The AJCC/UICC staging system is a major tool in oncology, currently used worldwide for clinical, pathological and recurrent disease staging. Squamous cell carcinomas (SCC) of the head and neck are a heterogeneous disease. The TNM classification offers a reliable method for estimating the prognosis of patients with cancer based on certain characteristics of the tumor. It is also used in planning treatment, and has helped to standardize the way cancer is staged and treatment results are reported around the world. Although the original TNM system was based solely on the anatomic extent of the tumor, other non-anatomic parameters have found their way into the staging paradigm. The objective of this communication is to present the characteristics of the TNM staging system and review the current modification in the 8th version of the head and neck cancer staging. The objective of this article is to describe the characteristics of the TNM staging system and review the changes made to head and neck cancer staging in the most recent (8th) edition.
El sistema de etapificación de la AJCC/UICC es una herramienta de gran utilidad en oncología y es actualmente utilizado en todo el mundo para la etapificación clínica, patológica y de la enfermedad recurrente. El cáncer de células escamosas de la cabeza y el cuello es una enfermedad heterogénea. La clasificación de TNM ofrece un método confiable para estimar el pronóstico de pacientes con cáncer basado en ciertas características del tumor. Sin embargo, también se utiliza en la planificación y selección de tratamiento y ha contribuido a estandarizar la forma de cáncer se etapifica y los resultados del tratamiento son registrados y reportados alrededor del mundo. Aunque el sistema TNM se basó inicialmente únicamente en la extensión anatómica del tumor, otros parámetros no anatómicos han ido encontrado su lugar en este sistema de etapificación. El objetivo de esta comunicación es describir las características del sistema de etapificación y revisar la actual modificación en la 8va versión de la etapificación del cáncer de cabeza y cuello.
The AJCC/UICC staging system is a major tool in oncology, currently used worldwide to describe the extent of disease at presentation prior to treatment, (clinical staging, cTNM) after surgical treatment pathological staging, pTNM and at disease recurrence (rTNM). Thus, TNM staging helps in planning treatment and assessing prognosis. Assessing prognosis in cancer remains its strongest use. The prediction of outcomes of oncologic treatment and prognosis is a fundamental need in the minds of patients and physicians alike. William Halsted, the father of surgical oncology, recognized that cancer progression follows an orderly stepwise process beginning from primary tumor formation to distant metastasis and passing through regional lymph nodes1. In 1905, in Germany, Steinthal made the first attempt to clinically stage breast cancer based on Halsted's theory2. This simple concept of stepwise tumor progression was widely used in the first part of the twentieth century and has essentially shaped the way we view and comprehend the behavior of a complex disease. It has also influenced the way we diagnose cancer, treat it and predict its course. The first systematic approach to stage cancer in a consistent way was done at Institut Gustave Roussy by Pierre Denoix. Between 1942 and 1952, Dr. Denoix developed a system to stage solid tumors based mainly on three anatomic characteristics: tumor (T), lymph node spread (N) and distant metastasis (M)3. In 1953, a Special Committee on Clinical Stage Classification was established by the International Union Against Cancer (UICC) under the leadership of Dr. Denoix4. In 1959, the American Joint Committee on Cancer (AJCC) was established to “formulate and publish systems of classification of cancer, including staging and end results reporting, which will be acceptable to and used by the medical profession for selecting the most effective treatment, determining prognosis, and continuing evaluation of cancer control measures”5. After an initial course that was independent of each other and often contradictory, both organizations have worked in collaboration through the publication of the UICC-AJCC TNM classification since 1987, and this has helped to standardize the way cancer is staged and results of treatment are reported around the world6.
The TNM classification offers a reliable method for estimating the prognosis of patients with cancer based on certain anatomic characteristics of the tumor. Over the last 60 years, the TNM classification has been widely used to plan treatment, summarize prognostic information, evaluate treatment results and compare outcomes between institutions around the world7. Although the main T, N and M categories remain almost unchanged since their initial conception, the staging system has been periodically fine-tuned to incorporate newer anatomical prognostic factors. Thus, prior editions included more detailed T4 categories for head and neck cancers; the introduction of sentinel lymph node and isolated tumor cells in the N categories of breast cancer8,9; and the depth of invasion, ulceration, mitotic rate as major T determinants in malignant melanoma10. Although the original TNM system was based solely on the anatomic extent of the tumor, other non-anatomic parameters related to both the tumor and the host have found their way into the staging paradigm on a periodic basis. Examples of this trend include the incorporation of age and histology into thyroid cancer staging in 198311; histological grade into soft tissue sarcoma and bone tumor staging in 198812; and serum markers in testicular cancer, gestational trophoblastic tumors and prostate cancer in 199713,14.
The TNM system is the most widely accepted prognostic system currently used in clinical practice around the world because of its relative simple design and user-friendliness. Despite this popularity, criticism about the slow adoption of changes into the TNM staging system is common. It has been called anachronistic, and its use as the gold standard of cancer staging is accepted as “good enough” rather than optimal15. Many disadvantages have been highlighted: lack of predictive power, lack of balance and differentiation between groups, failure to account for other tumor and host factors. The majority of this criticism is predicated by the enormous amount of new prognostic information now available to clinicians from radiographic imaging, histopathological examination, immunohistochemical studies, molecular analysis, in addition to patient factors such as comorbidity and lifestyle (tobacco, alcohol, Human papillomavirus (HPV) status, HIV status, Epstein-Barr Virus (EBV) status, etc.). The design of each new edition unfortunately does not allow for the easy addition of new variables without compromising its biggest advantage: simplicity and user-friendliness. The challenge facing us now is to find a solution which can strike a balance between complexity and user-friendliness16.
As of 2018, eight editions of the cancer staging manual have been published, with each new edition improving over its predecessor and demonstrating better prognostic accuracy and predictability. The current edition (8th) includes uniform staging criteria for each head and neck cancer site accepted by the AJCC and UICC (Figure 1), with major changes introduced for oropharynx, oral cavity and thyroid cancer. The objective of this article is to review some of the fundamental characteristics and the process of development of the TNM system and offer insight into the new staging system changes.
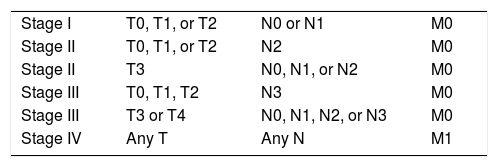
THE TNM STAGING SYSTEMThe TNM system is based on the assumption that cancers grow first at the site of the primary tumor, and then spread in a predictable, progressive fashion to regional lymph nodes and, finally, metastasize to distant sites in the body. Based on these three broad categories, the TNM system uses compartments or “bins” to segregate patients with cancer into distinct categories that predict outcome. The T category (Tis, T1, T2, T3, and T4), N category (N0, N1, N2, and N3) and the M category (M0 and M1) are then combined into different “bins”. For most tumors, excluding the M1 category, this amounts to around 20 bins (T=5 X N=4). Each of these bins is then assigned to one of four stage groups which reflect progressively advanced extent of disease, and therefore worsening prognosis. In recognition of the distinct implications of locoregional advanced diseased versus distant metastases, recent iterations of the system have stratified the advanced stage IV grouping into stage IVA (locoregionally advanced (surgically resectable) disease), stage IVB (locoregionally very advanced (surgically unresectable) disease), and stage IVC (advanced distant metastatic disease)17. Any individual patient can be slotted into a unique “bin” and stage, and therefore every patient should have representation in the bin model18.
ADVANTAGES OF THE TNM SYSTEMThe major advantage of the TNM system is its simple design, which requires only very basic information about the tumor and its spread. This information can generally be derived from clinical examination and radiographic imaging studies. The data points used for staging can be extracted from patient records even by personnel with minimal training, and this user-friendliness has been pivotal in its worldwide popularity. Ironically, the simplicity of the system is at once its main advantage and its greatest limitation.
LIMITATIONS OF THE TNM SYSTEMThe main drawback of the TNM system is its inability to easily adapt to advances in our understanding of cancer biology and incorporate new prognostic variables as they become available. The rigid “bin” configuration means that any attempts to include new variables or categories would exponentially increase the number of bins, multiply the stages, and make the system unmanageable. This would detract from its very core advantage of simplicity and user-friendliness. Any increase in the number of bins would also require enough number of patients in each bin in order to maintain the predictive value of the system. The addition of new variables would generate changes in the system, causing upward or downward “stage migration” and requiring a complete redefinition of the staging system18,19. For these mechanistic reasons, changing the current form of the TNM system has not been deemed feasible.
The main endpoint used by the TNM staging system is overall survival. However, the inclusion of other endpoints, such as disease-specific survival and local or regional control, are more relevant to the assessment of treatment results and differences among therapeutic options16. Although the TNM system remains a very good tool for estimating prognosis at diagnosis and immediately after the initial treatment of cancer, one of its most important flaws is its inability to use subsequent events in the patient's course to recalibrate prognosis during follow-up. Thus, each patient has an initial TNM stage assigned at diagnosis, and the system is unable to take into account the influence of freedom of recurrence on outcome. Therefore, the current TNM system is “static”, and not “dynamic”, representing the “current state of disease” at any given point during the natural history of the disease in a given patient.
However, before we consider changing a system that has served us so well for several decades, it would be useful to examine the characteristics of an ideal staging system.
CHARACTERISTICS OF AN IDEAL STAGING SYSTEMThe characteristics of an efficient and effective staging system have been defined by Groome et al20 as one which considers:
- 1.
Hazard consistency: or the ability to have internal homogeneity within each group with similar survival for all individual patients included in the group.
- 2.
Hazard discrimination: groups should be different in composition and have distinct prognosis.
- 3.
Balance: each group and stage should have a similar number of patients.
- 4.
Predictive power: the ability to predict an outcome of interest (overall survival, disease-specific survival, etc.).
It is easy to see how the addition of prognostic factors to the current TNM system would make it increasingly difficult to maintain homogeneity within each group and at the same time maintain hazard discrimination across groups. The central challenge in improving the staging paradigm is to understand this basic interaction between hazard consistency and hazard discrimination which maintains balance between groups and yet has optimal predictive power.
Besides these discrimination goals, complexity and compliance are two crucial issues that any staging system must address. A highly accurate predictive system, including all the possible factors to impact on outcomes, might be too complex to be practical, affecting its user-friendliness and decreasing its compliance between users. Thus, an ideal system must be complex enough to be predictive, but ensuring maximum compliance21.
ONGOING CHANGES IN THE TNM STAGING SYSTEMContrary to the relative stability in the structure and design of the TNM system, several new prognostic factors have come into common clinical use over the past several decades. The vast majority of these factors have not made their way into the staging system because of the limitations described above, but also because most of these variables do not predict outcome “independently” in multivariate models of prognostication. The potential prognostic categories that may be considered for inclusion in a revised staging system are summarized below.
Host-related factors: Demographic factors such as age, sex, race, familial history of cancer, socioeconomic status, lifestyle and habits (tobacco, alcohol and substance abuse) are only a few of the many factors that impact outcomes of treatment22–25. Clinicians regularly use this information in the decision-making process for the treatment of patients with cancer. However, with the exception of age, is used in the TNM staging system for thyroid cancer, none of the other host characteristics have representation in the current staging paradigm. Medical comorbidity is also a significant determinant of outcome, especially overall survival. Patients with significant medical problems may not be as capable of tolerating treatment as healthier patients may be. Comparing outcomes among patients with head and neck cancer without accounting for the influence of medical comorbidity therefore introduces significant bias in reporting. Another host-related characteristic that has come into prominence recently is the etiologic role of viruses in cancer pathogenesis (e.g., HPV, HPV for oropharyngeal cancer or EBV for nasopharyngeal cancer)26,27. HPV-related oropharyngeal cancer is now recognized to behave differently from its more traditional tobacco- and alcohol-related counterpart.
Histopathological variables: Many features of the tumor, such as gross characteristics volume, depth of invasion or thickness, histological subtype, microscopic pattern of invasion (invasive front, pushing border or invasive islands), lymphovascular and/or perineural invasion, and histological differentiation grade are prognostic factors that have been extensively analyzed but have not been included previously in the TNM staging system28–30. The impact of lymph node metastases on prognosis in patients with head and neck cancer is well recognized31. The TNM system accounts for the metastatic burden in lymph nodes by stratifying N stage using the size, number and laterality of involved nodes. The influence of other features of metastatic lymph nodes such as extranodal extension (ENE) has been finally considered for inclusion into the current staging system.
Biological and Molecular Markers:With advances in the understanding of cancer biology in general, and head and neck cancer in particular, we are now faced with a flood of information on molecular and genetic predictors of prognosis. Some molecular markers such as the epidermal growth factor (EGFR) have been shown to have prognostic influence in certain situations such as laryngeal cancer32,33. We now also have an improved understanding of etiopathology of some cancers; HPV infection in oropharyngeal cancer and EBV infection in nasopharyngeal cancer are two prime examples. HPV infection is now considered the major etiologic determinant of patients with oropharyngeal cancer, and specific treatment paradigms are being considered based on the HPV status of oropharyngeal cancers27, as patients who are HPV-positive have a significantly better prognosis than HPV-negative patients34.
MODIFICATIONS IN THE CURRENT TNM STAGING SYSTEMSeveral proposals have been published over the last 20 years to change the current TNM staging system for head and neck cancer. With each new edition, the staging system has evolved with the addition of new prognostic factors to the fundamental TNM system. The process of review and consideration of new information to be incorporated in the revision of the staging system starts 2-3 years prior to the final publication of the next edition. Task forces for several tumor sites made up from a multidisciplinary and comprehensive group of specialists from AJCC and UICC are in charge reviewing new published information which might have a potential impact on the staging of tumor. The collected data are validated using large databases from the National Cancer Database (NCDB) or Surveillance, Epidemiology, and End Results (SEER) Surveillance, Epidemiology, and End Results (SEER) Program in the United States. After a long process of revision of the system, the eighth edition of the AJCC-UICC staging manual was published in October 2016. The implementation of these criteria for staging began on January 1, 2018. This edition has incorporated more substantial and significant changes than have several previous editions. A detailed description of the new changes to the staging system for head and neck sites and its supporting data was published by Lydiatt et al in 201735. There are major changes with in the staging of oral cavity cancer, oropharynx cancer, and unknown primary cancers of head and neck. Significant changes in the staging system for non-melanoma skin cancer, nasopharynx cancer, and thyroid cancer.
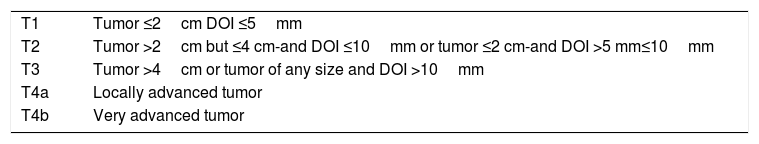
Oral cavity cancer:The most important and significant changes in oral cavity are the incorporation of pathologic features such as depth of invasion (DOI) in T staging of primary tumors of the oral cavity and the addition of extranodal extension (ENE) in N staging of cervical lymph node metastasis. Traditionally, T staging category was based on the surface dimensions of tumors. Significant information on the prognostic implication of the third dimension on the risk of occult metastases and disease-specific survival has resulted in the inclusion of depth of invasion in T staging of primary tumors36. Each 5-mm increase in depth of invasion upstages the tumor by one T stage (Table 1). Assessment using bimanual palpation is acceptale in assigning the primary tumor as thin (<5mm), thick (5–10mm), and very thick (>10mm) for clinical staging purposes.
Oropharynx Cancer:A rising incidence of oropharynx (OPC) cancers associated with HPV has been reported in the last 20 years. Currently, in most developed countries, it has the fastest rising incidence among all mucosal head and neck cancers34. HPV-related oropharyngeal cancer is a completely different disease from its traditional counterpart (tobacco-induced OPC), with a unique biological behavior. This has required that a separate staging system be developed for HPV-related cancer. HPV-related oropharynx cancers occur mostly in non-smoking, healthy young adults, show very good response to therapy and have excellent outcomes, even with advanced-stage disease. The T staging of HPV-related oropharynx cancers remains the same as in HPV-negative cancers. But, the N staging of HPV-related oropharynx cancer has changed, since several studies have shown that the volume and extent of nodal metastases in these tumors do not have the same negative impact on outcome. The new nodal staging for HPV-related OPC is shown in Table 2. Specifically, stage IV is now assigned to only those patients with distant metastases (Table 3).
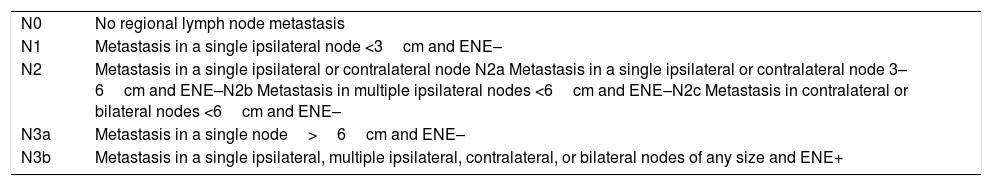
N Staging:Long-standing neck staging system was based on the size, number, and laterality of nodal metastases for assigning N stage. Increasing information on the extent of metastatic disease expressed by extranodal extension (ENE) led to its incorporation in the revision to the N staging for metastatic carcinoma37,38. This is so significant, that assigning a completely new category (N3b) to nodes with ENE was felt appropriate. The presence of gross ENE upstages the neck status to N3b (Table 4).
N staging for cervical lymph node metastases.
| N0 | No regional lymph node metastasis |
| N1 | Metastasis in a single ipsilateral node <3cm and ENE– |
| N2 | Metastasis in a single ipsilateral or contralateral node N2a Metastasis in a single ipsilateral or contralateral node 3–6cm and ENE–N2b Metastasis in multiple ipsilateral nodes <6cm and ENE–N2c Metastasis in contralateral or bilateral nodes <6cm and ENE– |
| N3a | Metastasis in a single node>6cm and ENE– |
| N3b | Metastasis in a single ipsilateral, multiple ipsilateral, contralateral, or bilateral nodes of any size and ENE+ |
Differentiated Carcinoma of the Thyroid Gland:There are significant changes in the staging of thyroid cancer- in age at diagnosis, T staging, and N staging. After several cohort studies showed that age 55 was a better discriminator of outcomes than 45, age 55 will now be used to risk-stratify patients between low- and high-risk groups. Microscopic extrathyroid extension will no longer affect T staging, after minor/microscopic extrathyroid extension (ETE) of primary tumors of the thyroid has shown no impact on local regional recurrence or disease-specific survival. The new T stages are shown in Table 5. Nodal metastases staging changes include histological confirmation and distribution of nodal metastases (Table 6). These changes in the staging of thyroid cancer result in a more accurate separation of survival curves with better discrimination39.
T staging for differentiated cancer of the thyroid.
| T1 | Tumor <2cm limited to the thyroid |
| T2 | Tumor 2–4cm limited to the thyroid |
| T3a | Tumor >4cm limited to the thyroid gland |
| T3b | Tumor of any size with gross ETE involving the strap muscles |
| T4a | Gross ETE involving the larynx, trachea, esophagus, recurrent nerve, or soft tissues |
| T4b | Gross ETE encasing the carotid artery, mediastinal vessels, or prevertebral fascia |
N staging for differentiated cancer of the thyroid.
| N0 | One or more cytologically or histologically confirmed benign lymph nodes |
| N1a | Metastases to level VI or VII lymph nodes (pretracheal, paratracheal, prelaryngeal/Delphian, or upper mediastinal) |
| N1b | Metastases to unilateral, bilateral, or contralateral lateral neck lymph nodes (levels I, II, III, IV, or V), or retropharyngeal lymph nodes |
Nasopharynx:New revisions to the T staging of primary tumors include clarification and expansion of the definition of each T stage, as is shown in Table 7.
T staging for cancer of the nasopharynx.
| T0 | No primary tumor but EBV+neck nodes |
|---|---|
| T1 | Tumor confined to nasopharynx or extended to the oropharynx, nasal cavity without parapharyngeal space invasion |
| T2 | Tumor with extension to parapharyngeal space and/or adjacent soft tissue involvement (pterygoids, prevertebral muscle) |
| T3 | Tumor with infiltration of the bony skull base, cervical vertebrae, pterygoid plates, or paranasal sinuses |
| T4 | Tumor with intracranial extension, invasion of cranial nerves, hypopharynx, orbit, parotid, or extensive soft tissue disease lateral to the lateral pterygoid muscle |
Non-Melanoma Skin Cancer:The main change is the addition of perineural invasion in T staging of primary skin cancer. The presence of perineural invasion upstages the tumor to T3 regardless of the tumor size. Thus, the new definition for T3 is tumors 4cm in diameter or larger, or with minor bone erosion, or with perineural invasion, or deep invasion >6mm, as is shown in Table 8.
FUTURE OF CANCER STAGING:BEYOND CURRENT TNM STAGINGThe ultimate goal of future staging systems is to develop a dynamic, personalized and accurate prognostic tool based on multiple tumor treatment (response to therapy) and host variables. Nomograms have emerged in the last 10 years as a potential alternative to current prognostication modeling, and have been extensively used and tested. A nomogram denotes the simple graphical representation or calculation device that is based on a statistical prediction model. This is a numeric probability of the occurrence of a clinical event based on a logistic or progression hazard analysis (Cox regression)40
Nomograms have been widely tested in a variety of cancers, with more than 1000 publications in the literature. Most have been developed for use in prostate cancer, and have focused on various applications such as predicting the probability of cancer in a biopsy, estimating prognosis before and after treatment, or predicting the risk of locoregional recurrence41. Nomograms have also been widely used in prognostic prediction in sarcoma, melanoma, and pancreatic cancer. Nomograms have been increasingly used for head and neck tumors to predict prognosis and adjuvant treatment decision-making in oral cancer42,43, to predict risk in thyroid cancer44, to select of treatment in patients with advanced-stage head and neck carcinoma based on factors such as tumor volume and other host variables45, and to predict overall survival and local failure in laryngeal cancer46.
Nomograms will likely be more widely used in cancer prognostication, especially with the availability of good quality data and increasing sophistication in computing software and technology.
CONCLUSIONSThe TNM classification has been fundamental in shaping oncology and cancer prognostication over more than half a century. It has been the standard and most widely accepted prognostic system. With improved understanding of the biologic behavior of cancer, the relevance of the traditional anatomic-based TNM system has been increasingly challenged. Although newer prognostic variables merit inclusion in the staging paradigm, our ability to change the TNM system has been guided by the need to keep the simplicity and user-friendliness that are the hallmark of the TNM system and yet have a tool that accurately incorporates all factors that might influence outcomes.
Declaración de interesesNada que declarar.