarium, Rhizoctonia and Alternaria18. Furthermore, S. maltophilia strain C3, a biocontrol agent of Bipolaris sorokiniana in turfgrass, produces at least two chitinases that are antifungal37. In S. maltophilia there are no reports about DSF regulation of chiA. However, in Xylella fastidiosa RpfF, which is phylogenetically closely related to the above organism, expression of chiA in the rpfF mutant is only 40% of that of the wild type20 and also synthesizes the cell-cell signaling molecule DSF.
The ability of C. albicans to switch its morphology between yeast and hyphal form is crucial to its ability to adhere to surfaces32. It has been reported that BDSF was more effective against C. albicans biofilm formation than farnesol and synthesized DSF, and that BDSF could block C. albicans biofilm formation by interfering with the morphological switch3,36. Since other members of the DSF family of signal molecules have been implicated in the interkingdom signaling between bacteria and C. albicans, it was suggested that DSF from S. maltophilia could also influence the yeast-hyphal transition30,33. As it was demonstrated in the present study, S. maltophilia K279a does interfere with C. albicans filamentation and the effect was dependent on DSF production. This interference could also contribute to the antagonistic effect of S. maltophilia on C. albicans biofilms. Finally, fatty acid signals have been shown to regulate a wide range of bacterial behaviors including biofilm dispersion. The QS signal cis-2-decenoic from P. aeruginosa is a member of the DSF family that is able to disperse C. albicans biofilms8. Thus, further studies are needed to determine whether the antagonistic effects of S. maltophilia on C. albicans biofilm mode of growth are on adhesion, killing of fungal sessile cells, or on biofilm dispersal.
In conclusion, this is the first study to report that S. maltophilia interferes with two key virulence factors of C. albicans, the yeast-to-hyphal transition and biofilm formation. DSF could be directly responsible for these effects or may induce the expression of genes involved in antifungal activity. A previous work has established the regulatory influence of DSF from S. maltophilia as an interspecies signal. P. aeruginosa can respond to DSF by altering its biofilm architecture and increasing tolerance to the antibiotic polymixin31. Further studies are needed to determine the potential role of QS and DSF signal under in vivo conditions, for example, in the lungs of CF patients where S. maltophilia may coexist with C. albicans34.
Ethical disclosuresProtection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of data. The authors declare that no patient data appear in this article.
Right to privacy and informed consent. The authors declare that no patient data appear in this article.
Conflicts of interestThe authors declare that they have no conflicts of interest.
This research was supported by grants 20020100100773 and 20020130100371BA from UBACYT, Argentina. The authors thank Maxwell Dow (BIOMERIT Research Centre, Department of Microbiology, Biosciences Institute, National University of Ireland, Cork, Ireland) for providing S. maltophilia K279a and K279a rpfF strains; and Robert Kolter (Department of Microbiology and Immunobiology, Harvard Medical School, Boston, Massachusetts, USA) for providing mutant C. albicans tup1. The authors are grateful to Tomás Santa Coloma [Instituto de Investigaciones Biomédicas (BIOMED) Pontificia Universidad Católica Argentina (UCA)-Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET)] for help provided with confocal microscopy.