Streptococcus uberis has become one of the most important environmental pathogens associated with clinical and subclinical bovine mastitis. Biofilm confers to bacteria more resistance to physical and chemical agents as well as to different mechanisms of the innate immune system. The aim of this work was to evaluate the ability of in vitro biofilm production in 32 S. uberis isolates from bovine mastitis and identified by biochemical tests and subsequently confirmed by the amplification of the pauA gene. The isolates were cultivated in TMP broth and TMP broth with the addition of 0.5% glucose, 1% sucrose, 1% lactose or 0.5% skim milk in microtiter plates stained with crystal violet. We demonstrated that S. uberis isolated from bovine mastitis are able to produce biofilms in TMP broth and, also that biofilm formation by S. uberis can be significantly enhanced by the addition of 0.5% glucose or 1% sucrose to TMP broth. This may suggest that the carbohydrates in milk or within the ruminant gut might affect the growth mode of S. uberis. In addition, our results showed that in vitro biofilm production under different conditions of supplementation displays variation among the isolates and that each isolate shows a particular profile of biofilm production. This phenotypic heterogeneity in biofilm production exhibited by S. uberis could at least partly explain why this bacterium has the ability to adapt to different niches facilitating survival to diverse and stressful conditions.
Streptococcus uberis es uno de los más importantes patógenos medioambientales asociados a la mastitis bovina clínica y subclínica. El biofilm confiere a las bacterias resistencia a agentes físicos y químicos, como así también a diferentes mecanismos del sistema inmune innato. El objetivo del presente estudio fue evaluar la habilidad de producción de biofilm in vitro de 32 aislamientos de S. uberis recuperados de mastitis bovina, previamente identificados por pruebas bioquímicas y confirmados por la amplificación del gen pauA. Los aislamientos fueron cultivados en caldo TMP sin carbohidratos, y además en caldo TMP con la adición de 0,5% de glucosa, 1% de sacarosa, 1% de lactosa o 0,5% de leche descremada, en placas de microtitulación teñidas con cristal violeta. Se demostró que dichos aislamientos son capaces de producir biofilm en caldo TMP, y además se observó un incremento significativo en la producción de biofilm en caldo TMP suplementado con 0,5% de glucosa o con 1% de sacarosa. Así, los carbohidratos de la leche o los presentes dentro del intestino de los rumiantes podrían afectar el modo de crecimiento de S. uberis. Además, nuestros hallazgos mostraron que la producción de biofilm in vitro en diferentes condiciones de suplementación presenta variabilidad entre los aislamientos de S. uberis y que cada aislamiento muestra un perfil particular de producción de biofilm. Esta heterogeneidad fenotípica en la producción de biofilm de S. uberis podría explicar, al menos en parte, por qué esta bacteria tiene la habilidad de adaptarse a diferentes nichos, lo que le facilita la supervivencia frente a condiciones diversas y estresantes.
Streptococcus uberis is commensal at many body sites and has been isolated from the skin, gut, tonsils and genital tract of asymptomatic ruminants such as bovines21. In recent decades, S. uberis has become one of the most important environmental pathogens associated with clinical and subclinical bovine mastitis24, given the constant challenge of the environment with multiple strains of S. uberis which have previously colonized the ruminant gut and later gain access to the mammary gland16,21. S. uberis infections may persist in the same cow for up to 5 months11, and they have been observed during both non-lactating and lactating periods as well as during the antimicrobial treatment17,23. One of the major reasons for recurrence of infection is bacterial biofilm formation inside the udder tissue15. Biofilm formation makes bacteria more resistant to physical and chemical agents as well as to innate immune mechanisms5. Biofilm is a structured community of bacterial cells added and embedded in an extracellular polymeric matrix composed of polysaccharides, proteins and/or extracellular DNA7,9. Biofilm formation is caused in response to fluctuating environmental conditions19. Previous studies evidenced that the biofilm-forming capacity of S. uberis strains varies from nonproducing strains to strong biofilm-producing strains in supplemented media with milk or carbohydrates1,4,12,20. Varhimo et al.20, demonstrated that extracellular proteins play a crucial role in biofilm formation by S. uberis strains. In addition, sugar metabolism has been suggested to be an important process in the early growth of biofilm in S. uberis 0140J4. As yet, nothing has been reported about in vitro biofilm formation among S. uberis isolates from cattle with mastitis in Argentina. The purpose of this study was to investigate the ability of in vitro biofilm production in S. uberis isolated from bovine mastitis in TMP broth without carbohydrates, and also in response to supplements such as glucose, lactose or sucrose, and skim milk.
Materials and methodsBacterial isolatesA total of 32 S. uberis isolates collected from 19 herds located in the central dairy region of Argentina were used in this study. One to three isolates were isolated from each herd.
The isolates were obtained from individual mammary quarters of cows with clinical and subclinical mastitis with a somatic cell count of (SCC)>250000cells/ml between December 2014 and March 2015. These isolates were presumably identified as S. uberis by biochemical tests of hippurate hydrolysis, esculin hydrolysis, growth on 6.5% sodium chloride and growth on bile13,14, and were confirmed by the amplification of the pauA gene by PCR18.
Biofilm assayThe ability of S. uberis to form in vitro biofilm was determined using 96-well polystyrene microtiter plates, according to what was described by Christensen3, with minor changes. A preculture of each isolate was carried out in 6ml of TMP broth (1.5% tryptone, 0.3% meat peptone, 0.5% sodium chloride, 0.25% dibasic potassium phosphate) (Britania, BA, Argentina), and following overnight incubation at 37°C, a 1/100 dilution in TMP broth was performed. The optical density (OD660 nm) was adjusted to 0.009, and a volume of 10μl of the culture was transferred to the wells of a microplate (Nunc, Roskilde, Denmark) containing 190μl of TMP broth or TMP broth supplemented with 0.5% glucose, 1% sucrose, 1% lactose or 0.5% skim milk (Sigma–Aldrich, MO, USA). Following incubation at 37°C for 24h, the medium and the planktonic cells were removed, and the wells of each plate were gently washed three times with sterile PBS. Each microplate was set at 60°C for 1h and stained using 100μl of Hucker's crystal violet solution (2%, p/v) (Britania, BA, Argentina). Finally, 100μl of 100% ethanol was added, and an OD560nm reading was done using an ELISA reader (Labsystems Multiskan MS). Each isolate was tested in triplicate, and the assay was repeated two times. Staphylococcus aureus strain V329 Staphylococcus epidermidis ATCC 12228 were used as positive and negative controls, respectively. The optical density (ODs) of each isolate was obtained by the arithmetic mean of the absorbance of three wells and this value was compared with the mean absorbance of negative controls (ODnc). The following classification was used for the determination of in vitro biofilm formation: no biofilm production (ODs≤ODnc), weak biofilm production (ODnc<ODs≤2 ODnc), moderate biofilm production (2 ODnc<ODs≤4 ODnc) and strong biofilm production (4 ODnc<ODs).
Statistical analysisData analyses were performed using the InfoStat statistical software6. Descriptive statistics (the arithmetic mean, standard deviation and standard error) were used to assess in vitro biofilm production in different media (TMP broth and TMP broth supplemented with 0.5% glucose, 1% sucrose, 1% lactose or 0.5% skim milk). The statistical analysis was performed using one-way factorial ANOVA with the least significant difference (LSD) test for comparison between multiple groups. p-values of<0.05 were considered to be statistically significant.
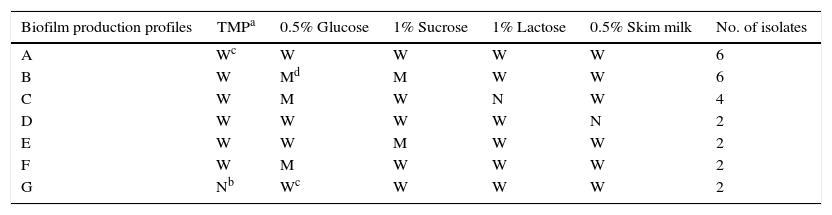
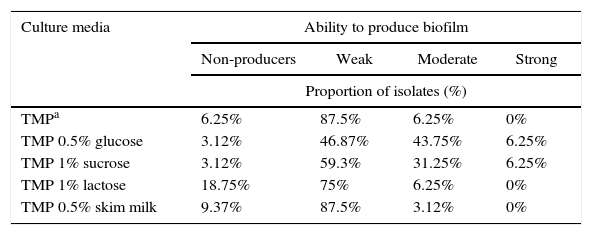
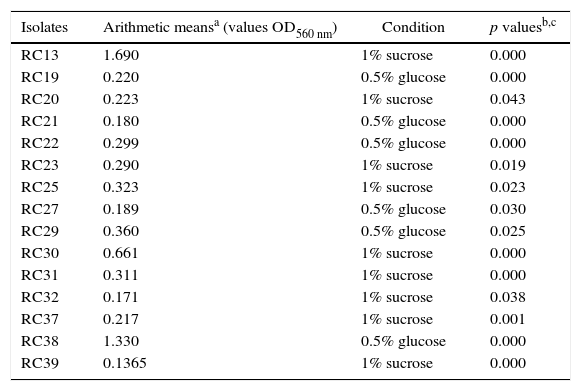
ResultsIn this study, we evaluated for the first time in vitro biofilm production of S. uberis in TMP broth, without a carbohydrate source. We also determined the response of the isolates to different carbohydrates or skim milk added to this medium on in vitro biofilm formation by using the crystal violet staining method in 96-well polystyrene plates. All S. uberis isolates showed different ability to produce in vitro biofilm in TMP broth or TMP broth with different supplements. Further analysis showed that 15 different biofilm profiles, named with a capital letter from A to O, were found in all 32 S. uberis isolates, and 16 (50%) strains belonged to the three most frequent profiles. Data regarding these three most frequent biofilm profiles are summarized in Table 1. In general, it was observed that a high percentage of isolates was classified as weak biofilm producers in a wide range of values (46.7–87.5%), considering all culture media tested. The percentage of isolates, which were non-biofilm producers, was equal or lower than 18.75% in all culture media used, while strong and moderate biofilm producers were observed in 50% or less of the isolates (Table 2). The quantitative analysis was carried out in 32 S. uberis isolates growing in all the above-mentioned conditions. The addition of 0.5% glucose or 1% sucrose to cultures in TMP broth significantly improved in vitro biofilm formation, exhibiting arithmetic means of 0.179 and 0.180, respectively. According to our results, biofilm formation by S. uberis isolates in the presence of 1% lactose (OD560 0.050) was observed. Importantly, there was no significant difference in the arithmetic mean values of the biofilm production as compared with those obtained in TMP broth (OD560 0.063) or with skim milk (OD560 0.042). Fifteen of the 32 isolates (46.8%) showed OD560 nm values with statistical significance (p<0.05) in at least one of the media tested (Table 3). Nine of the 15 isolates exhibited a significant in vitro biofilm production in TMP broth supplemented with 1% sucrose, while the remaining isolates showed this behavior in TMP broth supplemented with 0.5% glucose. In contrast, none of the isolates showed OD560 nm values with statistically significant differences in TMP broth and in TMP broth supplemented with 1% lactose or 0.5% skim milk. In order to assess whether there are differences in the effect caused by glucose or sucrose on in vitro biofilm production, all isolates that demonstrated a positive effect to glucose and sucrose were pooled, and a simple way ANOVA test with two levels (0.5% glucose and 1% sucrose) was performed. This analysis led to the conclusion that there was not a significant difference (p=0.868) between the mean values of the isolates in TMP broth supplemented with sucrose or glucose. A multiple contrast test revealed a greater ability to produce in vitro biofilm in 3 isolates selected among 15 isolates that showed a significant increase in their ability to form in vitro biofilm in TMP broth supplemented with 0.5% glucose or 1% sucrose.
Proportion of in vitro biofilm production by 32 Streptococcus uberis isolates in TMP broth and in response to carbohydrates and skim milk according to the qualitative analysis
| Culture media | Ability to produce biofilm | |||
|---|---|---|---|---|
| Non-producers | Weak | Moderate | Strong | |
| Proportion of isolates (%) | ||||
| TMPa | 6.25% | 87.5% | 6.25% | 0% |
| TMP 0.5% glucose | 3.12% | 46.87% | 43.75% | 6.25% |
| TMP 1% sucrose | 3.12% | 59.3% | 31.25% | 6.25% |
| TMP 1% lactose | 18.75% | 75% | 6.25% | 0% |
| TMP 0.5% skim milk | 9.37% | 87.5% | 3.12% | 0% |
Streptococcus uberis isolates with statistically significant in vitro biofilm production
| Isolates | Arithmetic meansa (values OD560 nm) | Condition | p valuesb,c |
|---|---|---|---|
| RC13 | 1.690 | 1% sucrose | 0.000 |
| RC19 | 0.220 | 0.5% glucose | 0.000 |
| RC20 | 0.223 | 1% sucrose | 0.043 |
| RC21 | 0.180 | 0.5% glucose | 0.000 |
| RC22 | 0.299 | 0.5% glucose | 0.000 |
| RC23 | 0.290 | 1% sucrose | 0.019 |
| RC25 | 0.323 | 1% sucrose | 0.023 |
| RC27 | 0.189 | 0.5% glucose | 0.030 |
| RC29 | 0.360 | 0.5% glucose | 0.025 |
| RC30 | 0.661 | 1% sucrose | 0.000 |
| RC31 | 0.311 | 1% sucrose | 0.000 |
| RC32 | 0.171 | 1% sucrose | 0.038 |
| RC37 | 0.217 | 1% sucrose | 0.001 |
| RC38 | 1.330 | 0.5% glucose | 0.000 |
| RC39 | 0.1365 | 1% sucrose | 0.000 |
Biofilms are sessile and attached forms of bacterial growth that enable better survival in hostile environments such as antimicrobial treatments or the immune response of the host, and to colonize new niches through dispersal mechanisms9,25. Cell signaling allows bacteria to sense and phenotypically respond to their environment, for example, environmental chemical cues7. The nutrient content of the growth medium regulates the development in vitro of biofilms in several organisms2,8,10. Bovine milk is the natural growth medium of S. uberis, and lactose is the major carbohydrate constituent in bovine milk. A diversity of metabolic pathways involved in the utilization of different carbohydrates available within the bovine gut and also in mammary gland secretions have been identified in S. uberis7,21. Thus, the effect of carbohydrates present in milk or in different environmental niches on S. uberis biofilm formation is of great importance. The purpose of this study was to investigate the ability of in vitro biofilm production in S. uberis isolated from bovine mastitis in TMP broth, and also in response to supplements such as glucose, lactose or sucrose, and skim milk. Thirty-two S. uberis isolates were tested for their ability to form in vitro biofilms on 96-well polystyrene microtiter plates using crystal violet staining. It should be also pointed out that the results of the present study were not strictly comparable to those obtained in previous studies. As yet, nothing has been reported about in vitro biofilm formation in S. uberis in broth medium without carbohydrate such as TMP broth. Previous studies determined biofilm formation in conventional Tryptic Soy Broth (TSB) or Todd–Hewitt broth (THB) media1,4,12 or Todd–Hewitt broth with 1% yeast extract (THY)20. Our findings demonstrate that S. uberis isolates are able to produce biofilms in TMP broth. Furthermore, in vitro biofilm formation by S. uberis was enhanced when TMP broth was supplemented with 0.5% glucose. Here, a low glucose concentration as found in bovine milk, was sufficient to improve in vitro biofilm formation by S. uberis. Previous results obtained with S. uberis support this idea by indicating that the glucose (1–2%) added to THB medium can have a significant increase in biofilm formation1. A significant increase in biofilm formation in the presence of 1% sucrose was observed in this study. This result agrees with the work of Abureema1, who reported a significant increase in vitro biofilm formation in the presence of sucrose at a similar concentration. In contrast to our results, Moore12 did not find an effect on biofilm production by S. uberis when sucrose was added to TSB medium. A possible explanation for this inconsistency could be due to the different concentrations of sucrose that could lead to different results in the amount of biofilm production. The latter study was performed using 5% sucrose, while the current study used 1% sucrose. In the present study, the biofilm generated in the presence of 0.5% glucose was not significantly different from that obtained with 1% sucrose. Less biofilm formation by S. uberis in the presence of 1% lactose as compared with the above-mentioned carbohydrates was observed in this study. This result is consistent with previous studies that suggested that 1% or 2% lactose in THB markedly reduced biofilm formation1, or did not have an effect on biofilm production by S. uberis when 0.5% lactose was added to TSB medium12. A possible explanation of why lactose did not show an inductive effect on biofilm formation in several investigations including ours could be due to a low concentration of this sugar added to the medium, as compared with approximately 5% lactose naturally found in bovine milk. Since adhesion is a prerequisite for biofilm formation, a host factor such as bovine milk could be useful in establishing biofilms. The inductive effect of sterile skim milk at high concentrations on S. uberis biofilm formation has been described previously by Aburema1 and Crowley4. However, these milk concentrations led to the coagulation of milk proteins in the 96-well microtiter plates in the present study (data not shown). Thus, the effect of 0.5% sterile skim milk on in vitro biofilm formation was investigated in this study. The results showed less biofilm formation by S. uberis in the presence of 0.5% skim milk as compared with glucose or sucrose. In the present study, the results of in vitro biofilm formation in the presence of skim milk are inconsistent with those described by other authors1,4. Crowley et al.4 demonstrated in vitro biofilm formation in all S. uberis isolates when they were grown with 20% sterile skim milk in TSB medium without glucose, whereas Abureema1 showed a statistical significant production of biofilm by S. uberis in THB medium at higher milk concentrations (12.5%, 25% and 50%) than those used in our study. A possible explanation as to why skim milk showed a lower inductive effect on biofilm formation in our research could be due to a markedly lower concentration of milk added in the medium compared to other studies mentioned before1,4. In the present study, the biofilm generated in the presence of 0.5% skim milk was not significantly different from those obtained in TMP or TMP with 1% lactose. Moreover, a previous study suggested that the extracellular proteolytic activity of S. uberis contributes to an increased biofilm formation20. In this work, we showed that 100% of S. uberis harbored the gene pauA (data not shown). This gene codes for plasminogen activator which is able to activate bovine plasminogen to the serine protease plasmin22. Varhimo et al.20 demonstrated that serine-type proteolytic activity is necessary for biofilm formation in S. uberis due to the inhibition of milk-stimulated biofilm formation by 1-mM serine-(dichloroisocoumarin) protease inhibitor. In this study, at least to our knowledge, it is the first time that in vitro biofilm formation by S. uberis growing in TMP broth without carbohydrates has been reported. Furthermore, the response to supplements such as glucose, lactose or sucrose, and skim milk in biofilm formation has been investigated. In conclusion, we demonstrated that S. uberis isolated from bovine mastitis are able to produce biofilms in TMP broth and, also that biofilm formation by S. uberis can be enhanced by the addition of glucose or sucrose to TMP broth. This may suggest that the carbohydrates in milk or present within the ruminant gut might affect the growth mode of S. uberis. In addition, our results showed that in vitro biofilm production under different conditions of supplementation displays variation among the isolates and each isolate shows a particular profile in biofilm production. This phenotypic heterogeneity in biofilm production exhibited by S. uberis could at least partly explain why this bacterium has the ability to adapt to different niches facilitating survival to diverse and stressful conditions.
Ethical disclosuresProtection of human and animal subjectsThe authors state that no human or animal experiments have been performed for this research.
Confidentiality of dataThe authors state that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestNone of the authors of this paper has any financial or personal relationship with other people or organizations that could inappropriately influence or bias the content of the paper.
We thank M.V. Liliana Tirante (LactoDiagnóstico Sur, Olivos, Buenos Aires) for providing the isolates. This work was supported by grants from SECyT (Secretaría de Ciencia y Técnica, Universidad Nacional de Río Cuarto) and FONCyT (Agencia Nacional de Promoción Científica y Tecnológica). S. Dieser holds a postdoctoral fellowship with the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), A. Fessia holds a doctoral fellowship with the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET).