Invasive candidiasis by Candida albicans is associated with high morbidity and mortality, due in part to the late implementation of an appropriate antifungal therapy hindered by the lack of an early diagnosis.
AimsWe aimed to evaluate the in vitro antifungal activity of the antibodies against C. albicans germ tubes (CAGTA) raised in a rabbit model of candidemia.
MethodsWe measured the effect of CAGTA activity by colorimetric XTT and crystal violet assays, and colony forming units count, both on C. albicans planktonic cells and during the course of biofilm formation and maturation. Viability and cell morphology were assessed by optical, fluorescent or scanning electron microscopy.
ResultsCAGTA ≥50μg/ml caused a strong inhibition of C. albicans blastospores growth, and DiBAC fluorescent staining evidenced a fungicidal activity. Moreover, electron microscopy images revealed that CAGTA induced morphological alterations of the surface of C. albicans germ tubes grown free as well as in biofilm. Interestingly, CAGTA ≥80μg/ml reduced the amount of C. albicans biofilm, and this effect started at the initial adhesion stage of the biofilm formation, during the first 90min.
ConclusionsThis is the first report showing that CAGTA reduce C. albicans growth, and impair its metabolic activity and ability to form biofilm in vitro. The antigens recognized by CAGTA could be the basis for the development of immunization protocols that might protect against Candida infections.
La infección invasora por Candida albicans está asociada a altas tasas de morbimortalidad, en parte debido al retraso en la instauración de una terapia antifúngica adecuada, dificultada a su vez por la falta de un diagnóstico precoz.
ObjetivosEvaluar la actividad antifúngica de los anticuerpos contra tubos germinales de C. albicans (CAGTA) obtenidos a partir de un modelo animal de candidemia en conejo.
MétodosEl efecto de los CAGTA se evaluó mediante los ensayos colorimétricos XTT y cristal violeta, así como mediante el recuento de unidades formadoras de colonias, tanto en células planctónicas de C. albicans como en distintos estadios de formación y maduración de biopelículas. La viabilidad y la morfología de las células tratadas con CAGTA se determinó mediante microscopía óptica, de fluorescencia o electrónica (SEM).
ResultadosConcentraciones de CAGTA≥50μg/ml generaban una fuerte inhibición del crecimiento de C. albicans, y su actividad se mostró fungicida. Los CAGTA producían alteraciones en la superficie de los tubos germinales desarrollados tanto a partir de células en suspensión como de células en biopelículas. Además, concentraciones de CAGTA≥80μg/ml redujeron la biomasa de biopelículas de Candida, y este efecto se desencadenaba en los primeros 90min de su formación.
ConclusionesEste es el primer estudio que demuestra la capacidad de los CAGTA para reducir el crecimiento de C. albicans y su actividad metabólica, así como para alterar la formación de biopelículas in vitro. Los antígenos reconocidos por los CAGTA podrían servir de base para el desarrollo de protocolos de inmunización protectores frente a infecciones por Candida.
Invasive candidiasis (IC) concerns more than 250,000 people in the world and causes above 50,000 deaths every year.15 Despite the introduction of new auxiliary tests, the diagnosis of IC is difficult because there are no pathognomonic signs and it is hard to distinguish between colonization and invasion. Therefore, therapy starts late, and partly explains the high mortality associated with IC.25,28 In addition, the opportunistic fungal pathogen Candida albicans is able to organize into biofilm on both abiotic (i.e. medical devices) and biotic (i.e. oral mucosal) surfaces,13 what enhances its resistance to antifungal therapy and host immune mediated defenses.14
Our group of research developed an indirect immunofluorescence assay to detect antibodies (Abs) that react specifically with superficial antigens of the germ tubes of C. albicans (CAGTA) in patients with IC9,12,21 and such antibodies can also be found in a rabbit model of IC.31 Interestingly, the mortality rate of ICU patients receiving an antifungal treatment was significantly reduced in CAGTA-positive individuals.34 Pitarch and colleagues27 associated the best prognosis of IC patients with those showing the highest titer of specific Abs such as anti-Met6 (methionine synthase), anti-Hsp90 (heat-shock protein 90kDa) and anti-Pgk1 (phosphoglycerate kinase 1). In a previous proteomic study of our group31 we identified some C. albicans antigens recognized by CAGTA: Met6, Ino1, Eno1, Pgk1, Adh1, 14-3-3 and Edg2. Moreover, Huertas et al. reported that, when infected with C. albicans, mice previously colonized with this yeast showed higher survival rates than non-colonized control counterpart.11 The group of animals with good outcome presented high titers of IgG specific for Eno1 (enolase), Met6, Hsp70 (heat-shock protein 70kDa), Pdc11 (pyruvate decarboxylase), Pgk1 and Cdc19 (pyruvate kinase).11
The aim of vaccination is to develop protective specific antibodies and immune memory against a particular pathogen. In recent years, studies in animal models are confirming the immunogenicity and efficacy of certain antigenic components of C. albicans as vaccines, including β-d-glucan and mannoproteins, or the recombinant proteins enolase (Eno1), hyphally regulated protein (Hyr1), hyphal wall protein (Hwp1), agglutinin-like sequence (Als3), secreted aspartyl proteinases (Sap) or heat-shock protein 90 (Hsp90).19,33 CAGTA recognize most of these components,31 consequently if these antibodies prove a protective role against C. albicans, other antigens reacting with them could add new candidates for a future Candida vaccine. Thus, the main objective of this study was to evaluate the antifungal activity of CAGTA on C. albicans growth and on its ability to form biofilm.
Material and methodsStrain and culture conditionsCandida albicans SC5314 (Stanford DNA Sequencing and Technology Center, Stanford, USA) was routinely grown on Sabouraud dextrose agar (SDA, Difco, Sparks, MD, USA) at 24°C for 48h. To obtain germ tubes, two or three colonies of C. albicans freshly grown on SDA at 24°C were inoculated into TC199 medium (Sigma-Aldrich, St. Louis, MO, USA) and incubated overnight at 24°C with shaking (120rpm). The resulting blastospores were harvested and suspended in four volumes of TC199 medium pre-heated to 37°C. Germ tubes were collected after 4h of incubation at 37°C and 120rpm.31
Immune sera: fractionation, quantification and purification of CAGTAImmune sera were obtained from White New Zealand female rabbits infected intravenously with C. albicans blastospores, as described previously,31 and were fractionated to run the different assays. Whole IgG fraction from immune serum (total-IgG) included Abs against the superficial antigens of C. albicans blastospores (anti-Bl) and filamentous cells, including germ tubes, pseudo-hyphae and hyphae, together with non-specific Abs (CAGTA-enr fraction).
The CAGTA enriched fraction of serum (CAGTA-enr) was obtained after incubating the immune serum with an equal volume of heat-killed C. albicans blastospores (1010cell/ml of PBS) for 2h at room temperature, followed by centrifugation at 2500rpm for 5min to remove the blastospores. The anti-blastospore antibodies (anti-Bl) adsorbed onto the surface of the yeast cells were eluted by gentle shaking in 2.5M sodium iodide (Sigma-Aldrich, St. Louis, MO, USA) at room temperature for 1h, and then blastospores were eliminated by centrifugation. The eluted anti-Bl antibodies were dialyzed (MWCO 12,000–14,000Da; Medicell International, London, UK) against PBS and concentrated with polyethylene glycol 20,000 (Merck, Hohenbrunn, Germany).31
Purified CAGTA were obtained from C. albicans germ tubes that had been incubated with an equal volume of CAGTA-enr serum fraction at room temperature for 1h with gentle agitation. The cell pellet was washed with PBS, and CAGTA were eluted, dialyzed and concentrated following the same protocol described for anti-Bl antibodies.
The IgG class antibodies were purified with Melon™ Gel IgG Spin Purification Kit (ThermoFisher Scientific, St. Louis, MO, USA).
The protein concentration of serum fractions was estimated with Pierce™ Coomassie Plus (Bradford) Assay Kit (ThermoFisher Scientific, St. Louis, MO, USA) according to the manufacturer's instructions.
Evaluation of CAGTA activity against Candida albicans planktonic cellsThe effect of the antibodies on C. albicans was evaluated by measuring the cell metabolic activity with the colorimetric XTT assay, and colony forming units (CFU) count.
Candida albicans blastospores grown overnight in Sabouraud broth (Difco, Sparks, MD, USA) at 30°C in an orbital shaker (120rpm) were suspended in fresh medium 106cell/ml, and distributed in a U-bottom 96-well plate (Costar, Corning Life Science, Lowell, MA, USA), 50μl per well. Each well was supplemented with 50μl of Sabouraud broth containing Abs to reach different final concentrations (50, 100 or 200μg/ml). Plates were incubated at 37°C for 2.5h with gentle shaking. Then, for CFU estimation, each well content was homogenized and a 50μl aliquot was inoculated onto SDA plates and incubated at 37°C for 48h. For the XTT assay, plates were centrifuged at 2500rpm, the supernatant was discarded and the metabolic activity of cells was estimated with the 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)2H-tetrazolium-5-carboxanilide-inner salt (XTT; Sigma-Aldrich, St. Louis, MO, USA), according to the protocol of Henriques et al.10 Briefly, cell pellets in each well were supplemented with 90μl of XTT 0.75mg/ml in PBS and 10μl of phenazine methasulfate (PMS; Sigma-Aldrich, St. Louis, MO, USA) 0.32mg/ml in ultrapure water, and they were incubated at 37°C for 2h. Then each well content was homogenized and the absorbance at 492nm (Abs 492nm) was registered using a spectrophotometer (Microplate Autoreader Elx808, Bio-Tek Instruments). Two different controls were included in every experiment, one without antibodies, and a second one with an equivalent concentration of an irrelevant non-specific rabbit IgG (irr-IgG; Sigma-Aldrich, St. Louis, MO, USA).
Evaluation of CAGTA activity against Candida albicans biofilmThe day before each experiment, C. albicans was inoculated onto SDA plates that were incubated at 37°C. Blastospores were harvested, washed, suspended at 106cell/ml in RPMI 1640 (Gibco, Grand Island, NY, USA) supplemented with 10% heat-inactivated fetal bovine serum (Defined Hyclone, Logan, UT, USA) and distributed (100μl/well) into flat-bottom 96-well plates (Corning Incorporated, NY, USA). Plates were incubated at 37°C and, according to the protocols shown below, CAGTA or irr-IgG were added at different times in order to test their effect at different stages of the biofilm formation process.
Protocol I: effect on biofilm formation. Irr-IgG or CAGTA (20–160μg/ml) were added at time zero, and the effect on biofilm was checked after 24h of incubation.
Protocol II: effect on biofilm maturation. Irr-IgG or CAGTA (20–160μg/ml) were added to a 24h-old biofilm and their effect was assessed after an additional 24h incubation period at 37°C.
Protocol III: effect on C. albicans adhesion. C. albicans cells were exposed to CAGTA 80μg/ml for the first 90min during the adhesion process, and then cells were washed and incubated without antibodies up to 24h, to allow biofilm formation.
Protocol IV: effect on biofilm development. C. albicans cells were incubated for 90min at 37°C allowing the cells to adhere to the bottom of the plate, and then they were supplemented with CAGTA 80μg/ml and incubated up to 24h prior to biofilm assessment.
At the end of each experiment, non-adherent fungal cells were removed and total biofilm mass was assessed with crystal violet (CV, Abs 540nm), while the metabolic activity was estimated with the XTT assay (Abs 492nm), as described elsewhere.17,26 Two negative controls were included as explained for the planktonic cells experiments.
Optical and fluorescent microscopy analysisCell growth and morphology of C. albicans treated with CAGTA-enr serum fraction 6.25–200μg/ml for 2.5h at 37°C were assessed by optical microscopy. Growth conditions of planktonic cells were the same as indicated for XTT and CFU assays. Two controls were included in every experiment, one without antibodies, and a second one with an equivalent concentration of an Irr-IgG.
Cell viability of C. albicans incubated with purified CAGTA 20μg/ml for 2.5h at 37°C was assessed with the fluorescent dyes 5,(6)-carboxyfluorescein diacetate (CFDA; Molecular Probes, Eugene, OR, USA) and bis-(1,3-dibutylbarbituric acid) trimethine oxonol (DiBAC; Molecular Probes, Eugene, OR, USA), following the method described by Bowman et al.1 Briefly, a stock solution of CFDA 5mg/ml in dimethyl sulfoxide was diluted to 50μg/ml with MOPS 3 (0.1M MOPS–50mM citric acid at pH 3.0), and cells were incubated with the dye in the dark at 37°C for 45min with gentle shaking and then stored on ice until analysis. DiBAC 2μg/ml in MOPS 7 (0.1M MOPS at pH 7.0) was prepared from a 1mg/ml stock solution in ethanol, and cells were treated at room temperature for 1h in the dark, washed twice with MOPS 7 and stored on ice until analysis.
Electron microscopy analysisCandida albicans germ tubes and blastospores were grown in Sabouraud broth with CAGTA 40μg/ml for 2.5h at 37°C, and biofilms were formed in RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum and CAGTA 80μg/ml for 24h at 37°C. Negative controls were run without CAGTA. Then, cells were washed in Sorenson's buffer [0.133M Na2HPO4, 0.133M KH2PO4 (4:1, v/v), pH 7.2], treated with fixing solution (2% glutaraldehyde in Sorenson's buffer) at room temperature for 1h, and washed three times with 6% sucrose in Sorenson's buffer. Cells were dehydrated using graded ethanol solutions (50%, 70% and 100%) for 5min each. Then membranes were washed twice with 5ml hexamethyldisilazane (Electron Microscopy Sciences, Hatfield, PA, USA) for 5min each, and hexamethyldisilazane was removed by vacuum filtration. Membranes were left to dry in the air. Finally, the samples were processed for electron microscopy at the Service of Analytic and High Resolution Microscopy in Biomedicine (SGIker, University of the Basque Country).
Statistical analysisThe statistical analysis was performed using the GraphPad Prism software (version 6. GraphPad Software, Inc., La Jolla, CA, USA). For the analyses, the results of the different treatments were transformed into a percentage with reference to the mean value of the untreated control. Then variables were analyzed with the one-way ANOVA test or the Student's t-test for unrelated samples. p values <0.05 were considered statistically significant. All assays were performed at least three times and each assay was assessed in triplicate.
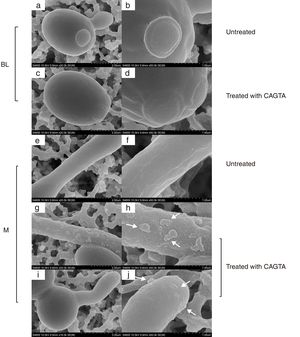
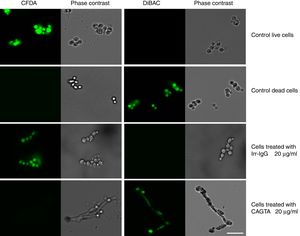
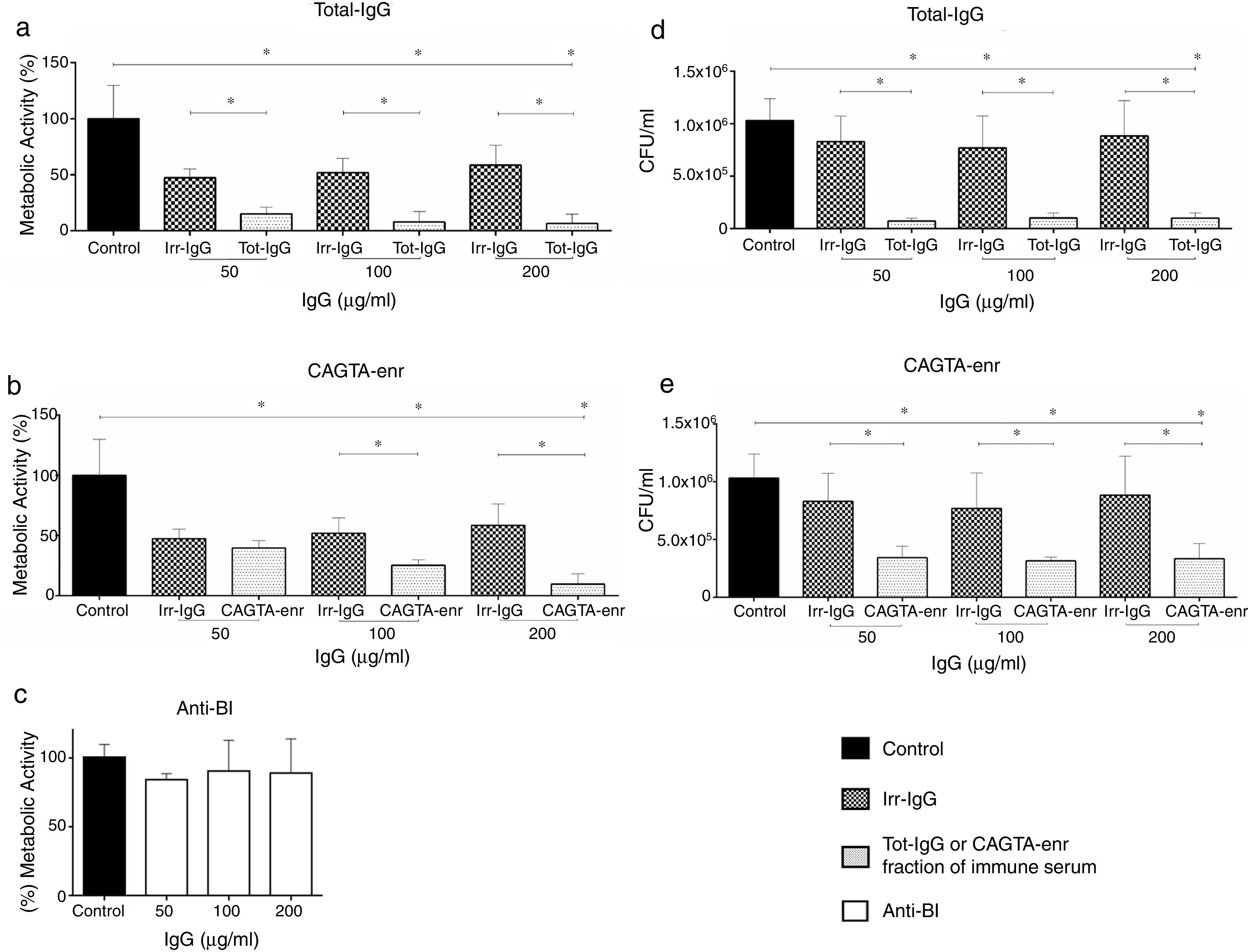
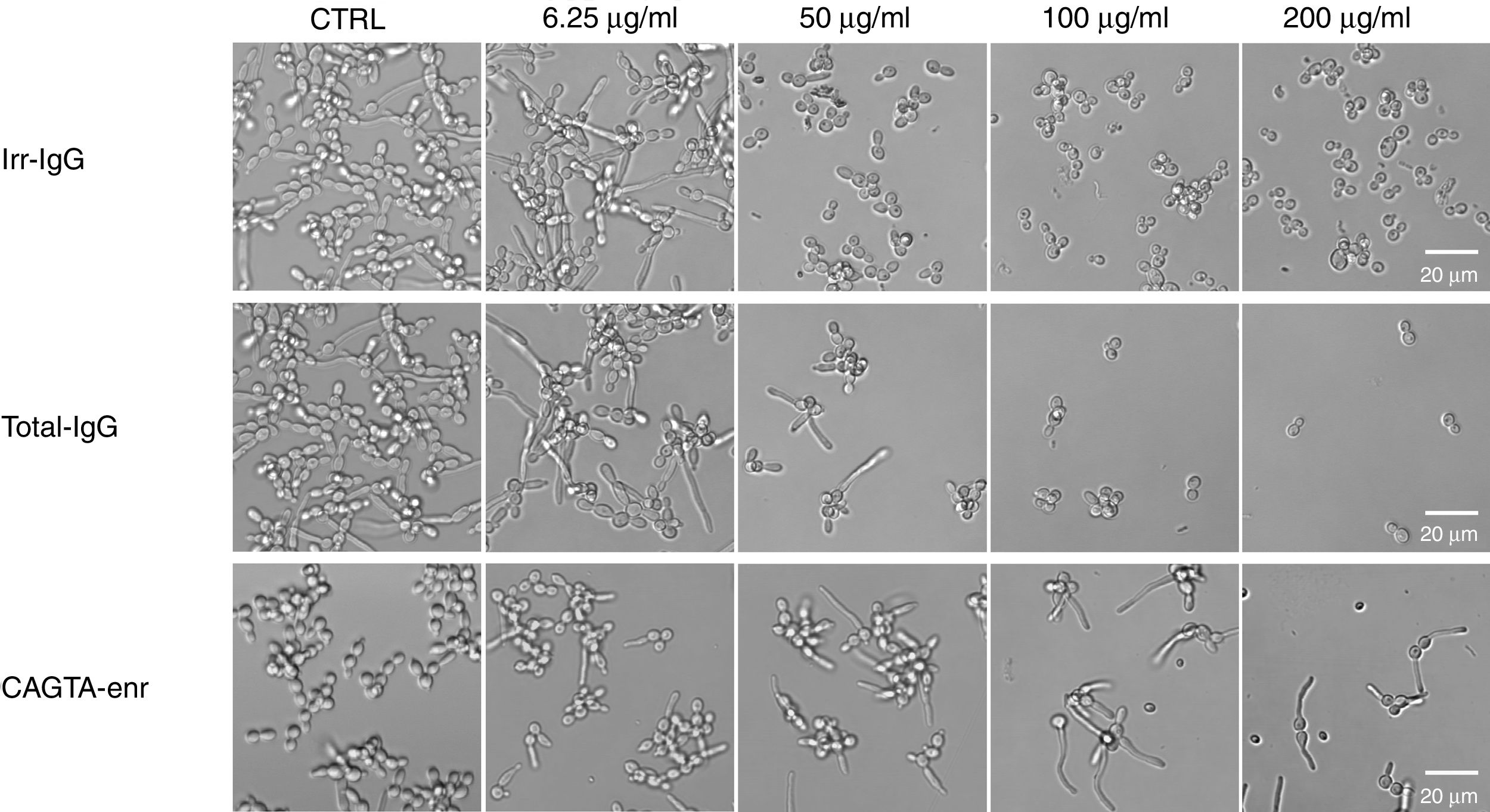
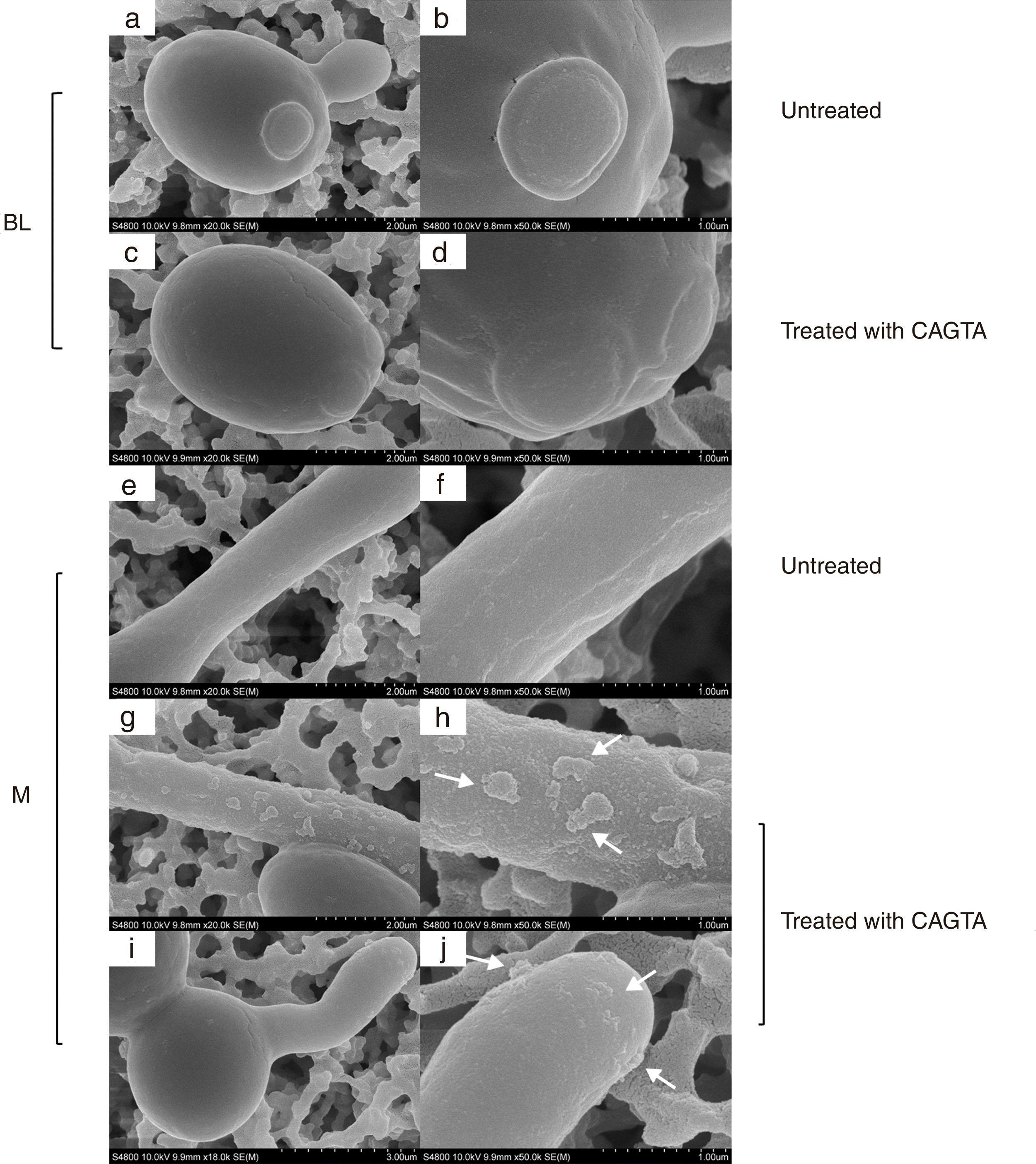
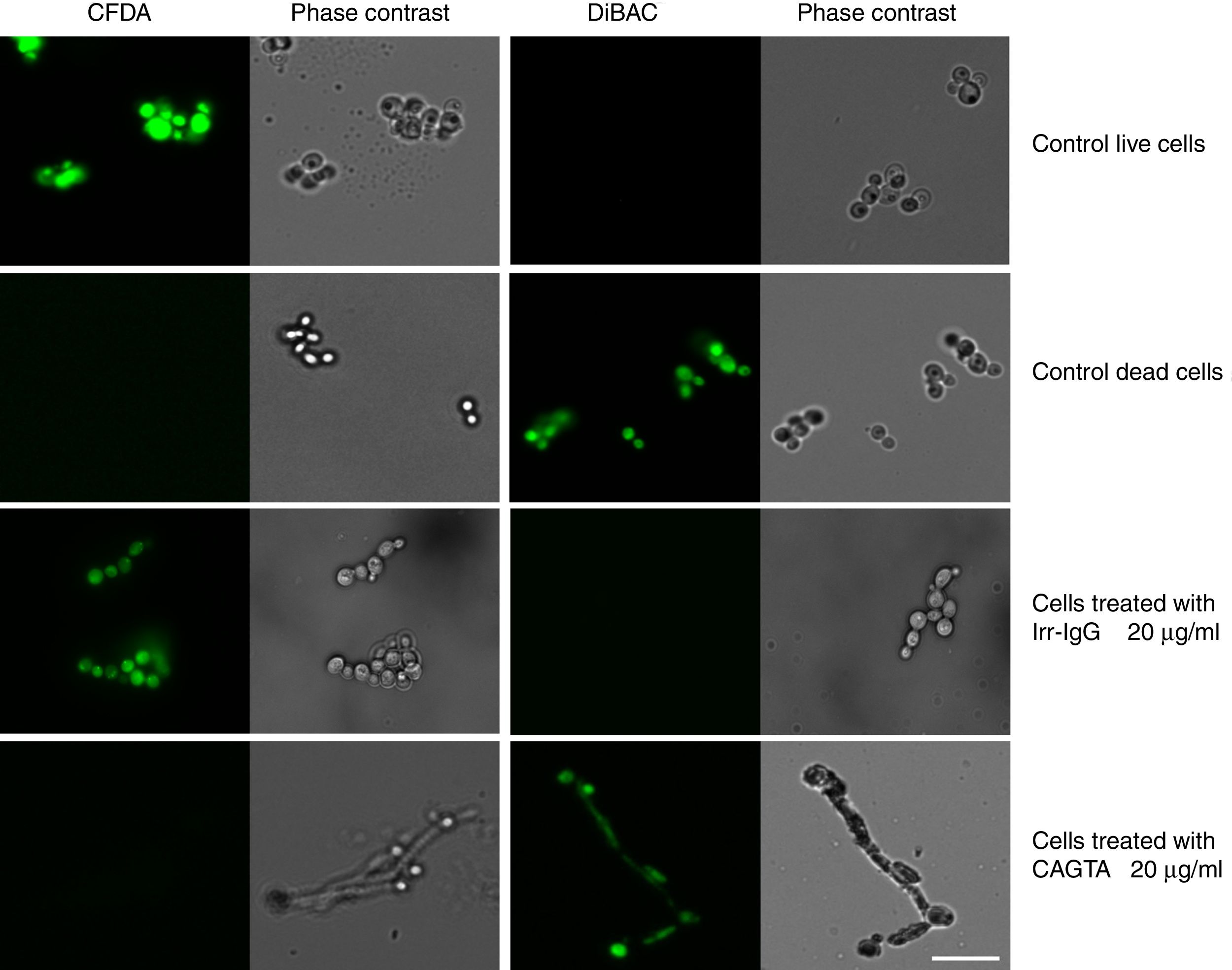
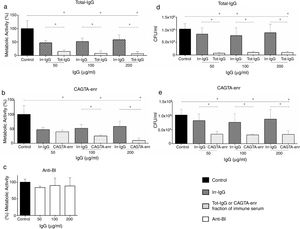
ResultsEffect of CAGTA on Candida albicans planktonic cellsCandida albicans planktonic yeast cells incubated with whole IgG from rabbit immune sera showed a drastic reduction of their metabolic activity, as evaluated by the XTT assay, and the effect was concentration dependent (Fig. 1a). The reduction was statistically significant with respect to untreated cells and cells treated with an equivalent amount of a commercialized Irr-IgG. A parallel experiment with IgG from the CAGTA enriched serum fraction (CAGTA-enr) gave a similar result (Fig. 1b). However, anti-Bl ≤200μg/ml did not show any significant effect on the metabolic activity of the yeast cells and germ tubes which appeared after 2.5h of incubation (Fig. 1c). The effect of the different serum antibody fractions on the metabolic activity of C. albicans planktonic cells was confirmed by the CFU assay, where whole IgG and CAGTA-enr fractions caused a dramatic decrease in the number of colonies at IgG concentrations ≥50μg/ml (Fig. 1d and e). These data were also corroborated with the optical microscopy images of C. albicans planktonic cells incubated with increasing concentrations of different IgG fractions (Fig. 2). Whole IgG from rabbit immune serum reduced the growth of cells in a concentration dependent manner. However, CAGTA-enr IgG induced a slight reduction of C. albicans growth and, even at the highest concentration (200μg/ml), short germ tubes were observed. This paradoxical effect did not correlate the results of XTT and CFU assays. Nevertheless, electron microscopy images of untreated control cells (Fig. 3a, b, e, and f), as well as those of blastospores treated with CAGTA (Fig. 3c and d), appeared with regular and smooth surface, while filamentous phase forms of C. albicans treated with CAGTA-enr IgG 40μg/ml showed an altered surface (Fig. 3g–j). In addition, fluorescent DiBAC and CFDA staining of C. albicans cells treated with CAGTA-enr IgG 20μg/ml revealed that these antibodies exerted a fungicidal effect (Fig. 4).
Effect of antibodies on planktonic cells of C. albicans SC5314 grown at 37°C for 2.5h in Sabouraud broth supplemented with antibodies raised in a rabbit model of candidemia: total IgG (Tot-IgG), CAGTA enriched fraction (CAGTA-enr) and anti-blastospores antibodies (Anti-Bl). Metabolic activity was measured with XTT assay (a, b and c). Viability is expressed as CFU (d and e). Bars represent mean value±SD from three independent experiments. Statistical significance *p<0.05.
Phase contrast microscopy images of C. albicans SC5314 grown at 37°C for 2.5h in Sabouraud broth supplemented with different concentrations of IgG antibodies (irrelevant IgG [irr-IgG], rabbit immune serum total IgG [Tot-IgG] or CAGTA enriched serum fraction [CAGTA-enr]). Control cells (CTRL) were incubated without antibodies.
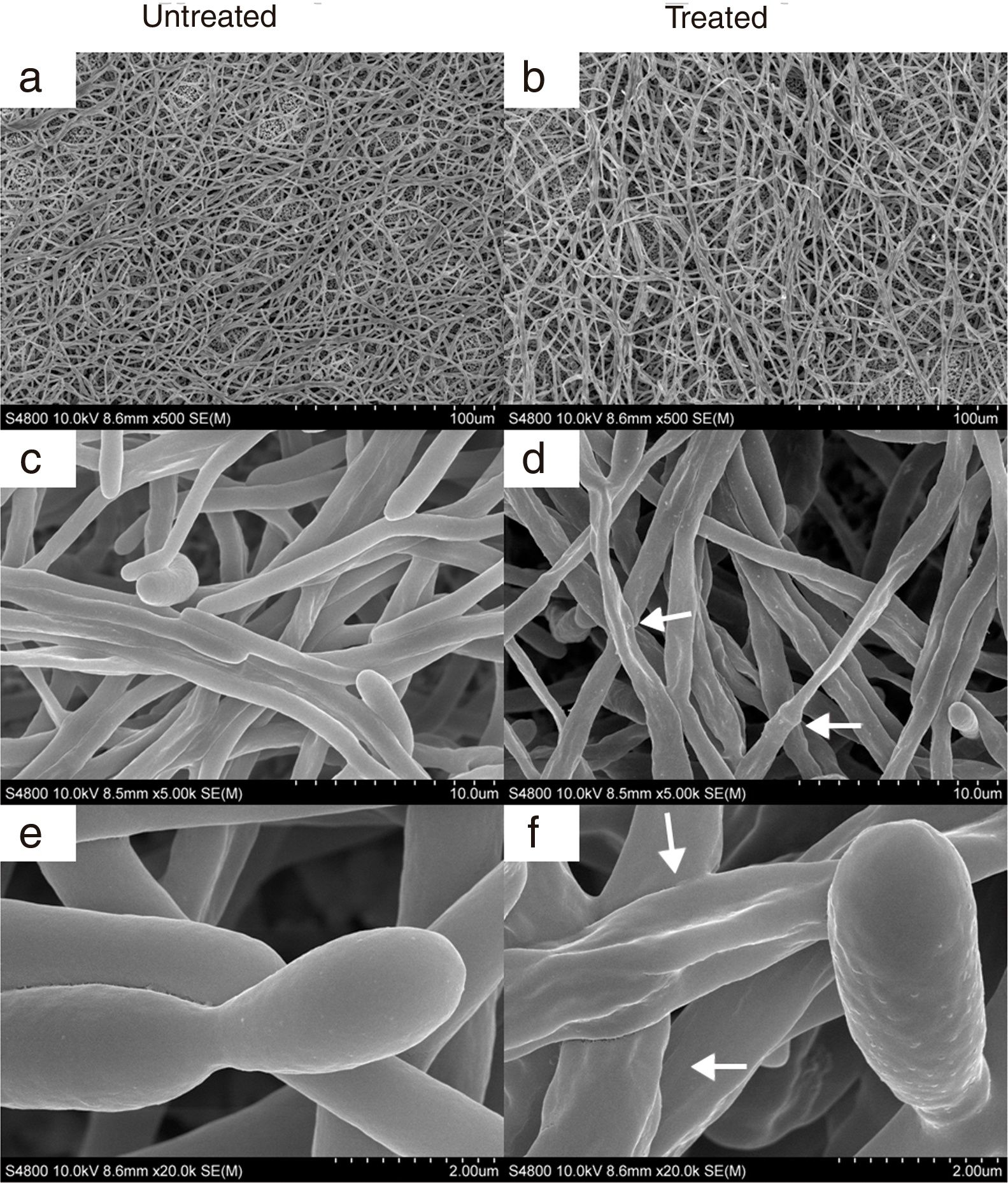
SEM images of C. albicans SC5314 blastospores (BL) and mycelia (M) grown at 37°C for 2.5h in Sabouraud broth with CAGTA 40μg/ml (c, d, g–j) or without antibodies (a, b, e and f). Magnification: ×20,000 (left column) and ×50,000 (right column) for details. Arrows highlight surface protuberances.
Photomicrographs of C. albicans SC5314 grown at 37°C for 2.5h in Sabouraud broth supplemented with CAGTA 20μg/ml and then stained with the fluorescent dyes CFDA and DiBAC. A rabbit irrelevant IgG (Irr-IgG) did not affect C. albicans viability. Staining control consisted of live and dead (heat-killed) yeast cells grown in Sabouraud broth without antibodies. Paired images depict epifluorescent and phase contrast microscopy of the same field. Bar 20μm.
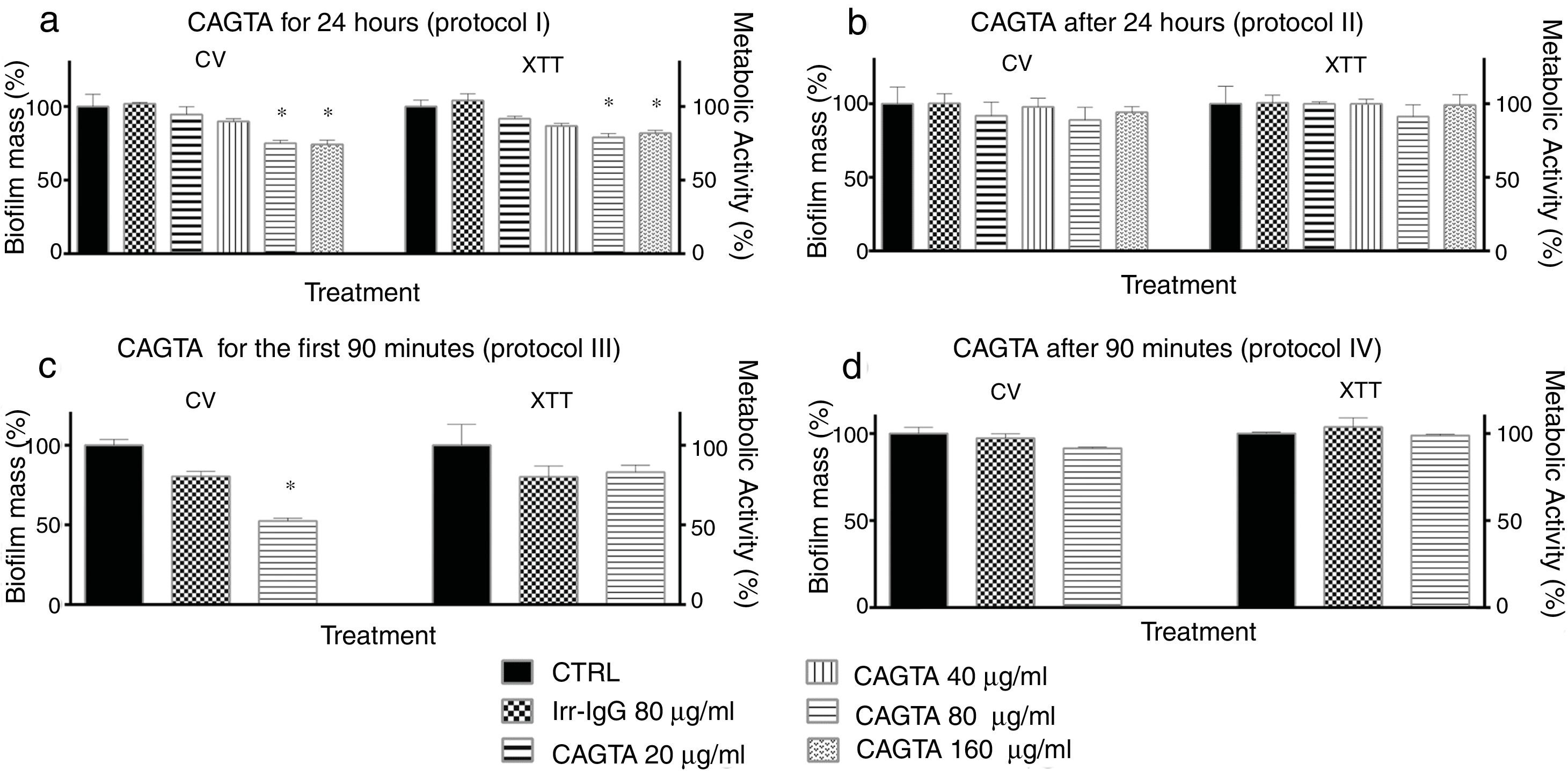
The formation of biofilm by C. albicans can be divided in phases that were analyzed following different time-based protocols of treatment with CAGTA. Yeast cells of C. albicans exposed to CAGTA for the first 24h of incubation (protocol I) experienced a statistically significant reduction of biofilm production at CAGTA concentrations ≥80μg/ml (Fig. 5a), while no differences were observed at the lowest CAGTA concentrations or in the presence of the Irr-IgG (80μg/ml). Similar profiles were obtained with the CV estimation of biofilm mass and metabolic activity according to the XTT assay. When CAGTA (20–160μg/ml) were added onto the 24h-old preformed biofilm, according to protocol II, the maturation of the biofilm was not affected after an additional 24-h incubation period (Fig. 5b).
Effect of CAGTA on metabolic activity (XTT assay) and biofilm mass (CV assay) of C. albicans SC5314 at different stages of biofilm formation. Experimental protocols are described in Material and Methods section: (a) Protocol I, (b) Protocol II, (c) Protocol III, and (d) Protocol IV. Bars represent mean value±SD from three independent experiments. Statistical significance *p<0.05.
To further characterize the effect of CAGTA on C. albicans, two additional experimental protocols were performed focusing on the early phases of the biofilm formation process. On the one hand, the presence of CAGTA only during the first 90min of incubation (protocol III), corresponding to the C. albicans adhesion step, significantly reduced the biofilm biomass to 48% while the metabolic activity remained close to the control group values (Fig. 5c). On the other hand, when CAGTA were added after the first 90min of incubation, no significant effect on the development of the 24h-old biofilm was recorded (protocol IV; Fig. 5d).
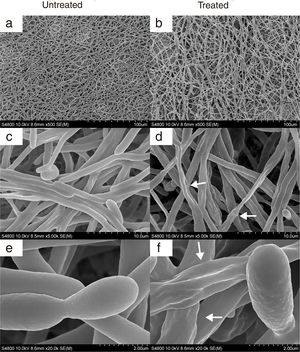
In agreement with the observed effect of CAGTA on C. albicans biofilm formation (protocol I), electron micrographs of 24h-old biofilms exhibited a reduction of the microbial structure density of treated cells (Fig. 6b) when compared to the untreated control (Fig. 6a). Moreover, micrographs revealed that CAGTA treatment had altered the cell wall surface of fungal filaments, that appeared rough and with protuberances (Fig. 6d and f), while the control group cells showed regular and smooth surfaces (Fig. 6c and e).
SEM images of 24-h biofilm of C. albicans SC5314 developed at 37°C in RPMI+FBS10% with CAGTA 80μg/ml (right panel) or without antibodies (left panel). Magnification: ×500 (a, b), ×5000 (c, d) and ×20,000 (e, f). Arrows highlight the altered surface and protuberances of CAGTA treated cells.
The opportunistic pathogen C. albicans can cause invasive infections that are associated with its capacity to grow as hyphae. Several virulence factors related to the cell wall are known to interact with host epithelial cells, which in turn begin and develop an immune response. Hence, an efficient anti-Candida response requires the cooperation of different mechanisms of the immune system that include specific antibodies.4,22,30
The development of an indirect immunofluorescence technique to detect specific antibodies against superficial components of the cell wall of the mycelial phase of C. albicans (CAGTA) has facilitated the diagnosis of IC in patients at risk, since it differentiates colonization from invasion.9,21 Moreover, increasing titers of CAGTA have been related with a better prognosis for patients with IC admitted at the ICU.34 Based on this premise, the present study was designed to investigate the antifungal activity of CAGTA against C. albicans.
In the animal model of IC, C. albicans blastospores were inoculated intravenously and developed germ tubes in the body environment, where they encountered predisposing factors such as temperature >35°C, neutral pH and serum components. Consequently, immune sera contained specific antibodies raised against C. albicans germ tube surface antigens (CAGTA). Whole IgG antibodies of rabbit immune serum reduced the metabolic activity and viability of Candida planktonic cells in vitro, and the effect was concentration dependent. However, when serum IgG content was fractionated, the anti-Bl fraction did not show any effect on planktonic cells, while the CAGTA-enr fraction appeared as the main responsible for the observed inhibitory effect. These results are in agreement with those of Fujibayashi et al.8; these authors registered a reduction in the growth and metabolic activity of C. albicans SC5314 when treated with polyclonal Abs produced in a chicken immunized with that same strain of Candida. Furthermore, Brena et al. observed that the monoclonal antibody C7 (MAb C7), obtained from BALB/c mice immunized with C.albicans,20 reduced the metabolic activity and the CFU count of C. albicans, demonstrating the fungicidal activity of such monoclonal antibody.3
Microphotographs of C. albicans cells incubated with the CAGTA-enr serum fraction evidenced the reduction of cell growth, although germ tubes emerged to some extent. This paradoxical behavior could be explained by the lack of anti-Bl Abs in the CAGTA-enr fraction of serum, which would allow the growth of blastospores to some extent and the emergence of germ tubes. However, purified CAGTA eventually killed C. albicans, as evidenced by DiBAC staining. Moreover, when treated with CAGTA-enr IgG, the surface of germ tubes appeared altered with protuberances while blastospores retained their original smooth surface. Similar results were obtained using the MAb C7 that affected the surface of Candida blastospores,5 and altered the iron uptake pathway.2 Although CAGTA seemed to affect only the surface of C. albicans mycelia, cell wall alteration is relevant for the fungus viability since it may disturb the traffic of nutrients, the osmotic stability and eventually the cell wall organization.5 Along these lines, Ribeiro et al. also observed that C. albicans exposed to a peptide from chilli pepper seeds caused severe alterations in bud formation, the cell wall and the cytoplasmic membrane of the yeast.29
At this point, we would like to explain the unexpected effect of the Irr-IgG from rabbit included in our experiments as a control antibody. Although the metabolic activity of Candida yeast cells was significantly reduced in the presence of this antibody, it never reached the extent of the effect of total-IgG or CAGTA-enr serum fractions, and it was not a concentration dependent effect. Moreover, the CFU counting experiment revealed that cell viability was not affected by the irr-IgG when compared to the untreated cells. A similar inhibitory effect with another Irr-IgG also from rabbit has been reported by Rodier et al.30
While there is evidence of the inhibitory activity of CAGTA against Candida planktonic cells, no data are still available on the ability of such Abs to prevent biofilm formation, one of the major virulence factors of C. albicans. To the best of our knowledge, this is the first report showing that CAGTA reduce Candida biofilm formation. In particular, our data indicate that CAGTA inhibit the adherence of the cells to the surface and, consequently, impair biofilm formation, mainly at an early stage, within the first 90min after C. albicans inoculation; in contrast, CAGTA showed no activity against an already structured 24h-old biofilm. This observation is in accordance with the fact that fungal cells embedded in biofilms are less susceptible to conventional antifungal drugs as well as to antibodies.6,7 Nevertheless, the biofilm formation was significantly impaired by the continuous presence of CAGTA, and the surface of the hyphal cell walls appeared altered. Other studies have reported comparable changes of Candida cells morphology when exposed to peptide P-113Du and P-113Tri16 or marine polyunsaturated fatty acids.32
Our results agree with previous evidence: other monoclonal and polyclonal antibodies, MAb 7D7,18 anti-C3-RP antibody and MAb OKM1,4 also altered the development of C. albicans biofilm. Interestingly, recent data demonstrate that antibody-derived peptides, such as the Killer Peptide (KP), and molecules contained in commercial mouthwashes, are able to significantly impair C. albicans biofilm formation and maturation in terms of both biomass and cell metabolic activity.23,24 Taken together, these findings open new ways for alternative strategies to counteract fungal biofilm formation.
In conclusion, CAGTA inhibit the growth of C. albicans planktonic cells as well as the formation of biofilm, alter the surface of the cell wall of hyphae developed in their presence, and eventually induce planktonic cells to die. The results presented in this work warrant further studies to evaluate the potential protective role of CAGTA, alone or combined with antifungal compounds, in an animal model of invasive candidiasis. Moreover, future research on the antigens recognized by CAGTA could help to find candidates for the development of immunization protocols that might protect against Candida infections.
Transparency declarationsNone to declare.
Conflict of interestThe authors declare that they have no conflict of interest.
We are grateful for the technical and human support provided by SGIker Analytical and High-Resolution Microscopy in Biomedicine Service of UPV/EHU and European funding (ERDF and ESF). The authors are very grateful to Anna Castagnoli for her technical assistance. G. Carrano was the recipient of a pre-doctoral grant from the Basque Government (Spain). This work was supported by grants of the Universidad del País Vasco UPV/EHU (UFI11/25), and the Consejería de Educación, Universidades e Investigación (GIC12/184_IT-788-13, and GIC15/103_IT-913-16) of Gobierno Vasco – Eusko Jaurlaritza.






![Phase contrast microscopy images of C. albicans SC5314 grown at 37°C for 2.5h in Sabouraud broth supplemented with different concentrations of IgG antibodies (irrelevant IgG [irr-IgG], rabbit immune serum total IgG [Tot-IgG] or CAGTA enriched serum fraction [CAGTA-enr]). Control cells (CTRL) were incubated without antibodies. Phase contrast microscopy images of C. albicans SC5314 grown at 37°C for 2.5h in Sabouraud broth supplemented with different concentrations of IgG antibodies (irrelevant IgG [irr-IgG], rabbit immune serum total IgG [Tot-IgG] or CAGTA enriched serum fraction [CAGTA-enr]). Control cells (CTRL) were incubated without antibodies.](https://static.elsevier.es/multimedia/11301406/0000003600000001/v3_201905020854/S1130140618300743/v3_201905020854/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)