Prismatomeris memecyloides Craib (Rubiaceae) is a medicinal plant traditionally used by ethnic minorities in Vietnam for the treatment of erectile dysfunction (ED). The aim of this study was to investigate the chemical compositions and screen in silico its possible inhibitory effect against PDE-5 which reduced cyclic guanosine-3′,5′-monophosphate (cGMP) levels and indirectly caused the male ED.
MethodsSeparation of natural compounds were carried out on chromatographic column with silica gel or reversed phase materials, eluting with different solvent gradients. The structures of all isolated compounds were elucidated on the basis of spectroscopic data (HR-MS, 1D/2D-NMR). Docking simulation study of compound (1–7) was performed by using flexible side chains protocol based on Iterated Local Search Global Optimizer Algorithm of AutoDock/Vina v.1.1.2. Pharmacokinetic parameters and toxicity prediction were also calculated by appropriate softwares.
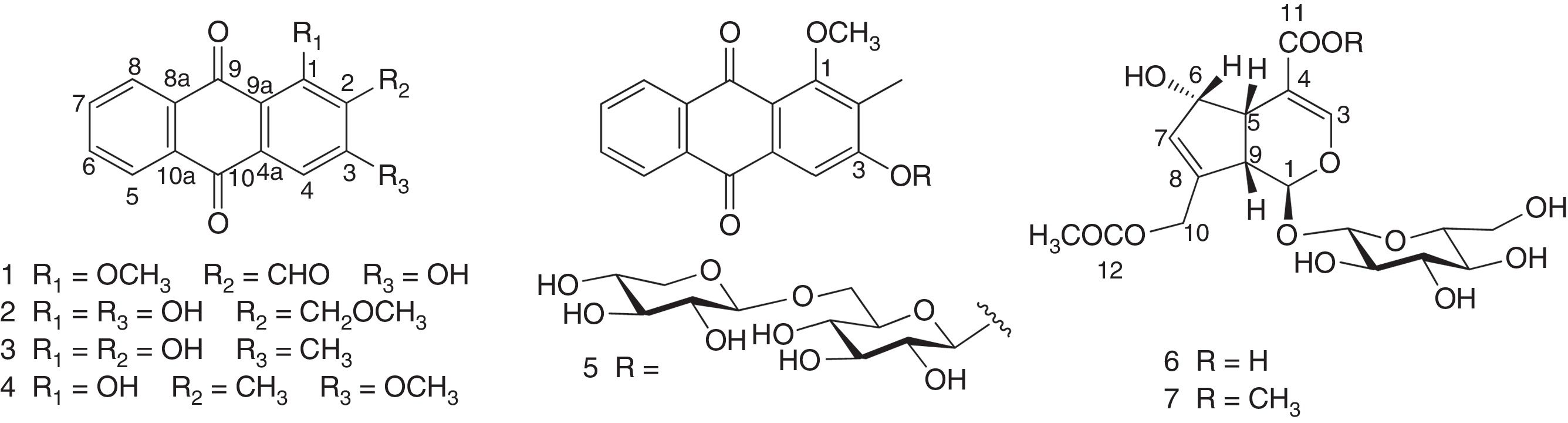
ResultsFrom the methanol extract of roots of P. memecyloides collected in Vietnam, seven compounds including four anthraquinone/one anthraquinone glycoside namely damnacanthal (1), lucidin-ω-methyl ether (2), 3-methylalizarin (3), rubiadin-3-methyl ether (4), and 1-O-methylrubiadin 3-O-primeveroside (5) along with two iridoid glucosides, asperulosidic acid (6) and aitchisonide A (7) were isolated. The molecular modeling results showed that 5 anthraquinone compounds possess the lowest binding energies to PDE-5. The anthraquinone glucoside 1-O-methylrubiadin 3-O-primeveroside (5) potentially inhibited PDE-5 similarly to commercial PDE-5Is sildenafil (SLD) and tadalafil (TLD). Calculated pharmacokinetic results like pIC50,pred; miLogP, TPSA, enzyme inhibitory of anthraquinone glucoside (5) were similar and even higher to those of the commercial PDE-5 inhibitors. Especially the predictive toxicity of 1-O-methylrubiadin 3-O-primeveroside (5) was even lower than those of SLD and TLD.
ConclusionThis is the first study to find a scientific-based evidence for the ethnic use of P. memecyloides as medicinal plant for the treatment of ED. The result indicates that the anthraquinones (damnacanthal (1), lucidin-ω-methyl ether (2), 3-methylalizarin (3) and rubiadin-3-methyl ether (4)), especially anthraquinone glycoside (1-O-methylrubiadin 3-O-primeveroside (5)) are compounds of potential novel drug class for the ED treatment.
Prismatomeris memecyloides Craib (Rubiaceae) es una planta medicinal utilizada tradicionalmente por las minorías étnicas en Vietnam para el tratamiento de la disfunción eréctil (DE). El objetivo de este estudio fue investigar sus composiciones químicas y realizar un cribado virtual de su posible efecto inhibitorio frente a PDE-5 con niveles reducidos de guanosín-3′,5′-monofosfato cíclico (cGMP) y la causa indirecta de la DE en los varones.
MétodosSe realizó la separación de los componentes naturales en una columna cromatográfica con gel de silicio o materiales de cambio de fase, diluyéndolos con diferentes gradientes de disolventes. Se dilucidaron las estructuras de los componentes aislados sobre la base de los datos espectroscópicos (HR-MS, 1D/2D-NMR). Se realizó el estudio de simulación de acoplamiento del componente (1–7) utilizando el protocolo de cadenas laterales flexibles basado en el algoritmo «Iterated Local Search Global Optimizer» de los parámetros farmacocinéticos AutoDock Vina v.1.1.2., calculándose asímismo la predicción de toxicidad mediante los softwares adecuados.
ResultadosA partir de los extractos de metanol de las raíces de P. memecyloides recolectadas en Vietnam se aislaron 7 componentes, incluyendo 4 antraquinonas/un glucósido antraquinona denominado damnacantal (1), lucidín-ω-metil éter (2), 3-metilalizarín (3), rubiadín-3-metil éter (4) y 1-O-metilrubiadín 3-O-primeverosida (5) junto con 2 glucósidos iridoides, ácido asperulosídico (6) andaitquisonida A (7). Los resultados de modelación molecular reflejaron que 5 componentes de antraquinona poseen las menores energías de ligamiento a PDE-5. El glucósido antraquinona 1-O-metilrubiadín 3-O-primeverosida (5) inhibió potencialmente PDE-5 de manera similar a las PDE-5Is comerciales sildenafil (SLD) y tadalafil (TLD). Los resultados farmacocinéticos calculados como pIC50, pred, miLogP, TPSA, enzima inhibidora de antraquinona glucósido, 1-O-metilrubiadín 3-O-primoverosida (5), fueron similares e incluso más altos que los de los inhibidores comerciales de PDE-5. Especialmente, la toxicidad predictiva de (5) fue incluso menor que la de SLD y TLD.
ConclusiónEste es el primer estudio que encuentra una evidencia con base científica para el uso étnico de Prismatormeris memecyloides como planta medicinal para el tratamiento de la DE. El resultado indica que las antraquinonas (damnacantal (1), lucidín-ω-metil éter (2), 3-metilalizarín (3) y rubiadín-3-metil éter (4)), y en especial el glucósido antraquinona (1-O-metilrubiadín 3-O-primeverosida (5)) son componentes de clase farmacológica novel potencial para el tratamiento de la DE.
Erectile dysfunction (ED) is a sexual dysfunction characterized by the inability to develop or maintain an erection of the penis during sexual activity in humans. For treatment of this dysfunction, several psychological, physical and cellular-level principal methods have been developed.1 Currently, phosphodiesterase 5 inhibitors (PDE-5Is) for the therapeutic treatment of ED have attracted great attention since the notable introduction of commercial PDE-5Is drugs including sildenafil citrate (Viagra®) in 1998, tadalafil (Cialis®) and vardenafil (Levitra®) in 2003.1,2 These PDE-5Is have become the first-line therapy for ED, as recommended by the American Urological Association (AUA) and the European Association of Urology (EAU).3
In order to develop new PDE-5Is, many studies based on virtual screening and molecular docking modeling of the protein inhibitor binding of both synthetic4 and natural-occurring bioactive compounds from natural sources or from traditional medicine database5 have performed to identify potential candidates. A new insight into the protein–inhibitor interactions is helpful in designing potent and selective PDE-5Is for the treatment of ED.6 Increasing both the steric and electrostatic factors for designing novel PDE-5 inhibitors is expected to induce higher inhibitory activity and higher selectivity to PDE-5.2 Numerous interesting results have been obtained from the molecular docking performance guiding for more potent and selective PDE-5 inhibitors, such as several candidates from the TCM database,5 pyridopurinone derivatives,6 several sildenafil analogs,7l-tryptophane tadalafil analogues,8 benzyl derivative (NSC 619),9 tetrahydro-β-carboline derivatives (THBCs),10 etc., which might be benefits for the drug development of novel ED therapeutic agents.
The search for PDE-5Is from medicinal plants and herbal remedies is one of efficient choices for ED management. The plant genus Prismatomeris worldwide includes about 29 species growing mainly in tropical areas 11. Phytochemical investigations of several Prismatomeris plants such as P. malayana, P. tetrandra, P. fragrans, P. connata, etc. led to the isolation and structural elucidation of mono anthraquinones,11 anthraquinone glucosides,12 iridoids, iridoid glucosides and triterpenoids.13–15 The biological activities of these substances are anti-cancer activity against prostate cancer, lung cancer (both small and normal forms), anti-malarial, anti-fungal, anti-bacterial and anti-tuberculosis activities.11 In China, the Prismatomeris connata roots (called Huang-gen) were used for treatment of hepatitis and allergic cough (pneumoconiosis).12 In Malaysia, the aqueous extract of the dried roots of P. glabra (as local name Ajisamat) has been traditionally used by the aborigines and certain rural Malays for wellness, as tonic and aphrodisiac agent.16 In Vietnam, there are 10 Prismatomeris species found, mostly in the mountainous provinces in north Vietnam.14,17 These Prismatomeris species have been used in traditional medicine to heal wounds, for treatment of knee backache, asthma, epilepsy, arthritis and diuretic.14,18 Especially, Prismatomeris memecyloides Craib (local name “Congselen”) is widely used as traditional remedies for treatment of ED by the ethnic minorities as Thai, H’mong, Muong in Son La province in Vietnam.17,19P. memecyloides is a small tree with glabrous limb, 2-cm-long pedicel filiform and 2-cm-long corolla.18 As far as our knowledge, no scientific evidences are documentally published about the chemical compositions and pharmacological activity of P. memecyloides. The aim of this study was therefore to investigate the chemical constituents of P. memecyloides and to find out the main compounds leading to the therapeutic value in management of ED of this plant. In the paper, we describe the isolation and structures of seven known compounds (1–7) including five anthraquinones/anthraquinone glycoside along with two iridoid glucosides from the roots of P. memecyloides. The structures of all isolated compounds (1–7) were elucidated on the basis of spectroscopic data (HR-MS, 1D/2D-NMR) as well as by comparison with spectral values published in the literature. The inhibitory activity against PDE-5 was investigated using in computer-based molecular modeling. Using AutoDock software, the isolated compounds were subjected to docking simulation in order to investigate their possible interactions into active binding sites of PDE-5 and leading to inhibit the enzyme. The results indicated that 5 anthraquinone compounds possess the lowest binding energies to PDE-5. Calculated pharmacokinetic results like pIC50,pred, miLogP, TPSA, enzyme inhibitory of anthraquinone glucoside, 1-O-methylrubiadin 3-O-primeveroside (5), were similar and even higher to those of the commercial PDE-5 inhibitors. Especially the predictive toxicity of (5) was even lower than those of SLD and TLD. The findings might be worthy in order to further develop anthraquinones/anthraquinone glucosides as novel drug class for treatment of ED.
Material and methodsGeneral experimental procedures1H NMR (500MHz), 13C NMR (125MHz) spectra were measured on a Bruker AVANCE 500 spectrometer. The ESI-MS spectra were obtained with a ESI-MicroQ-TOF III (Bruker Daltonics Inc.) and a FT-ESI-MS (Varian Inc.) mass spectrometer. UV and IR spectra were obtained on a JASCO V-630 and an Impact 410 Nicolet FT-IR spectrometer, respectively. Column chromatography (CC) was carried out on silica gel (Si 60 F254, 230–400 mesh, Merck). All solvents were distilled before use. Precoated plates of silica gel 60 F254 were used for analytical purposes. Compounds were visualized under UV radiation (254, 365nm) and by spraying plates with 10% H2SO4 followed by heating with a heat gun.
Plant materialThe roots of Prismatomeris memcyloides Craib, were collected in Son La province, North Vietnam. The plant was identified by the botanist Dr. Ngo Van Trai, National Institute of Medicinal Materials (NIMM). A voucher specimen (C-521) is deposited in the herbarium of the Institute of Natural Products Chemistry, VAST, Hanoi, Vietnam.
Extraction and isolationDried powdered roots of P. memecyloides (1.0kg) were extracted with MeOH (3× 3L) over the period of 5 days at room temperature and concentrated under reduced pressure to yield a black crude MeOH extract (80g). The crude MeOH extract was suspended in hot water (1:1, v/v) and successively partitioned with chloroform (C) and ethyl acetate (EtOAc). The resulting fractions were concentrated under reduced pressure to give the corresponding fractions of chloroform (10.2g), EtOAc (0.8g) and water (60.1g).
The chloroform fraction (10.2g) was subjected to chromatographic column (CC) on flash silica gel column (400–630 mesh) with gradient solvents of n-hexane/acetone (1:0, 40:1, 20:1, 10:1, 5:1 and 0:1, v/v, 1.0L/each) to afford 6 fractions (A1–A6). The fraction A3 (0.6g) was subjected to CC on a silica gel column (230–400 mesh), using n-hexane/acetone (7:1, v/v) as eluting solvents to obtain 12 subfractions (A3.1–A3.12). The subfraction A3.3 (100mg) was rechromatographed on a silica gel column eluting with n-hexan:CH2Cl2:CH3OH (10:1:0.1, v/v/v) to yield compound 1 (5mg). The fraction A3.10 (1.4g) was further chromatographed on a silica gel column eluting with DCM 100% to get compound 2 (56mg) as white powder.
The fraction A5 (2.5g) was subjected to CC on a silica gel column (230–400 mesh), using n-hexane/acetone (7:1, v/v) to yield compound 3 (28mg) as yellow powder. The fraction A5.2 (300mg) was further chromatographed on a silica gel column eluting with n-hexane/acetone (7:1, v/v) to yield compound 4 (5mg).
The water fraction (60.1g) was subjected to CC on a dianion column using MeOH:H2O (3:1, v/v) as eluting solvent to get 3 fractions (10A–10C). The fraction 10B (4.1g) was consequently rechromatographed over 3 columns: silica gel eluting with CHCl3/MeOH/water (4:1,2:1, v/v/v)/EtOAC/MeOH/water (15:1:0,1, v/v), Sephadex LH-20 eluting with MeOH/water (1:1, v/v), and RP-C18 eluting with MeOH/water (5:1; 4:1, v/v) to yield compound 5 (600mg) and 6 (56mg).
Spectroscopic dataDamnacanthal (1): white powder, soluble in MeOH (C16H10O5, M=576); 1H NMR (DMSO, 500MHz): 7.50 (1H, s, H-4), 8.14 (1H, dd, 1.25; 7.75Hz, H-5), 7.88 (1H, dt, 1.5; 7.5Hz, H-6), 7.94 (1H, dt, 1.5; 7.5Hz, H-7), 8.18 (1H, dd, 1.25; 7.75Hz, H-8), 3.98 (3H, s, OCH3), 10.39 (1H, s, CHO); 13C NMR (DMSO, 125MHz): 164.5 (C-1), 120.2 (C-2), 165.2 (C-3), 111.2 (C-4), 140.1 (C-4a), 126.4 (C-5), 133.7 (C-6), 134.6 (C-7), 126.8 (C-8), 135.0 (C-8a), 179.6 (C-9), 117.8 (C-9a), 181.8 (C-10), 132.1 (C-10a), 63.7 (OCH3), 192.8 (CHO).
Lucidin-ω-methyl ether (2): white powder, soluble in CHCl3 (C16H12O5, M=424); 1H NMR (DMSO, 500MHz): 7.21 (1H, s, H-4), 8.09 (1H, dd, 1.5; 7.25Hz, H-5), 7.85 (1H, td, 1.5; 7.5Hz, H-6), 7.88 (1H, td, 1.5; 7.5Hz, H-7), 8.15 (1H, dd, 1.5; 7.5Hz, H-8), 4.40 (2H, s, CH2O), 3.35 (3H, s, OCH3), 13.18 (1H, s, OH); 13C NMR (DMSO, 125MHz), δ (ppm): 163.7 (C-1), 116.6 (C-2), 164.1 (C-3), 107.5 (C-4), 133.8 (C-4a), 126.7 (C-5), 134.4 (C-6), 134.6 (C-7), 126.3 (C-8), 132.9 (C-8a), 186.1 (C-9), 109.0 (C-9a), 181.7 (C-10), 132.7 (C-10a), 61.0 (CH2O), 57.6 (OCH3).
3-Methylalizalin (3): white powder, soluble in MeOH (C15H10O4, M=283); 1H NMR (DMSO, 500MHz): 7.22 (1H, s, H-4), 8.10 (1H, dd, 2.0; 7.0Hz, H-5), 7.88 (2H, m, 1.5; 7.25Hz, H-6,7), 8.16 (1H, dd, 2.0; 7.0Hz, H-8), 13.08 (1H, s, OH), 2.05 (3H, s, CH3); 13C NMR (DMSO, 125MHz): 162.8 (C-1), 162.4 (C-2), 133.0 (C-3), 107.3 (C-4), 131.7 (C-4a), 126.4 (C-5), 134.5 (C-6), 134.4 (C-7), 126.7 (C-8), 117.3 (C-8a), 186.3 (C-9), 109.0 (C-9a), 181.8 (C-10), 132.9 (C-10a), 8.0 (CH3).
Rubiadin-3-methyl ether (4): white powder, soluble in MeOH (C16H12O4, M=92); 1H NMR (DMSO, 500MHz): 7.50 (1H, s, H-4), 8.10 (1H, dd, 1.25; 7.5Hz, H-5), 7.83 (1H, dt, 1.5; 7.5Hz, H-6), 7.88 (1H, dt, 1.0; 7.5Hz, H-7), 8.15 (1H, dd, 1.0; 7.5Hz, H-8), 11.09 (1H, s, OH), 3.79 (3H, s, OCH3), 2.15 (3H, s, CH3); 13C NMR (DMSO, 125MHz): 161.5 (C-1), 117.9 (C-2), 160.6 (C-3), 109.0 (C-4), 133.7 (C-4a), 126.0 (C-5), 133.3 (C-6), 134.5 (C-7), 126.6 (C-8), 134.5 (C-8a), 180.3 (C-9), 126.1 (C-9a), 182.6 (C-10), 132.0 (C-10a), 60.6 (OCH3), 9.0 (CH3).
1-O-methylrubiadin 3-O-primeveroside (5): Yellow powder, soluble in MeOH (C26H28O14, M=564.5); 1H NMR (DMSO, 500MHz): 3.81 (s, 1-OCH3), 2.25, (s, 2-CH3 7.70 (s, H-4), 8.15 (dd, 8.0, 3.0Hz, H-5), 7.88 (m, H-6), 7.88 (m, H-7), 8.15 (dd, 8.0, 3.0Hz, H-8), 5.14 (d, 5.5Hz, H-1′), 2.98–3.97 (m, H-2′), 2.98–3.97 (m, H-3′), 2.98–3.97 (m, H-4′), 2.98–3.97 (m, H-5′), 3.95 (brd, 12.5Hz, H-6′), 4.14 (d, 7.0Hz, H-1″), 2.98–3.97 (m, H-2″), 2.98–3.97 (m, H-3″), 2.98–3.97 (m, H-4″), 2.98–3.97 (m, H-5″). 13C NMR (DMSO, 125MHz): 159.85 (C-1), 60.82 (1-OCH3), 128.90 (C-2), 9.30 (2-CH3), 160.01 (C-3), 108.25 (C-4), 132.10 (C-4a), 126.20 (C-5), 133.56 (C-6), 134.30 (C-7), 126.64 (C-8), 134.55 (C-8a), 180.55 (C-9), 120.14 (C-9a), 182.23 (C-10), 133.89 (C-10a), 100.33 (C-1′), 73.3 (C-2′), 75.67 (C-3′), 69.17 (C-4′), 76.12 (C-5′), 68.01 (C-6′), 104.02 (C-1″), 73.14 (C-2″), 76.4 (C-3″), 69.50 (C-4″), 65.60 (C-5″).
Asperulosidic acid (6): white powder, soluble in MeOH (C18H24O12, M=432); 1H NMR (CD3OD, 500MHz): 5.09 (1H, d, 9.0, H-1), 7.66 (1H, d, 1.5Hz, H-3), 3.04 (1H, 1.0; 7.0Hz, H-5), 4.82 (1H, overlap, H-6), 6.04 (1H, s, H-7), 2.66 (1H, t, 8.0Hz, H-9), 4.98 (1H, d, 15.5Hz, Ha-10), 4.81 (1H, br s, Hb-10), 2.11 (3H, s, 12-COCH3), 4.74 (1H, d, 7.5Hz, H-1′), 3.27 (1H, m, H-2′), 3.40 (1H, t, 9.0Hz, H-3′), 3.27 (2H, m, H-4′, 5′), 3.64 (1H, dd, 6.0; 12.0Hz, Ha-6′), 3.88 (1H, dd, 1.5; 12.0Hz, Hb-6′); 13C NMR (CD3OD, 125MHz): 101.3 (C-1), 155.2 (C-3), 108.1 (C-4), 42.5 (C-5), 75.4 (C-6), 131.9 (C-7), 145.9 (C-8), 46.3 (C-9), 63.8 (C-10), 169.3 (C-11), 172.5 (C-12), 20.7 (12-COCH3), 100.6 (C-1′), 74.9 (C-2′), 78.6 (C-3′), 71.6 (C-4′), 77.9 (C-5′), 63.0 (C-6′).
Aitchisonide A (7): white powder, soluble in MeOH (C19H25O12, M=446); 1H NMR (CD3OD, 500MHz): 5.09 (1H, d, 9.0Hz, H-1), 7.68 (1H, d, 1.5Hz, H-3), 3.06 (1H, dtd, 1.5; 1.5; 1.5Hz, H-5), 4.85 (1H, overlap, H-6), 6.04 (1H, d, 1.5Hz, H-7), 2.66 (1H, t, 8.0Hz, H-9), 4.96 (1H, d, 15.5Hz, Ha-10), 4.82 (1H, m, Hb-10), 3.77 (3H, s, 11-OCH3), 2.11 (3H, s, 12-COCH3), 4.74 (1H, d, 8.0Hz, H-1′), 3.27 (1H, m, H-2′), 3.40 (1H, t, 9.0Hz, H-3′), 3.27 (2H, m, H-4′, 5′), 3.64 (1H, dd, 6.0; 12.0Hz, Hb-6′), 3.88 (1H, dd, 1.5; 12.0Hz, Ha-6′); 13C NMR (CD3OD, 125MHz): 101.3 (C-1), 155.4 (C-3), 108.1 (C-4), 42.4 (C-5), 75.4 (C-6), 131.8 (C-7), 145.9 (C-8), 46.3 (C-9), 63.8 (C-10), 169.3 (C-11), 172.5 (C-12), 51.8 (OCH3), 20.7 (CH3), 100.6 (C-1′), 74.9 (C-2′), 78.6 (C-3′), 71.6 (C-4′), 77.9 (C-5′), 63.0 (C-6′).
Computational analysisDocking simulation study of compound (1–7) was performed by using flexible side chains protocol based on Iterated Local Search Global Optimizer Algorithm of AutoDock/Vina v.1.1.2.20 Ligands were docked into the PDE-5 catalytic site and employed in the model building and refinement, by taking into account the inhibitor specific steric and chemical features.21 The crystal structures of PDE-5 complexed with sildenafil, IBMX and tadalafil were downloaded from Brookhaeven Protein Data Bank (entry 1UDT, 1RKP and 1UDU) (www.rcsb.org/pdb). All hydrogen atoms and gasteiger charges in the protein structures as well as ligands were removed from original protein were added using AutoDockTools. Docking simulation of proteinstructures and built new ligand were performed using the Lamarckian Genetic Algorithm and runs 30 number. Ligand structures were prepared using ChemBioDraw Ultra 12.0 (HyperChem™ Professional 7.51, Hypercube, Inc., 1115 NW 4th Street, Gainesville, Florida 32601, USA) and the energy minimizations of the prepared ligands were carried out with Chem3D Ultra, while the pdbqt format essential for docking simulation were generated by using script of Molecular Graphics Laboratory (MGL) tools.22 The computational software was downloaded from the website http://scripps.edu, operated under Microsoft Windows 8, installed on an Intel i7 PC with a 3.2GHz processor and 8 GB RAM. The ligand molecules were docked into refined PDE-5 enzyme using AutoDock/Vina v.1.1.2.20 The generation and affinity grid maps, viewing of docking poses, and analysis of virtual screening results were done by using AutoDock plug-in of PyMOL (The PyMOL Molecular Graphics System, Version 1.7.4 Schrödinger, LLC). A grid was generated at active site, identified on the bases of already co-crystallized ligand (SLD, TLD and IBMX) to the PDE-5 receptor. The docking protocol set to be standardized for all co-crystallized ligands and isolated compounds into the PDE-5 protein structure. The AutoDock output results represented the docking scores as Gibbs free energy of binding (ΔG) values, further converted to the predicted inhibition constants (Ki,pred) and pIC50,pred based on the formula Ki=IC50/1+[S]/Km=exp(ΔG/R*T), IC50=exp(ΔG/R*T)*1+[S]/Km (IC50∼Ki) and pIC50=−lgIC50. Each amino acid residue present at the binding site was computationally mutated to alanine and the ligand interaction energy recalculated for each mutant. The interactive three-dimensional maps were constructed by using LigPlot+ bioinformatics tool.23 The Molinspiration24 and OpenTox cheminformatics server25 was exploited to predict bioactivity and evaluate the acute toxicity of all research compounds.
ResultsChemical compositions of P. memecyloidesThe methanolic extract from P. memecyloides roots was suspended with water and subsequently fractioned with solvents with different polarities: chloroform, ethyl acetate and water. Repeated chromatography on silica gel, diaion HP-20 or reversed phase RP-18 led to the yield of 5 known compounds (1–5): four anthraquinones and one anthraquinone glucoside including damnacanthal (1),26,27 lucidin-ω-methyl ether (2),27,28 3-methylalizarin (3),29 rubiadin-3-methyl ether (4),30 1-O-methylrubiadin 3-O-primeveroside (5)12 along with two iridoid glucosides asperulosidic acid (6) and aitchisonide A (7)31 (Fig. 1). The structures of all isolated compounds (1–7) were elucidated on the basis of spectroscopic data (HR-MS, 1D/2D-NMR).
Molecular simulation: the active site binding pocket of PDE-5Since P. memecyloides is widely used among ethnic minorities to manage ED, the chemical components of the plant are aimed to screen in silico their ability to inhibit the type-5 phosphodiesterase (PDE-5), which indirectly causes the ED. The binding mechanisms of the compounds to the PDE-5 enzyme were investigated using AutoDock/Vina molecular docking software, where each compound was manually docked at the active site of the PDE-5 (complexed with ligands).
Using three different commercial ligands (SLD, TLD and IBMX) attached to the PDE-5 crystals, the active binding sites of the PDE-5 were explored by that the three protein complexes were aligned to obtain their structure fitting. It was found that, a good super imposition and an identical conformation of the complex were observed with the three different ligands attached to the PDE-5 crystals (Fig. 1, Supporting Information: available in on-line supplementary material). In this way we can evaluate the docking position of the three compounds to the binding sites as well as residues that form temporary bonds with the substrate in the active binding site.
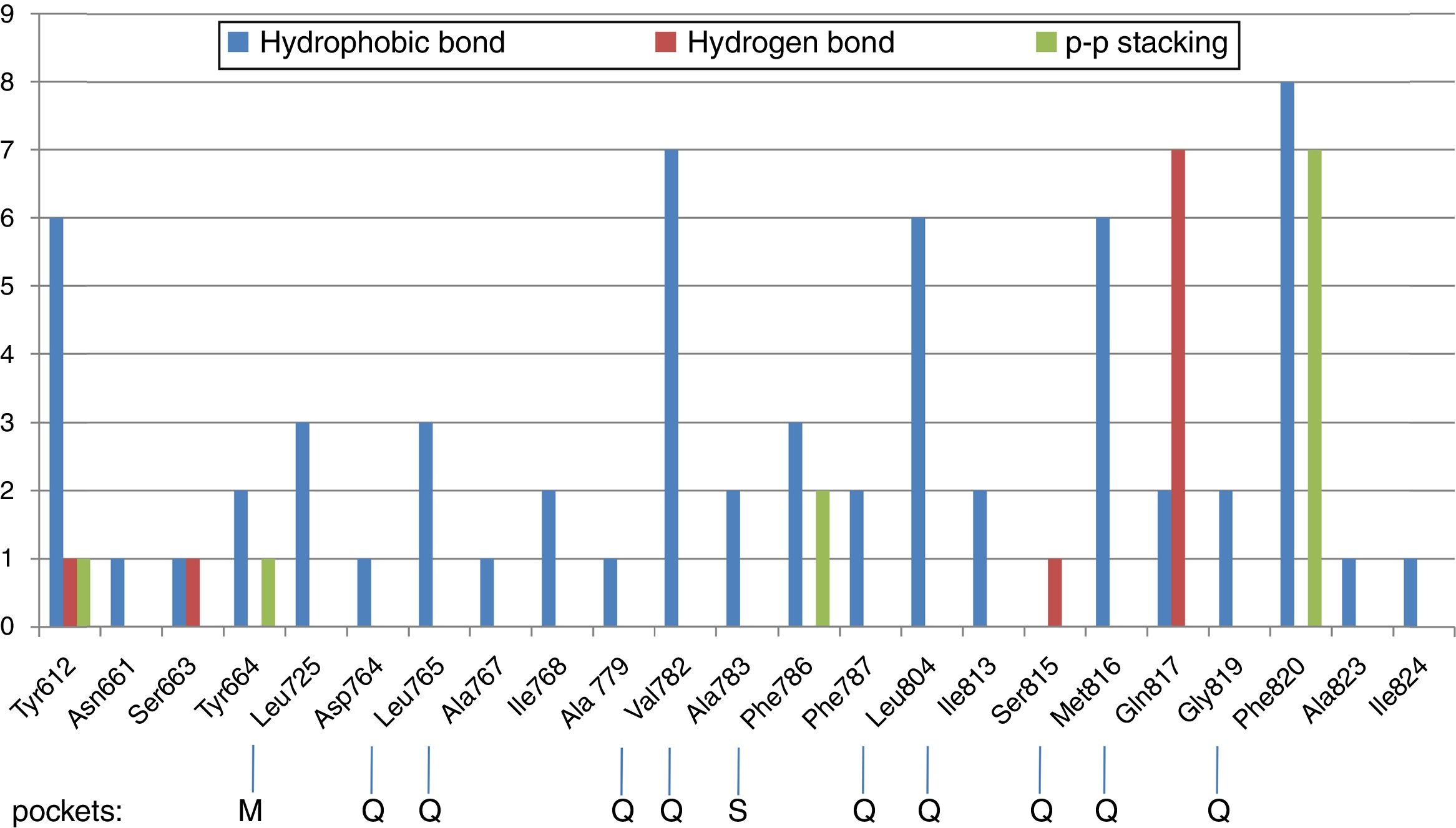
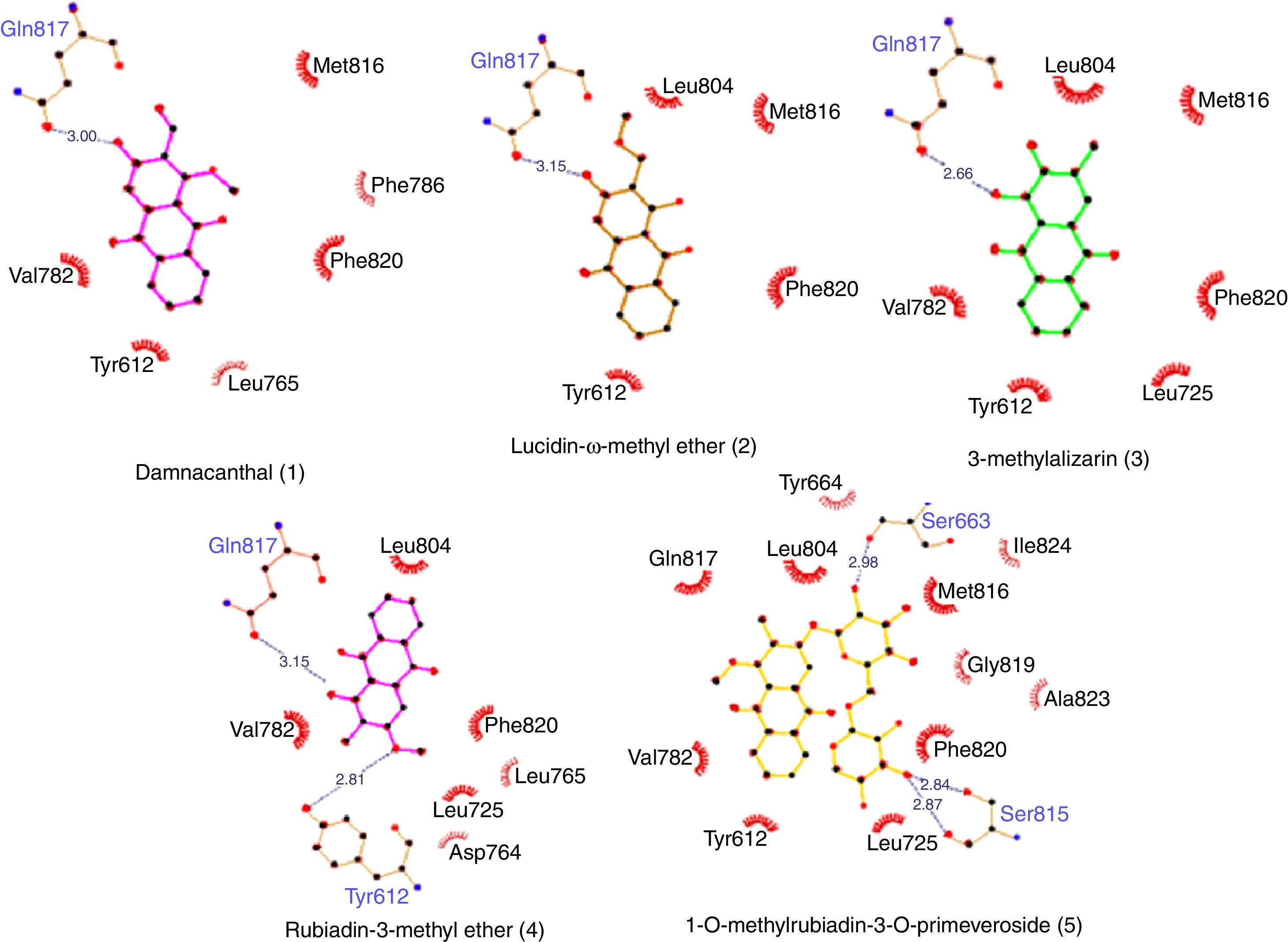
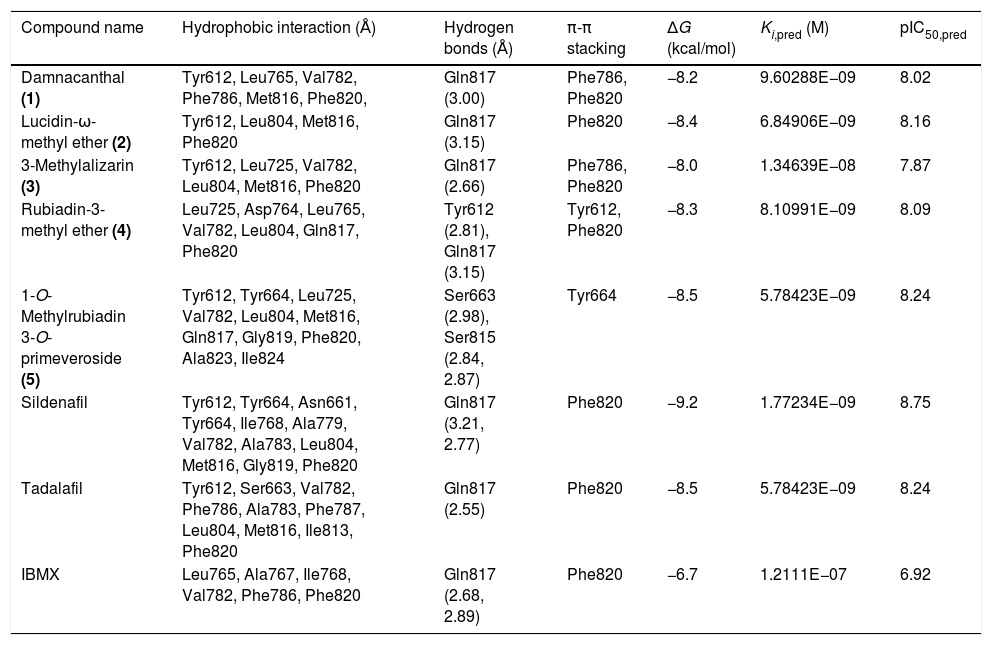
Molecular docking of isolated compounds (1–7) isolated from P. memecyloidesAll seven isolated compounds from P. memecyloides were used for docking simulation against the predicted active site using AutoDock/Vina package. A standardized docking protocol used for the three commercial compounds was also applied for all studied compounds. The docking simulation results in Table 1 highlight that all five anthraquinones/anthraquinone glucoside posed inside a catalytic region, subdivided in a metal binding (M), solvent-filled side (S) and hydrophobic (Q) pockets as suggested by Cichero et al.,21 with binding energies similarly to those of the commercial compounds. Most compounds bind to their PDE-5 by means of intermolecular bonds including hydrogen bonds, hydrophobic interactions and π-π stacking as suggested by LigPlot+ network analysis of three-dimensional maps23 (Figs. 2–5). A summary of protein–ligand interactions between aminoacidic residues and studied compounds represented in Fig. 2 and Table 1 showed that the tested and control compounds interact rarely-to-no with the highly conserved M pockets, but mostly to hydrophobic, containing a purine-selected glutamin Q-pocket. Only tadalafil and IBMX interact with S-pocket residue (Phe786) (Table 1). The ΔG binding energy values of these anthraquinones were represented in Table 1 (and Fig. 3, Supporting Information: available in on-line supplementary material). Calculated pharmacokinetic parameters and toxicity prediction of the research compounds were presented in Table 2.
Network of interactions and ΔG binding energies by Molecular Docking simulation.
| Compound name | Hydrophobic interaction (Å) | Hydrogen bonds (Å) | π-π stacking | ΔG (kcal/mol) | Ki,pred (M) | pIC50,pred |
|---|---|---|---|---|---|---|
| Damnacanthal (1) | Tyr612, Leu765, Val782, Phe786, Met816, Phe820, | Gln817 (3.00) | Phe786, Phe820 | −8.2 | 9.60288E−09 | 8.02 |
| Lucidin-ω-methyl ether (2) | Tyr612, Leu804, Met816, Phe820 | Gln817 (3.15) | Phe820 | −8.4 | 6.84906E−09 | 8.16 |
| 3-Methylalizarin (3) | Tyr612, Leu725, Val782, Leu804, Met816, Phe820 | Gln817 (2.66) | Phe786, Phe820 | −8.0 | 1.34639E−08 | 7.87 |
| Rubiadin-3-methyl ether (4) | Leu725, Asp764, Leu765, Val782, Leu804, Gln817, Phe820 | Tyr612 (2.81), Gln817 (3.15) | Tyr612, Phe820 | −8.3 | 8.10991E−09 | 8.09 |
| 1-O-Methylrubiadin 3-O-primeveroside (5) | Tyr612, Tyr664, Leu725, Val782, Leu804, Met816, Gln817, Gly819, Phe820, Ala823, Ile824 | Ser663 (2.98), Ser815 (2.84, 2.87) | Tyr664 | −8.5 | 5.78423E−09 | 8.24 |
| Sildenafil | Tyr612, Tyr664, Asn661, Tyr664, Ile768, Ala779, Val782, Ala783, Leu804, Met816, Gly819, Phe820 | Gln817 (3.21, 2.77) | Phe820 | −9.2 | 1.77234E−09 | 8.75 |
| Tadalafil | Tyr612, Ser663, Val782, Phe786, Ala783, Phe787, Leu804, Met816, Ile813, Phe820 | Gln817 (2.55) | Phe820 | −8.5 | 5.78423E−09 | 8.24 |
| IBMX | Leu765, Ala767, Ile768, Val782, Phe786, Phe820 | Gln817 (2.68, 2.89) | Phe820 | −6.7 | 1.2111E−07 | 6.92 |
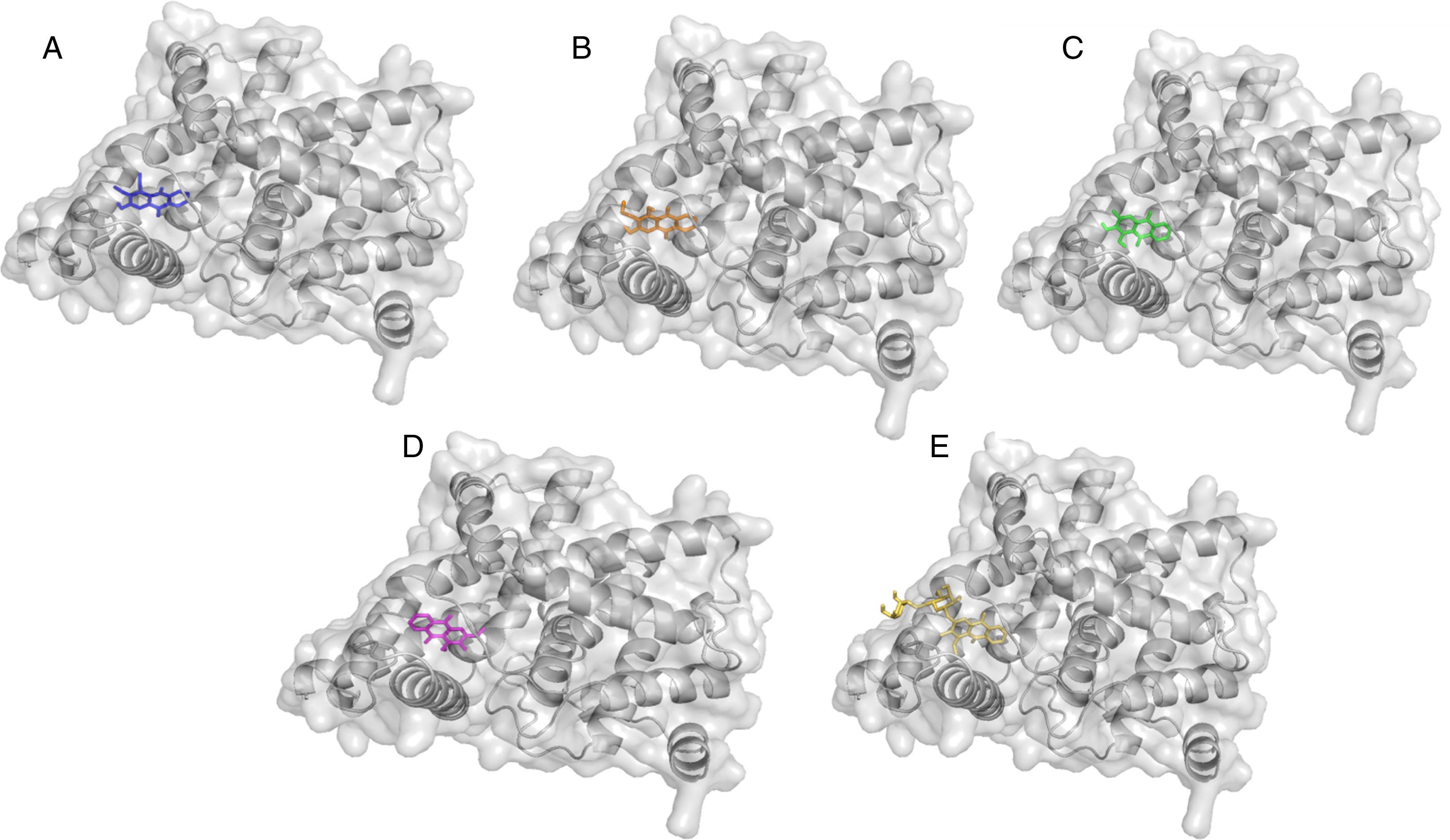
Cartoon representation of PDE5 in complex with isolated compound from Prismatomeris memecyloides in coulored sticks. (A) Damnacanthal (1) in blue; (B) Lucidin-ω-methyl ether (2) in orange; (C) 3-methylalizarin (3) in green; (D) Rubiadin-3-methyl ether (4) in magenta; (E) 1-O-methylrubiadin 3-O-primeveroside (5) in yellow.
Erectile dysfunction (ED) is one of the most prevalent male sexual dysfunction that affects anticipates increasing from 152 million in 1995 to 322 million by 20,251. Several treatment methods that consist of psychological therapy (healthy psycho and nervous system that conducts nerve impulses in the brain, spinal column, and penis), external therapy (impulse magnetic field therapy, low-intensity extracorporeal shockwave therapy (LI-ESWT), local devices (vacuum erectile devices (VEDs), prosthetic implants, penile vibrators), penile injection (intracavernosal injections (ICI)), or intraurethral suppositories, penile prosthesis), oral therapy (herbal treatment, natural drugs), as well as cellular based treatment methods including stem cells, soluble guanylate cyclase (sGC) activators, cGMP, testosterol and especially phosphodiesterase 5 inhibitors (PDE5Is) therapy.1
Herbal medicine is considered to be a natural drug resource for treatment of human health including sexual health. Several oriental herbs are usually used to enhance sexual function including Panax ginseng, Tongkat Ali, Ginkgo biloba, Schisandra chinensis, Epimedium koreanum, Lepidiummeyenii, Bombyx mori (male silkworm extract), Artemisia capillaris, Cuscuta chinensis, etc.1P. memecyloides is also a medicinal plant, widely used by ethnic minorities of Vietnam for the treatment of male erectile dysfunction. A water decoction of the whole plant (aerial part, roots and stems), in dosage of 30–50g/day and in duration of 10–50 days is usually prescribed by ethnic traditional healers to the patients, who surfer from male hypogonadism.17 The management of ED can be achieved by in silico inhibitory screening of PDE-5, an enzyme responsible for cGMP degradation in the corpus cavernosum.4 In this paper, the binding mechanisms of the compounds to the PDE-5 enzyme were investigated using AutoDock/Vina molecular docking software, where each compound was manually docked at the active site of the PDE-5 (complexed with ligands).
The active binding pockets of PDE-5 were identified on the bases of already co-crystallized commercial PDE-5Is including sildenafil (SLD), tadalafil (TLD) and IBMX posed in PDE-5. Their analogy with the isolated compounds from P. memecyloides provide initially give us the opportunity to choose the active conformation as starting structure for docking analysis. These co-crystallized compounds, which were known to bind within the active site of the enzyme, and thus preventing the hydrolysis of the cGMP substrate, were re-docked on to the PDE-5 protein. A good super-imposition and an identical conformation of the complex were observed with the three different ligands attached to the PDE-5 crystals (Fig. 1, Supporting Information: available in on-line supplementary material). Structure of PDE-5 enzyme includes two identical submits and each contains a catalytic and a regulatory domain.32 The docking results suggest that the commercial ligands SLD, TLD and IBMX intend to pose in the same active binding pockets in the catalytic domain of PDE-5 (Fig. 2, Supporting Information: available in on-line supplementary material). The binding region, obtained by docking results, is analog to the catalytic site of PDE-5 including in particular the hydrophobic clamp named Q pocket and those named M and S pockets.21 The active site pocket of the enzyme consists of some typical amino acidic residues including tyrosine (Tyr612, Tyr664), leucine (Leu725, Leu765, Leu804), valine (Val806), glutamine (Gln817), serine (Srn663), methionine (Met816), phenyl alanine (Phe820), which were similar to published literature.4,5
Further, as described in Table 1, by using LigPlot+ bioinformatic tool,23 the five anthraquinones were engaged in hydrophobic interactions, hydrogen bonds and π-π stacking involving the same aminoacidic residues as observed for commercial compounds (SLD, TLD and IBMX). A summary of aminoacidic residues that involved in hydrophobic interactions with studied compounds is presented in Fig. 2, where almost all compounds have hydrophobic interactions to Tyr612 and residues of Q-pocket: Leu765, Val782, Leu804, Met816 and Phe820 (Table 1). Hydrogen bond interactions found between PDE-5 and the ligands interacted mainly to three residues including Gln817 (glutamine Q-pocket), Ser663 and Tyr612 (Fig. 2). These findings were also fairly similar to the interactions observed between PDE-5 residues to the docking candidates from traditional Chinese plants reported by Chen.5 Only tadalafil and BMBX interact hydrophobically with S-pocket residue (Phe786) (Table 1).
Particularly interesting is that the anthraquinones (1–4) showed similar hydrogen bonds or hydrophobic interaction (5) (ranking from 2.6 to 3.15Å) to the Gln817 residue, which is reported to be one of the most active aminoacids possessed important interactions with ligands.4 The interactions between Gln817 in PDE-5 and anthraquinones were the same as those of SLD, TLD, IBMX, pyrazolopyrimidinone analogs,4 benzyl derivative NSC1699 and isochlorogenic acid b from TCM database.5 However, the bindings of anthraquinones (1–4) to Gln817 residue were only single hydrogen bonds while those of SLD and pyrazolopyrimidinone analogs were observed as bidentate interactions with larger H-bonds in SLD (3.21 and 2.77Å) (Table 1) and smaller H-bonds (1.6Å and 1.97Å) in pyrazolopyrimidinone group.4
Furthermore, all five anthraquinones are always involved in a π-π stacking with aromatic amino acidic residues such as Phe820, Phe786 and Tyr612, similarly to commercial compounds and pyrazolopyrimidinone analogs4 (Table 1, Fig. 2). All these interacting residues together exerted hydrophobic interactions/hydrogen bonds/and π-π stacking and stabilized the protein–ligand complex, characterized by its free binding energy (ΔG).
Thus, taking into accounts all these analyses showed that, the PDE-5 Q pocket could probably be occupied by bulky groups substituted in the inhibitor compounds. And proved to Cichero et al.,21 we found that by means of proper H-bond contacts, a good selectivity level could be achieved using the PDE-5 residues, especially those of Q-pocket, as an anchoring point for the inhibitor recognition.
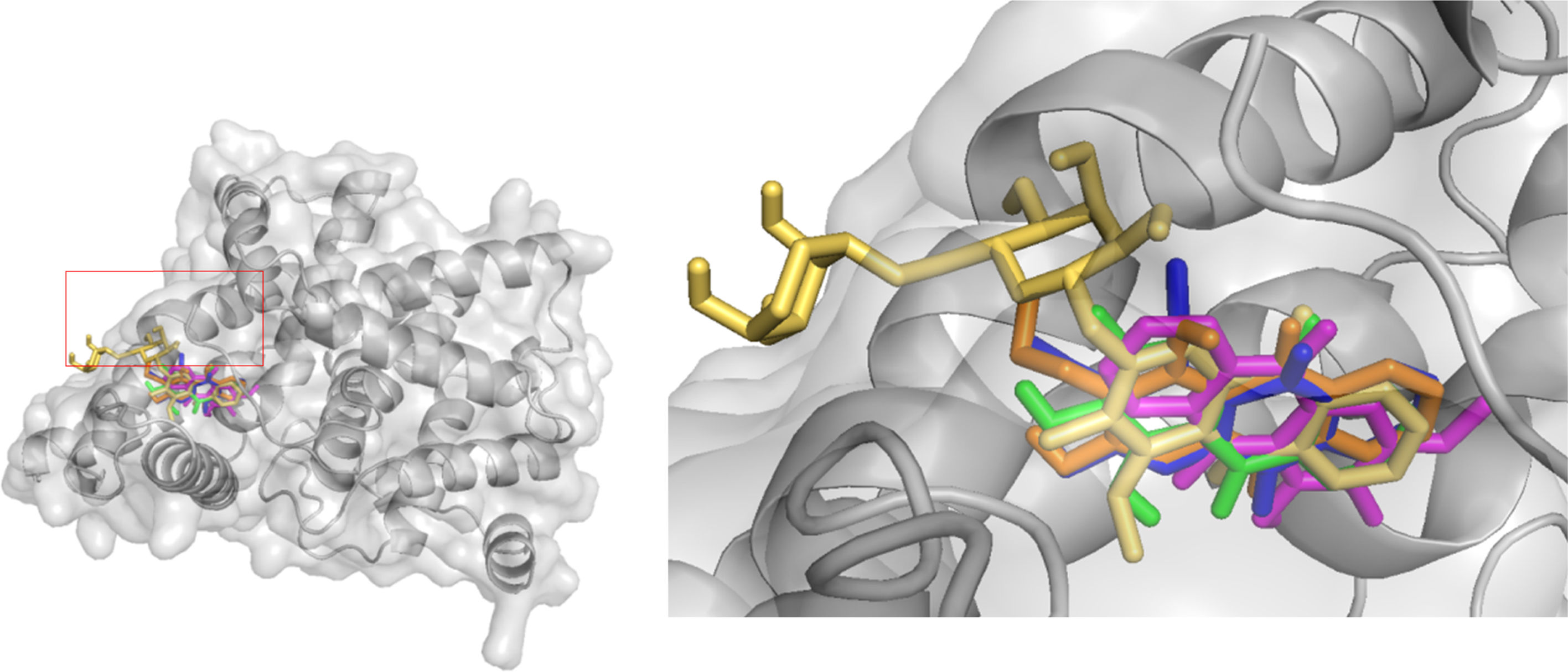
The AutoDock output results represented the docking scores as Gibbs free binding energy were shown in Table 2 (Fig. 2, Supporting Information: available in on-line supplementary material), where during the docking process, the protein was considered to be rigid while the ligands and those aminoacids inside the pocket were flexible. All tested anthraquinones (1–5) were found to have excellent binding affinity to the enzyme, showing binding energies as ΔG values in range of −8.0 to −8.5kcal/mol, which were very similar to those values of sildenafil (−9.2kcal/mol) and tadalafil (−8.5kcal/mol) but higher than those of pyridopurinone analogs (ranging from −12.1 to −17.4kcal/mol).6 The small variance in ΔG values and poses inside the pocket may be attributed due to the differences in the position of the functional groups in the selected compounds (Fig. 5). The more negative ΔG is, the more likely binding will take place. The negative values of ΔG indicate that the anthraquinones could bind to PDE-5 spontaneously and these values also proved that the compounds possess potential PDE-5 enzymatic inhibitory binding sites. The three compounds occupy the same cavity (Fig. 5) with only few differences in aminoacid residues involvement because of their dihedral rotation and conformational mismatch. The ΔG values were further converted to the predicted inhibition constants (pKi,pred) and pIC50,pred (Table 1). By comparing the Ki values inserted in Table 1, we can observe the same order of inhibitory magnitude of compounds (1, 2, 4 and 5) with SLD and TLD. Comparison of pIC50 values suggests increasing pIC50 in correspondence to increasing PDE-5 inhibitory ability of commercial and studied compounds as SLD>TLD∼1-O-methylrubiadin 3-O-primeveroside (5)>lucidin-ω-methyl ether (2)>rubiadin-3-methyl ether (4)>damnacanthal (1)>3-methylalizarin (3)>IBMX. These pIC50 values of studied compounds were also similar to those of several natural compounds from TCM database.5 All these findings support our assumption that the natural anthraquinone compounds and especially the anthraquinone glycoside were potential capability of acting in a similar way of commercial products.
Among those studied anthraquinones (1–5), we observe that probably the presence of glucoside moieties in compound (5) might be significant contribution to the PDE-5 inhibitory effect by only hydrophobic interactions because of its inability to perform H-bond with the conserved key glutamine. Anthraquinone glucoside (5) had also the lowest binding energy (−8.5kcal/mol) and the highest inhibitory activity due to additional hydrophobic interactions to Ala823 and Ile824. It was also observed that the –OH group of sugar moiety in compound (5) involved in interactions with Ser815 residue (2.84, 2.87) and Ser663 (2.98) (Fig. 3). The compound can be explained by a pharmacophore model,9,33 which contains four chemical features: a polycyclic aromatic ring (A), two hydrophobes (BH) (carbonyl groups), hydrogen acceptors (HA) (carbonyl groups, double bonds) and hydrogen donors (HD) (sugar OH, OCH3 groups) (Fig. 4). A closer look at Fig. 5 also revealed that the carbonyl groups, the polycyclic aromatic ring and the sugar OH groups in anthraquinone structures likely playing very important role for their inhibitory activity to PDE-5.
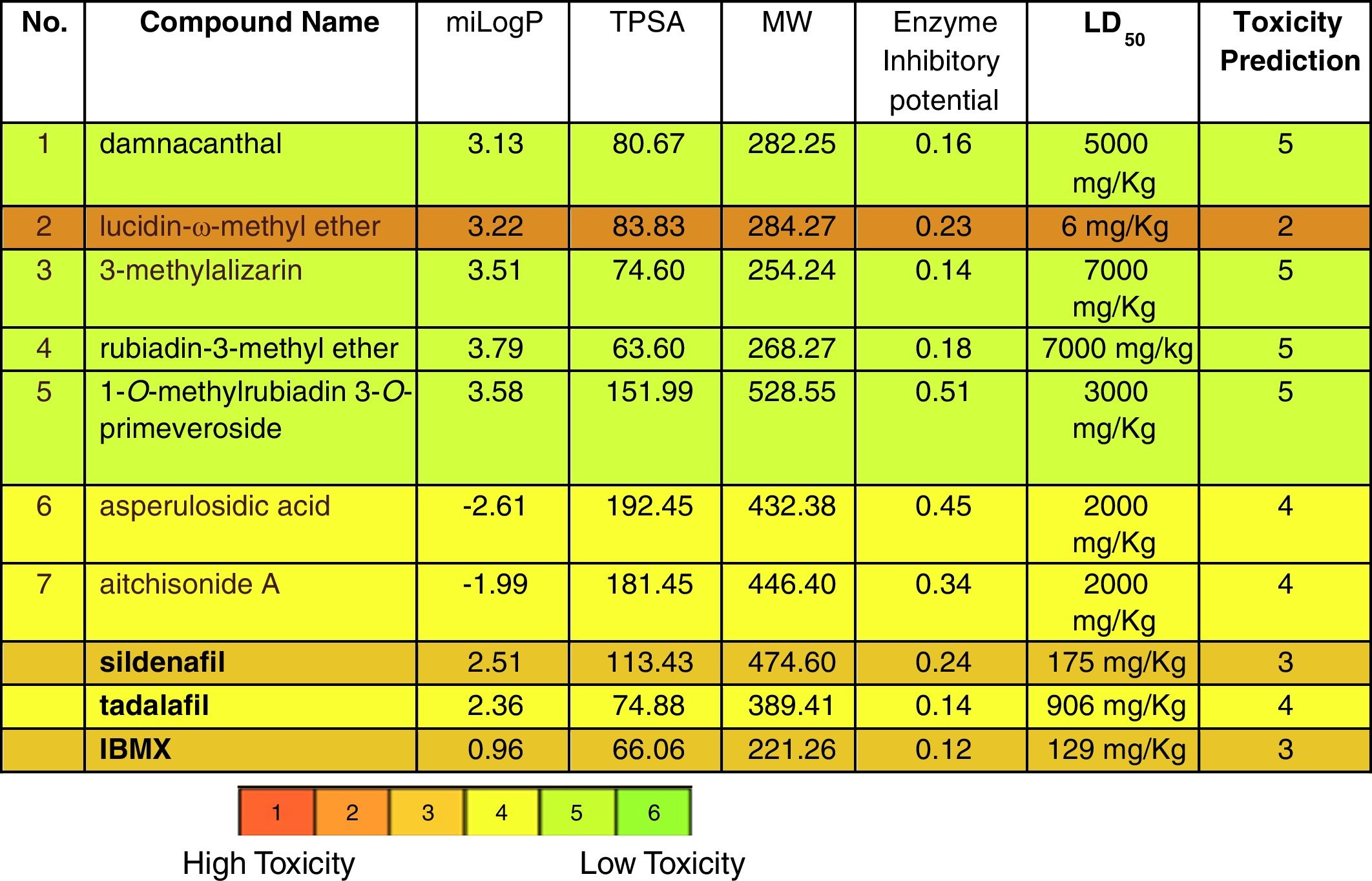
Docking analysis could prove the capability of P. memecyloides as traditional remedy for erectile dysfunction. In fact the computational results reveal as five of the seven isolated compounds from P. memecyloides exhibit similar binding energies in the same binding region. The bioactivities of five anthraquinone compounds were predicted using Molinspiration and OpenTox predictive tool (Table 2). The calculated docking results and pharmacokinetic parameters such as miLogP (octanol–water partition coefficient), TPSA (total molecular polar surface area), molecular weight and enzyme inhibitory potential. Although the TPSA of two compounds (6–7) were very high (192.45 and 181.45, respectively), their miLogP fall to minus values bring these compounds inability to bind to PDE-5 protein. The calculated properties and bioactivities of anthraquinones compounds (1–5) were comparable to the commercial PDE-5 inhibitors (SLD, TLD and IBMX). The calculated enzyme inhibitory potential of anthraquinone glycoside (5) was highest (0.51) and much higher than that of SLD (0.24) (Table 2). The calculated results are very interesting especially by consideration of the toxicity scale of studied compounds. In Table 2 we can observe how the anthraquinones isolated from P. memecyloides have a toxicity prediction lower than the three commercial available drugs. The highest toxicity with the lowest LD50 value was belonging to compound (3) (6mg/kg). Compounds (1), (3) and (4) with very high LD50 values as 5000, 7000 and 7000mg/kg, respectively were classified as non-toxic compounds. Anthraquinone glucoside (5) with LD50 value of 2000mg/kg was classified as low-toxic compound but was safer than SLD, TLD and IBMX with LD50 values of 175, 906 and 129mg/kg, respectively. All these results suggest the potential utilization of anthraquinones especially anthraquinone glycoside (5) for the treatment of erectile dysfunction.
The anthraquinones have similar structures as natural steroid hormones and might have function like these compounds.
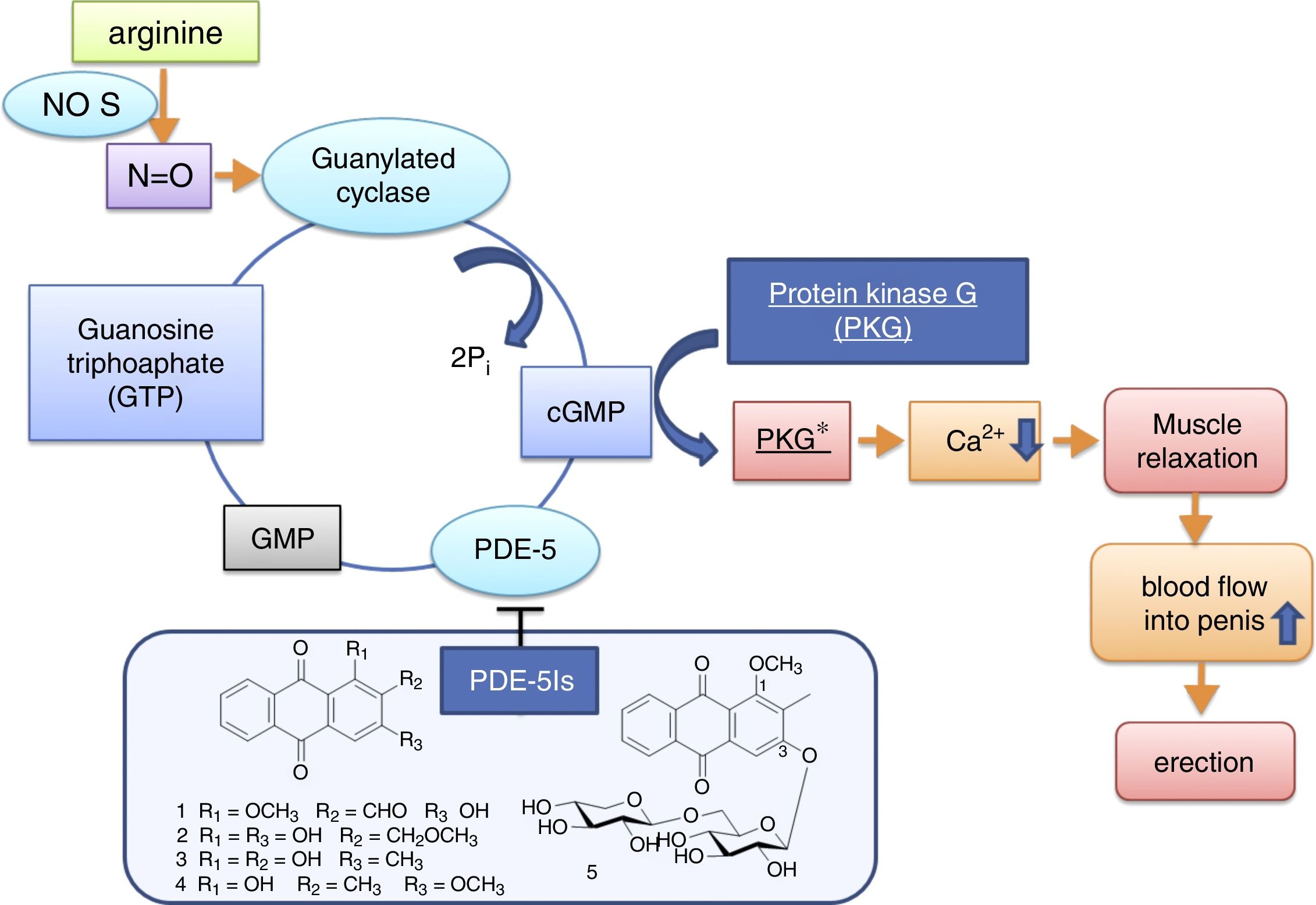
Phosphodiesterases 5 (PDE-5) belonging to the PDEs family is specified to hydrolyze phosphodiester bond at the 3′-position of cyclic GMP (cGMP) into their respective 5′-nucleotide monophosphate GMP. cGMP is functioned to initiate several reactions in the body including the initiation of protein kinase G (PKG), that phosphorylate various substrates responsible for regulation of many physiological processes particularly the activity of calcium channels as well as intracellular contractile proteins that affect the relaxation of smooth muscle.33 By inhibition of PDE-5 enzyme, the cGMP concentration will be increased, leading to the activation of protein phosphorylation cascades in the actin-smooth muscle myosin system. It causes a decrease in intracellular calcium within muscle cells and results in smooth muscle relaxation in penis, increased blood inflow and erection. So the possible molecular pathways that anthraquinone control PDE-5 is theoretically represented in Fig. 6.
ConclusionBy computational molecular simulation study, 4 anthraquinones and one anthraquinone glycoside along with two iridoid glycosides isolated from P. memecyloides, a medicinal plant for ED treatment, were explored to possess inhibitory effect to PDE-5. The results showed that the anthraquinones (damnacanthal (1), lucidin-ω-methyl ether (2), 3-methylalizarin (3) and rubiadin-3-methyl ether (4)), especially anthraquinone glycoside (1-O-methylrubiadin 3-O-primeveroside (5)) are a potential new class of compounds for ED treatment. Anthraquinones glycosides have a simpler structure than SLD but their mechanism of action and their calculated bioactivities such as Ki,pred, pIC50,pred, miLogP, TPSA and enzyme inhibitory potential were very similar to those of SLD. They have even lower predictive toxicity in comparison to SLD and TLD. These findings would be valuable for further study in order to develop a new ED treatment drug class, which based on anthraquinone leading structures. Further, pre- and clinical studies are required to control, approve and validate the procedure and potential restoration of the erectile normal function.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflict of interestThere is no conflict of interest in relation to the publication of this paper.
This study was financially supported by the Vietnam Academy of Science and Technology, Vietnam (VAST.ĐLT.03/15-16).