Urinary Candida infections in the hospital environment are frequent and need to be better understood.
AimsTo compare the results of antifungal susceptibility profiles of yeasts isolated from patients with urinary infections obtained by broth microdilution method (BM) and by disk diffusion (DD), and also evaluate the capacity of these yeasts to form biofilms.
MethodsOnly yeasts obtained from pure urine cultures with counts higher than 105 colony-forming units per milliliter, without bacteria development, of symptomatic patients were included. The isolates were identified by classical methods and the antifungal susceptibility tests were performed with the following drugs: amphotericin B, ketoconazole, fluconazole, itraconazole, voriconazole and caspofungin. The biofilm studies were carried out in polystyrene microtitration plates.
ResultsNinety-five yeasts isolates were analyzed, including 40 Candida albicans, 31 Candida glabrata, 24 Candida tropicalis. In general, the majority of the isolates were susceptible to the tested drugs but some resistance was observed, especially against fluconazole. Great variability in the antifungal susceptibility results was observed with the different tested drugs and a few discrepancies were observed between both methods. We suggest that in case of DD resistance this result should be confirmed by BM, the standard method. C. tropicalis isolates showed high biofilm production (91.7%) compared to C. albicans (82.5%) and C. glabrata (61.3%), with statistical significance (p=0.0129).
ConclusionsCandiduria in critical patients requires major attention and a better control. The different susceptibility results obtained in this study showed the need to identify yeasts up to the species level, especially in patients with urinary tract infection. The development of techniques of antifungal susceptibility tests can help the clinicians in the empiric treatment of candiduria.
Las infecciones urinarias producidas por especies del género Candida en el ámbito hospitalario son frecuentes, por lo que se requieren mayores conocimientos.
ObjetivosExaminar los resultados de los perfiles de sensibilidad de las levaduras aisladas de pacientes con infección urinaria a los fármacos antimicóticos, comparar los resultados obtenidos con las técnicas de microdilución en caldo y difusión en agar con disco, y valorar la capacidad de estas levaduras para producir biofilm.
MétodosSolo se incluyeron en el estudio las levaduras obtenidas a partir de urocultivos puros de pacientes sintomáticos con recuentos superiores a 105 unidades formadoras de colonias, sin el desarrollo de bacterias. Las levaduras se identificaron con técnicas clásicas y se realizaron pruebas de sensibilidad frente a los antimicóticos siguientes: anfotericina B, ketoconazol, fluconazol, itraconazol, voriconazol y caspofungina. Los exámenes de producción de biofilm se efectuaron en placas de microtitulación de poliestireno.
ResultadosSe analizaron 95 aislamientos de levaduras que incluían 40 Candida albicans, 31 Candida glabrata y 24 Candida tropicalis. En general, la mayoría de los aislamientos eran sensibles a los fármacos examinados, aunque se observaron algunas resistencias, en especial al fluconazol. Se observó una variabilidad considerable en los resultados de la sensibilidad a los diferentes antimicóticos examinados, detectándose algunas discrepancias entre ambos métodos de examen. Sugerimos que los casos valorados como resistentes por difusión con disco se confirmen mediante microdilución en caldo, que es el método de referencia. Los aislamientos de C. tropicalis mostraron una elevada producción de biofilm (91,7%) en comparación con C. albicans (82,5%) y C. glabrata (61,3%), siendo la diferencia estadísticamente significativa (p=0,0129).
ConclusionesEs preciso prestar mayor atención a la candiduria detectada en pacientes en estado crítico, al igual que un mejor control. Los diferentes resultados de sensibilidad a los antimicóticos obtenidos en el presente estudio demuestran la necesidad de identificar las especies de las levaduras aisladas de pacientes con infecciones del tracto urinario. El progreso de las técnicas de sensibilidad a los antimicóticos puede ayudar a los médicos en el tratamiento empírico de la candiduria.
Hospital infections are a major cause of mortality in developing countries, due to their high incidence and also the difficulty of early diagnosis.4 Although fungi were rarely involved in hospital infections during the 1980s, nowadays they are one of the main causes. Most hospital fungal infections are caused by yeasts of the genus Candida, and urinary infection is one of the most common types.2,5,25 Nevertheless, little is known about the characteristics of yeasts isolated from these infections.
No consensus exists as to whether yeasts disseminate from the urinary tract to the bloodstream; however, the association between fungal urinary-tract infections and high mortality rates is undeniable.19 A finding of candiduria is difficult to interpret, since it may indicate only a simple colonization, or alternatively a severe infectious process.
The recent increase in candiduria has been attributed to several factors, such as the length of hospital stay, indiscriminate use of antimicrobial agents, high frequency of use of invasive devices, the patient's degree of susceptibility,8,14,24,26,29 vaginal colonization can also be a risk to nosocomial candiduria.12,41
Urinary catheter use is the principal determining factor for the emergence of urinary yeast infections. The manipulation of this type of device by health professionals could facilitate the migration of yeasts to the bladder, contributing to the appearance of an infection.21 In addition, catheters are substrates that support the formation of biofilms, which protect the microorganisms against phagocytosis and antimicrobial action.10,16
In some cases, candiduria may resolve spontaneously, depending on the correction of risk factors, such as rational use of antibiotics, diabetes control, and removal of catheters.10,16,30,43 However, in some patients, the infectious condition persists, requiring treatment with antifungals, which is usually empirical. Antifungal therapy that is administered without previous determination of the agent incurs the risk of being ineffective, and could also contribute to the selection of resistant microorganisms.
Authors have shown an increase in the number of cases of candiduria due to non-Candida albicans Candida (NCAC) species,12,43 and some of them are naturally resistant to azoles. This situation has made necessary to identify the yeasts involved, in order to determine not only the species, but also its susceptibility profile to the available antifungals.4
The objectives of this study were to compare the results of antifungal susceptibility profiles of yeasts isolated from patients with urinary infections obtained by the broth microdilution method and by disk diffusion, and also to evaluate the capacity of these yeasts to form biofilms.
Materials and methodsOrganisms and culture conditionsA total of 95 yeast strains (40 samples of C. albicans, 31 Candida glabrata, and 24 Candida tropicalis), previously isolated from hospital patients with urinary infections in Maringá, Paraná, Brazil, were studied. After identification, the microorganisms were included in the pathogenic fungal collection (Medical Mycology Laboratory of State University of Maringá). They are maintained in Sabouraud Dextrose Broth (SDB) (Difco, Detroit, MI, USA) with glycerol at −80°C.
Only urine samples from patients with proven urinary-tract infections, according to criteria proposed by the Hospital Infection Control Committee (HICC), were included. Criteria for inclusion were urine cultures from patients with at least one of the following signs or symptoms, without other recognized cause: fever (38°C or higher, under antibiotics use), urinary urgency, increased urinary frequency, dysuria, or suprapubic tenderness.15,43,46 Laboratory parameters included pure cultures for yeasts, without the development of bacteria, and with counts higher than 105 colony-forming units per milliliter (CFU/mL). Patients with a urinary catheter had their urine collected 24h after the catheter was changed. Other markers utilized were detection of leukocyte esterase, nitrite-positive, and pyuria (10 or more leukocytes per mL).43
The yeasts were identified on the basis of their micromorphology in cornmeal supplemented with 1% Tween 80 agar and biochemical tests were performed with the commercial system ID 32C, bioMérieux Marcy l’Etoile, France.47 To carry out the tests, the yeasts stored were inoculated in SDB and incubated at 25°C for 48h. Next, they were grown in Sabouraud Dextrose Agar (SDA) (Difco, Detroit, MI, USA) and in CHROMagar Candida™ (CHROMagar Microbiology, Paris, France) to verify the viability and purity of the colonies.
Susceptibility testBroth microdilution “BM”: The antifungals amphotericin B (Bristol-Meyers-Squibb), ketoconazole (Janssen), itraconazole (Janssen), fluconazole (Pfizer), voriconazole (Pfizer) and caspofungin (Merck) were used. Stock solutions were prepared in dimethyl sulfoxide (DMSO) or water according to the solubility of each antifungal, and then serial dilutions were carried out according to the document M27-A3.7,31 The culture medium used was RPMI-1640 (Gibco, Detroit, Michigan, USA) buffered with morpholino propanesulfonic acid (Sigma, St. Louis, MO, USA) pH 7.0, supplemented with 2% glucose as proposed by EUCAST.11 To prepare the inoculum, yeasts were suspended in saline solution and the concentration was adjusted spectrophotometrically to 0.5–2.5×103cells/mL. The suspensions were tested with antifungal solution in 96-well microtitration plates (Nunclon, Delta, Nunc A/S, Roskilde, Denmark) incubated at 35°C for 48h. Candida parapsilosis ATCC 22019 was used as control. Absorbance was read using a microtitration reader (Expert plus – ASYS) set at 490nm. The Minimum Inhibitory Concentration (MIC) was defined as the lowest concentration of antifungal that provided 50% inhibition for azoles and caspofungin. For amphotericin B the MIC was the lowest drug concentration that prevented any discernible growth. The definition of breakpoints followed the document M27-A3,7 and for ketoconazole (not included in this document) it was based on Özçelik et al.27 The results were expressed as: susceptible, susceptible-dose-dependent (SDD), or resistant.
Disk diffusion “DD”: Amphotericin B (Cecon), ketoconazole (Cecon), itraconazole (Cecon), voriconazole (Oxoid) and fluconazole (Oxoid) disks were used. The test was performed according to document M44-A from CLSI.6 Müller-Hinton Agar (Difco) pH 7.2, supplemented with 2% glucose and 0.5μg/mL of methylene blue was utilized as the culture medium. The yeast suspension was adjusted to 1–5×106CFU/mL and was seeded onto the surface of the plates. The plates were incubated for 48h at 35°C, after culturing, the diameter of the zone of inhibition was measured in millimeters with a ruler and the interpretation was carried out according to the manufacturer's instructions.
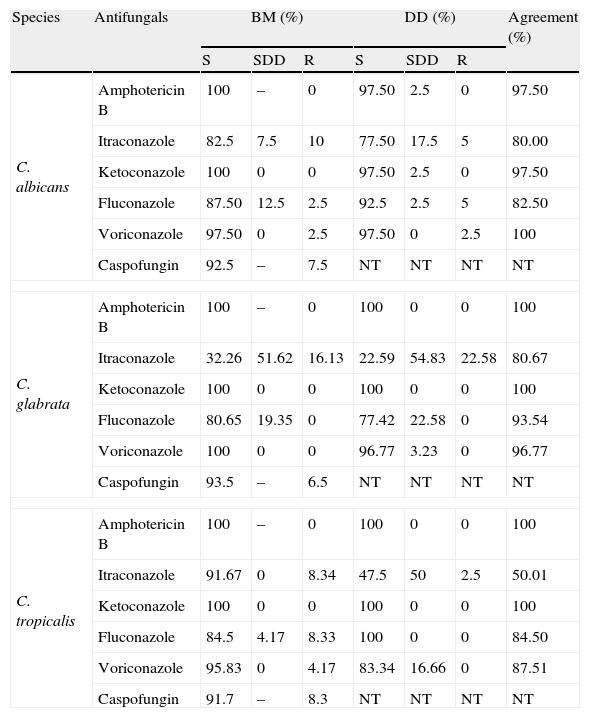
Results obtained by BM and DD methods, for each Candida species have been firstly classified in S, SDD or R according documents M27 A37 and M44A6, respectively. Next, the agreement rate was determined by simple comparison (percentage).
BiofilmThe capacity to produce biofilm was estimated as described by Shin et al. with some modifications.40 First, a yeast suspension was prepared and adjusted to 3.7×107CFU/mL. Then, 20μL of this suspension was placed in each well of 96-well polystyrene microtitration plates, and 180μL of SDB supplemented with glucose were added (final concentration 8%). The experiment was done in quadruplicate and C. albicans ATCC 90028 was used as control. The microtitration plate was incubated at 35°C for 24h. After that, the plates were washed twice with distilled water and the optical density (OD) was determined at 405nm in a Biochrom Asys Expert Plus microtitration plate reader. To quantify the biofilm of each yeast, OD values were converted to transmittance (T%) and subtracted from the negative value (without biofilm production). The interpretation scale used was: negative (T%<5), 1+ (T% 5–20), 2+ (T% 20–35), 3+ (T% 35–50), 4+ (T%≥50). To evaluate the difference, the t test was used; it was considered significantly different when the p value was ≤0.05.
Results and discussionAntifungal susceptibility profileOur results showed the presence of resistant yeasts (Table 1), especially to azoles. With respect to fluconazole, 12.5% of C. albicans were SDD and 2.5% were resistant; C. tropicalis also showed worrying results (4.2% SDD and 8.3% resistant isolates). None of the 31 C. glabrata isolates proved to be resistant, but 19.35% of them were considered SDD. These results are similar to those of Pfaller et al., who found that 1–2.1% of C. albicans, 12% of C. glabrata and 1.4–6.6% of C. tropicalis isolates were resistant to fluconazole.33–35
Comparison between broth microdilution and disk diffusion tests, for 95 Candida isolates from urinary nosocomial infections.
| Species | Antifungals | BM (%) | DD (%) | Agreement (%) | ||||
| S | SDD | R | S | SDD | R | |||
| C. albicans | Amphotericin B | 100 | – | 0 | 97.50 | 2.5 | 0 | 97.50 |
| Itraconazole | 82.5 | 7.5 | 10 | 77.50 | 17.5 | 5 | 80.00 | |
| Ketoconazole | 100 | 0 | 0 | 97.50 | 2.5 | 0 | 97.50 | |
| Fluconazole | 87.50 | 12.5 | 2.5 | 92.5 | 2.5 | 5 | 82.50 | |
| Voriconazole | 97.50 | 0 | 2.5 | 97.50 | 0 | 2.5 | 100 | |
| Caspofungin | 92.5 | – | 7.5 | NT | NT | NT | NT | |
| C. glabrata | Amphotericin B | 100 | – | 0 | 100 | 0 | 0 | 100 |
| Itraconazole | 32.26 | 51.62 | 16.13 | 22.59 | 54.83 | 22.58 | 80.67 | |
| Ketoconazole | 100 | 0 | 0 | 100 | 0 | 0 | 100 | |
| Fluconazole | 80.65 | 19.35 | 0 | 77.42 | 22.58 | 0 | 93.54 | |
| Voriconazole | 100 | 0 | 0 | 96.77 | 3.23 | 0 | 96.77 | |
| Caspofungin | 93.5 | – | 6.5 | NT | NT | NT | NT | |
| C. tropicalis | Amphotericin B | 100 | – | 0 | 100 | 0 | 0 | 100 |
| Itraconazole | 91.67 | 0 | 8.34 | 47.5 | 50 | 2.5 | 50.01 | |
| Ketoconazole | 100 | 0 | 0 | 100 | 0 | 0 | 100 | |
| Fluconazole | 84.5 | 4.17 | 8.33 | 100 | 0 | 0 | 84.50 | |
| Voriconazole | 95.83 | 0 | 4.17 | 83.34 | 16.66 | 0 | 87.51 | |
| Caspofungin | 91.7 | – | 8.3 | NT | NT | NT | NT | |
S=susceptible; SDD=susceptible-dose-dependent; R=resistant; NT=not tested; BM=Broth microdilution; DD=disk diffusion.
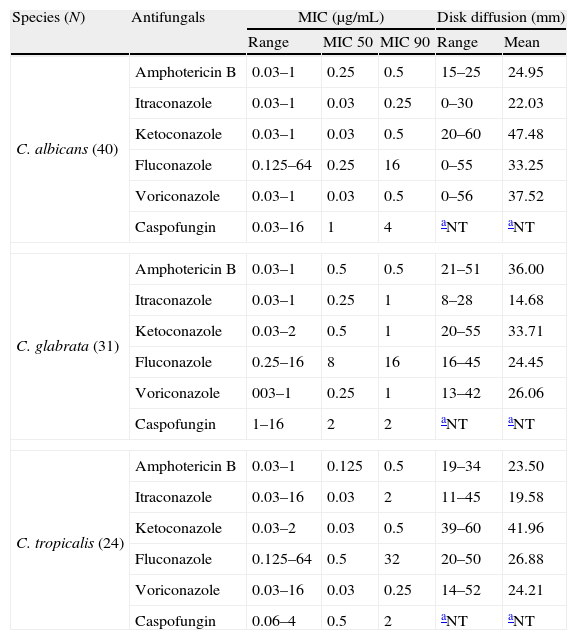
The breakpoint for Fluconazole, Itraconazole, Voriconazole, Amphotericin B and Caspofungin was established as proposed by document M27-A3.7 For Ketoconazole according to Özçelik et al.27 Interpretation: susceptible: ≤8μg/mL Ketoconazole; ≤1μg/mL Amphotericin B; ≤1μg/mL Voriconazole; ≤2μg/mL Caspofungin; susceptible-dose-dependent: 2μg/mL Voriconazole; resistant: >1μg/mL Amphotericin B; ≥4μg/mL Voriconazole; > 2μg/mL Caspofungin.
The increased MIC shown by different species of yeasts could be related to previous exposure to fluconazole,36 which is the first-choice drug, not only for prophylaxis but also for treatment, because of its favorable pharmacokinetics and low toxicity.39 The high number of SDD observed in C. glabrata was expected, since this species has low susceptibility to azoles.13,32,44 Bruder-Nascimento et al. observed even higher resistance rates than in the present study: C. glabrata to fluconazole (68%) and itraconazole (88%); and C. tropicalis to itraconazole (21.1%).3
In practice, voriconazole is normally more effective than fluconazole and itraconazole, as confirmed by in vitro tests.44 In this study, some resistant isolates were probably associated with crossing resistance, due to the similar chemical structure of these azoles, as voriconazole is not yet being used in this hospital, since normally it should be administered to patients with fluconazole-resistant yeasts.1,28,32
Regarding caspofungin our results suggest high sensitivity; in fact this is a parenteral echinocandin antifungal with known fungicidal activity against Candida species.31,42 This seems to be a good clinical option for candiduria management. Since according to Sobel et al. caspofungin has a higher concentration in the urine than in the blood plasma, it could be used in patients with azole-resistant yeast isolates.42
Among the antifungal drugs tested in this study, amphotericin B gave the best results, as also observed by Porte et al.37 Nevertheless, in clinical practice amphotericin B should be administered with caution, and it is only recommended for cases that are resistant to other drugs, due to the risk of toxicity.45
Comparison between broth microdilution and disk difusion methodsThe DD method is simpler and easier to perform than the BM method,9 but it is standardized and validated only for fluconazole. The present study included other antifungals used in clinical practice. Some results showed discrepancies in relation to the reference method, mainly for itraconazole.
According to Table 1, the DD test indicated intermediate resistance and susceptibility for C. glabrata, C. albicans and C. tropicalis. This false resistance, compared with BM, was highest for itraconazole, as previously described by Mock et al. These authors attributed the discrepancy to the difficulty of diffusion of the active principle in the agar.22 Therefore, “resistant” cases detected through DD should be confirmed by the BM method in order to avoid false-resistance results, mainly observed in NCAC.36
If only the results observed for DD were considered, itraconazole would be judged to be the least efficient antifungal, since 30.1% of the isolates were resistant, especially C. glabrata (Table 1). The same would apply as C. albicans as NCAC in relation to fluconazole and voriconazole, which are usually the drugs of choice to treat invasive infections. In contrast, the results for ketoconazole, which is not clinically indicated for candiduria, suggested that the yeasts were “susceptible”.
Even though resistant isolates were detected, most of them also proved to be susceptible by DD and BM. Pfaller et al.36 considered that false-susceptible results usually do not occur, and therefore the DD method could be included in the laboratory routine for antifungal activity trials, mainly for fluconazole. However, in cases where resistance is verified by DD, a confirmatory method such as BM is necessary (Table 2).
Results of broth microdilution and disk diffusion tests for Candida species.
| Species (N) | Antifungals | MIC (μg/mL) | Disk diffusion (mm) | |||
| Range | MIC 50 | MIC 90 | Range | Mean | ||
| C. albicans (40) | Amphotericin B | 0.03–1 | 0.25 | 0.5 | 15–25 | 24.95 |
| Itraconazole | 0.03–1 | 0.03 | 0.25 | 0–30 | 22.03 | |
| Ketoconazole | 0.03–1 | 0.03 | 0.5 | 20–60 | 47.48 | |
| Fluconazole | 0.125–64 | 0.25 | 16 | 0–55 | 33.25 | |
| Voriconazole | 0.03–1 | 0.03 | 0.5 | 0–56 | 37.52 | |
| Caspofungin | 0.03–16 | 1 | 4 | aNT | aNT | |
| C. glabrata (31) | Amphotericin B | 0.03–1 | 0.5 | 0.5 | 21–51 | 36.00 |
| Itraconazole | 0.03–1 | 0.25 | 1 | 8–28 | 14.68 | |
| Ketoconazole | 0.03–2 | 0.5 | 1 | 20–55 | 33.71 | |
| Fluconazole | 0.25–16 | 8 | 16 | 16–45 | 24.45 | |
| Voriconazole | 003–1 | 0.25 | 1 | 13–42 | 26.06 | |
| Caspofungin | 1–16 | 2 | 2 | aNT | aNT | |
| C. tropicalis (24) | Amphotericin B | 0.03–1 | 0.125 | 0.5 | 19–34 | 23.50 |
| Itraconazole | 0.03–16 | 0.03 | 2 | 11–45 | 19.58 | |
| Ketoconazole | 0.03–2 | 0.03 | 0.5 | 39–60 | 41.96 | |
| Fluconazole | 0.125–64 | 0.5 | 32 | 20–50 | 26.88 | |
| Voriconazole | 0.03–16 | 0.03 | 0.25 | 14–52 | 24.21 | |
| Caspofungin | 0.06–4 | 0.5 | 2 | aNT | aNT | |
Interestingly, some samples of C. tropicalis were resistant to most antifungals. Jang et al. observed that patients with candiduria caused by C. tropicalis developed systemic infections.17 Therefore it is important to give proper attention to this species, since in our hospital candiduria has been associated with high mortality rates (unpublished data). In fact, mortality rates are frequently associated with candiduria followed by candidemia.17,18
Biofilm formationIn our experiments, C. tropicalis showed a statistically greater capacity to form biofilms (91.7%) than C. albicans (82.5%) and C. glabrata (61.3%) (p=0.0129). Other investigators have also demonstrated the high ability of C. tropicalis to produce biofilms.23,40 This capability is important, since microorganisms organized in biofilms could become resistant to antifungals due to metabolic changes, reduction of their cell growth rate, expression of resistance genes, and the presence of an extracellular matrix.23,38
Health professionals should take special care when managing urinary catheters to prevent biofilm formation, since one of the main reasons for treatment failure stems from this capacity of fungi to produce biofilms on the surface of foreign bodies.20
In conclusion candiduria in critical patients requires major attention and a better control. The different susceptibility results obtained in this study showed the need to identify yeasts up to the species level, especially in patients with urinary tract infection. The development of techniques of antifungal susceptibility tests can help the clinicians in the empiric treatment of candiduria.
Conflict of interestThe authors have no conflict of interest to declare.
This study was supported by grants from the Fundação Araucária, protocol 8970.