Breast cancer is a malignant neoplasm that affects women worldwide, and cytotoxic chemotherapy remains a primary treatment modality. In breast cancer, many women experience therapeutic failure and unfavorable clinical outcomes due to mechanisms related to chemoresistance acquisition, which may include oxidative stress. In this study, we investigated the systemic oxidative stress profile of women diagnosed with chemoresistant breast cancer and evaluated the correlation of this profile with clinicopathological features.
MethodsThe oxidative stress levels were determined based on lipid peroxidation and nitric oxide metabolite (NOx) measurements. Chemoresistance was determined based on the Response Evaluation Criteria in Solid Tumors guidelines, and patients were categorized as responsive (complete response) or chemoresistant (partial or no response).
ResultsReduced lipid peroxide levels were observed independent of the pattern of chemotherapy response, without NOx variation. The type of drug schedule did not interfere with oxidative stress levels in the responsive patients. However, lipid peroxide levels were reduced in patients in the chemoresistant group receiving the combination of adryamicin+ciclofosfamide+Taxol. Additionally, lipid peroxidation strongly correlated with high histological grade and obesity in chemoresistant patients, while NOx correlated with disease stage, risk of death and recurrence, and menopausal status.
ConclusionThese findings highlight lipid peroxidation and NOx concentrations as putative markers of chemotherapy response in human breast cancer patients.
El cáncer de mama es una neoplasia maligna que afecta a las mujeres a nivel mundial, siendo la quimioterapia citotóxica una modalidad terapéutica primaria. En el cáncer de mama, muchas mujeres experimentan fallo terapéutico y resultados clínicos desfavorables debido a los mecanismos relacionados con la adquisición de quimiorresistencia, que pueden incluir estrés oxidativo. En este estudio, investigamos el perfil de estrés oxidativo sistémico de las mujeres diagnosticadas de cáncer de mama quimiorresistente, y evaluamos la correlación de este perfil con las características clínicopatológicas.
MétodosSe calcularon los niveles de estrés oxidativo sobre la base de las medidas de peroxidación lipídica y metabolitos de óxido nítrico (NOx). La quimiorresistencia se calculó sobre la base de las guías RECIST (Response Evaluation Criteria in Solid Tumors), categorizándose a las pacientes como receptivas (respuesta completa) o quimiorresistentes (respuesta parcial o nula).
ResultadosSe observó una reducción de los niveles de peróxido lipídico de manera independiente al patrón de respuesta a la quimioterapia, sin variación de NOx. El tipo de programa farmacológico no interfiere en los niveles de estrés oxidativo en las pacientes receptivas. Sin embargo, los niveles de peróxidos lipídicos se vieron reducidos en las pacientes del grupo quimiorresistente que recibieron la combinación de adriamicina+ciclofosfamida+Taxol. Además, la peroxidación lipídica se correlaciona fuertemente con el alto grado histológico y la obesidad en las pacientes quimiorresistentes, mientras que NOx se correlacionó con el estadio de la enfermedad, el riesgo de muerte y recidiva, y el estatus menopáusico.
ConclusiónEstos hallazgos subrayan a la peroxidación lipídica y las concentraciones de NOx como marcadores putativos de la respuesta a la quimioterapia en las pacientes de cáncer de mama en humanos.
Breast cancer is the most common malignant neoplasm affecting women worldwide, with approximately 2.3 million cases.1 Treating such patients involves a combination of surgical approaches, radiotherapy, and chemotherapy, which has a systemic scope, aiming to control disease and treat the appearance of distant metastases.2,3 Chemotherapy, known as neoadjuvant chemotherapy, can be administered to reduce the tumor size before performing a surgical procedure after the surgical procedure to reduce the probability of recurrence; this approach is described as adjuvant chemotherapy or even used to treat distant metastases and is known as palliative chemotherapy.4
In breast cancer, some women fail to respond to chemotherapy, characterizing the process of chemoresistance, which is based on the ability of malignant cells to adapt to chemotherapy treatment, resulting in the proliferation and spread of the disease through the formation of metastatic clones. This phenomenon is estimated to occur in almost 50% of patients with advanced breast cancer.5
Therapeutic failure refers to the ability of malignant cells to survive and proliferate without control, even in the presence of a chemical drug. The molecular mechanisms that impair the effectiveness of chemotherapy include reducing the intracellular accumulation of anticancer drugs by increasing efflux and/or decreasing absorption, drug sequestration, altering targets or activating detoxifying systems, increasing DNA damage repair, dysregulation of proliferative and apoptotic pathways, changes in xenobiotic metabolism, induction of epithelial–mesenchymal transition, and intercellular communication by exosomes.6,7
Most antineoplastic drugs act through mechanisms generated by reactive oxygen and nitrogen (RS) species, which results in oxidative stress due to the imbalance between oxidants and antioxidant neutralization.8 Excess oxidation can impact cells systemically and, with loss of function of several biomolecules, can generate oxidative damage to lipids, proteins, and even DNA.9 This oxidative damage, described as biomolecular damage caused by the attack of free radicals on the constituents of living organisms at high levels, can result not only from oxidative stress but also from the failure of repair or replacement systems.10
Moreover, the excessive proliferation of tumor cells is directly related to the high production of RS. However, carcinogenesis is adapted to increase antioxidant status to optimize cell proliferation and use these mechanisms in favor of cancer.11 For example, nitric oxide (NO) and its metabolites are known to play physiological roles in several processes but are dependent on their concentration. NO derived from tumor cells can promote tumor progression by inducing tumor cell invasion, proliferation, and the expression of angiogenic factors.12 Thus, all these mechanisms can impact the progression of cancer and the appearance of metastases,13 leading to high socioeconomic costs during the therapeutic process and unfavorable clinical outcomes, such as relapse and death.
Several studies have demonstrated the central role of oxidative stress in breast cancer, suggesting that the induction of a pro-oxidant environment systemically and locally14 is associated with unfavorable prognosis15 and the occurrence of metastases.16 However, little is known about the profile of systemic oxidative stress mediators in the context of treatment response. Thus, in this work, we investigated the systemic oxidative stress profile of patients with breast cancer in the context of chemoresistance.
MethodsStudy designThis work was an exploratory, retrospective, descriptive, and quantitative study. The Institutional Ethics Committee approved this proposal under CAAE 35524814.4.0000.0107, opinion number 810.501. All participants signed informed consent forms.
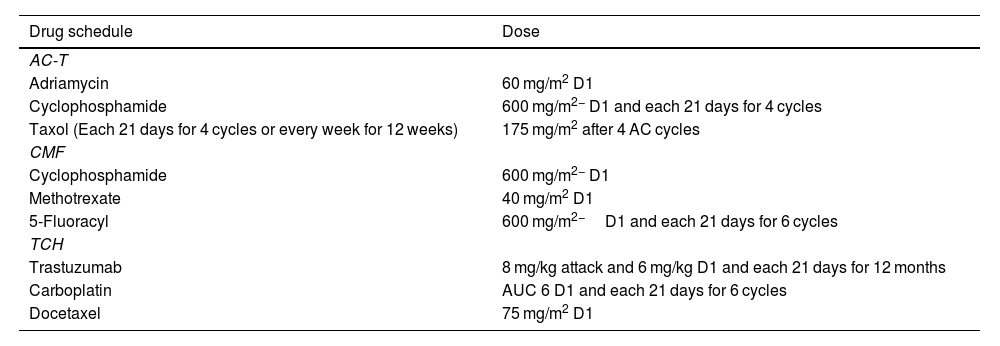
The study was carried out from May 2015 to April 2023. For this purpose, data were collected from medical records at the Francisco Beltrão Cancer Hospital (Ceonc, Francisco Beltrão, Paraná, Brazil). Patients diagnosed with early breast cancer, with follow-up for disease recurrence, and submitted to neoadjuvant chemotherapy were included in the study. Patients who recurred or had systemic disease received adjuvant treatment. Data on chemotherapy treatments were obtained through the Authorization for Outpatient Procedures system. The drug regimens are shown in Table 1.
Chemotherapy schedules from breast cancer patients included in the study.
| Drug schedule | Dose |
|---|---|
| AC-T | |
| Adriamycin | 60 mg/m2 D1 |
| Cyclophosphamide | 600 mg/m2− D1 and each 21 days for 4 cycles |
| Taxol (Each 21 days for 4 cycles or every week for 12 weeks) | 175 mg/m2 after 4 AC cycles |
| CMF | |
| Cyclophosphamide | 600 mg/m2− D1 |
| Methotrexate | 40 mg/m2 D1 |
| 5-Fluoracyl | 600 mg/m2−D1 and each 21 days for 6 cycles |
| TCH | |
| Trastuzumab | 8 mg/kg attack and 6 mg/kg D1 and each 21 days for 12 months |
| Carboplatin | AUC 6 D1 and each 21 days for 6 cycles |
| Docetaxel | 75 mg/m2 D1 |
For patient categorization, we considered all patients who failed to respond to neoadjuvant chemotherapy treatment as chemoresistant. We followed the RECIST (Response Evaluation Criteria in Solid Tumors, available at: https://recist.eortc.org/recist-1-1-2/) guideline criteria to determine chemoresistance.
RECIST guideline recommendations and reports of imaging tests (mammography, ultrasound at diagnosis, breast MR, CT scan, and PET-CT in the follow-up) were used to assess the baseline and follow-up images. The same image test was used for each patient to get the initial and final images.
The response criteria adopted were complete response (when all target lesions completely disappeared), partial response (when at least 30% of the size of the target lesions decreased, without new lesions detected), progressive disease (when an increase of at least 20% of the lesion was detected concerning the baseline lesions and/or new lesions appeared in the breast or distant organs), and stable disease (neither sufficient shrinkage nor sufficient increase to qualify for progressive disease). The total follow-up period of the patients included in this study was 5 years.
Based on this, patients were categorized as responsive (patients having a complete response) or chemoresistant (patients categorized as partial response, progressive disease, or stable disease).
Patient data and sample collectionThe following data were collected: beginning, end, dose, frequency of treatment, age at diagnosis, body mass index (BMI), Bi-RADS classification, molecular subtyping of tumors and sites of disease metastases, risk stratification for death and recurrence, as well as the entire history of the patients present in the medical records.
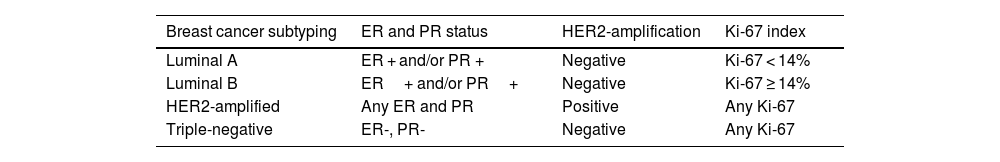
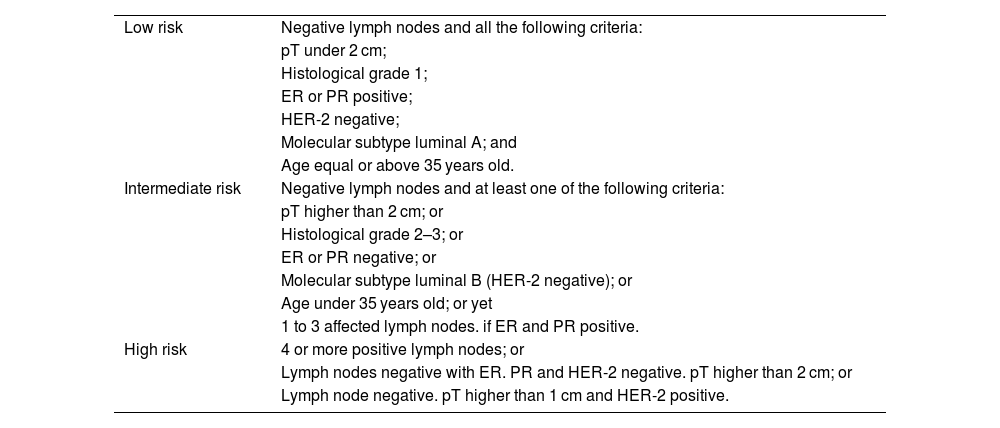
For tumor molecular subtyping, information concerning the estrogen receptor (ER), progesterone receptor (PR), Ki-67 index of proliferation, and human epidermal growth factor 2 receptor (HER2) status were collected from medical records. Based on the combined immunostaining results for ER, PR, and ki67, breast tumors were categorized following the St. Gallen classification (Goldhirsch et al., 2013), as shown in Table 2. Data concerning risk stratification for death and recurrence (Goldhirsch et al., 2007) are detailed in Table 3.
Breast cancer molecular subtyping.
| Breast cancer subtyping | ER and PR status | HER2-amplification | Ki-67 index |
|---|---|---|---|
| Luminal A | ER + and/or PR + | Negative | Ki-67 < 14% |
| Luminal B | ER+ and/or PR+ | Negative | Ki-67 ≥ 14% |
| HER2-amplified | Any ER and PR | Positive | Any Ki-67 |
| Triple-negative | ER-, PR- | Negative | Any Ki-67 |
Criteria for risk stratification of recurrence and death of patients diagnosed with breast cancer (Goldhirsch et al., 2007).
| Low risk | Negative lymph nodes and all the following criteria: |
| pT under 2 cm; | |
| Histological grade 1; | |
| ER or PR positive; | |
| HER-2 negative; | |
| Molecular subtype luminal A; and | |
| Age equal or above 35 years old. | |
| Intermediate risk | Negative lymph nodes and at least one of the following criteria: |
| pT higher than 2 cm; or | |
| Histological grade 2–3; or | |
| ER or PR negative; or | |
| Molecular subtype luminal B (HER-2 negative); or | |
| Age under 35 years old; or yet | |
| 1 to 3 affected lymph nodes. if ER and PR positive. | |
| High risk | 4 or more positive lymph nodes; or |
| Lymph nodes negative with ER. PR and HER-2 negative. pT higher than 2 cm; or | |
| Lymph node negative. pT higher than 1 cm and HER-2 positive. |
Blood samples were collected at diagnosis and after the treatment response evaluation (at the end of the neoadjuvant treatment or if they responded to the neoadjuvant chemotherapy but developed a new lesion during the 5-year follow-up). Blood was collected in a tube containing tetraethylenediamine acid. Ethylenediaminetetraacetic acid (EDTA), centrifuged (4000 rpm for 5 min), and plasma frozen at −20 °C until analysis.
Oxidative stress measurementTo quantify the levels of lipid peroxidation in the plasma samples by using the high-sensitivity chemiluminescence method, 855 μL of buffer solution (30 mM Na2HPO4, pH 7.4 at 37 °C) and 125 μL of plasma were mixed in a microtube. Then, 20 μL of 3 mM tert-butyl alcohol (at room temperature) was added, followed by homogenization and chemiluminescence reading. The results were expressed in relative units of light (RLU) by integrating the area under the curve or obtaining the maximum emission peak of each sample. NOx levels were determined as previously published by de Oliveira et al.16 Briefly, the samples were deproteinized, and the supernatant was incubated with copper-activated cadmium granules. After incubation, NOx was measured by adding Griess reagent and reading the samples at 550 nm.14 The results are expressed as μM.
Data analysisThe clinicopathological variables of the patients were categorized and tabulated in Microsoft Excel® spreadsheets. The frequencies of the categories of each variable were compared for patients belonging to both groups using the Chi-square test, considering a 5% statistical significance. Data were analyzed using GraphPad Prism 0.9.0 and SPSS 25.0.0 software. Data distribution was tested using the Shapiro–Wilk test. Thus, variables with normal distribution were analyzed using parametric tests. When the assumption of normality was not met, non-parametric tests were used. Statistical analysis, normality tests were performed, and paired or unpaired tests were subsequently applied according to the desired comparison and based on the variances of the groups. Student's t test was used for parametric data, and the Mann–Whitney test was used for non-parametric data. Analysis of variance (ANOVA) was used for comparisons of more than two groups, followed by the Bonferroni correction. A Kaplan–Meier survival curve of survival data concerning the patients' chemotherapy response profile, with a follow-up duration of 2 years after surgical resection of primary breast cancer was performed, comparing both groups by a Log rank test. p<.05 was considered to indicate statistical significance.
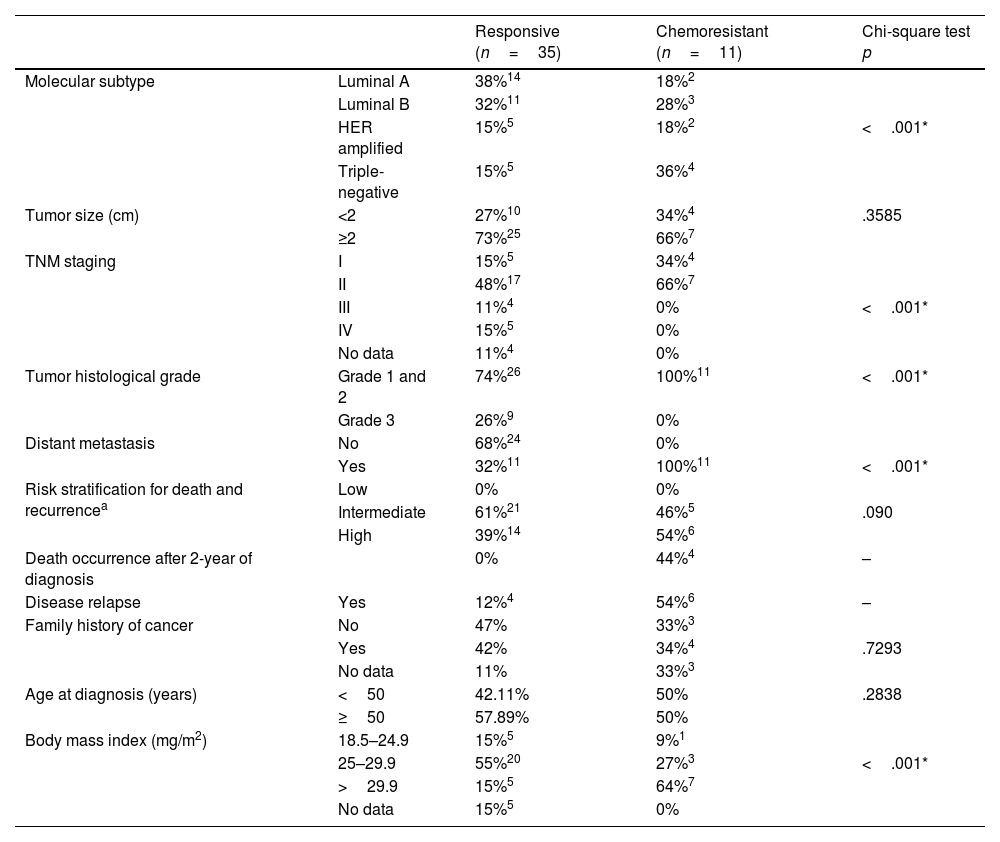
ResultsA total of 46 patients who had complete clinicopathological and laboratory data for the 5-year follow-up were included (35 responsive and 11 chemoresistant,Table 4) Patients were submitted to neoadjuvant or adjuvant chemotherapy. A majority of the responsive patients had luminal subtype A disease (38%), 55% were overweight (BMI 25–29.9 kg/m2), and 68% lacked distant metastasis. In the chemoresistant group, there was a predominance of TNM I and II in 100% of the patients, luminal B and triple negative subtypes, obesity in 64% of the group (BMI>29.9 kg/m2), distant metastases in 100% and death in 27%. About 54% of the chemoresistant patients subsequently relapsed. In the chemoresistant group, more than 70% of patients had progressive disease; about 20% had partial response, and about 10% showed stable disease. Molecular subtyping, TNM staging, tumor histological grade, distant metastasis, and body mass index were significantly different between groups (p<.001). All patients included in the responsive group presented a complete pathological response after treatment.
Table 4 - Clinicopathological data of patients.
| Responsive (n=35) | Chemoresistant (n=11) | Chi-square test p | ||
|---|---|---|---|---|
| Molecular subtype | Luminal A | 38%14 | 18%2 | |
| Luminal B | 32%11 | 28%3 | ||
| HER amplified | 15%5 | 18%2 | <.001* | |
| Triple-negative | 15%5 | 36%4 | ||
| Tumor size (cm) | <2 | 27%10 | 34%4 | .3585 |
| ≥2 | 73%25 | 66%7 | ||
| TNM staging | I | 15%5 | 34%4 | |
| II | 48%17 | 66%7 | ||
| III | 11%4 | 0% | <.001* | |
| IV | 15%5 | 0% | ||
| No data | 11%4 | 0% | ||
| Tumor histological grade | Grade 1 and 2 | 74%26 | 100%11 | <.001* |
| Grade 3 | 26%9 | 0% | ||
| Distant metastasis | No | 68%24 | 0% | |
| Yes | 32%11 | 100%11 | <.001* | |
| Risk stratification for death and recurrencea | Low | 0% | 0% | |
| Intermediate | 61%21 | 46%5 | .090 | |
| High | 39%14 | 54%6 | ||
| Death occurrence after 2-year of diagnosis | 0% | 44%4 | – | |
| Disease relapse | Yes | 12%4 | 54%6 | – |
| Family history of cancer | No | 47% | 33%3 | |
| Yes | 42% | 34%4 | .7293 | |
| No data | 11% | 33%3 | ||
| Age at diagnosis (years) | <50 | 42.11% | 50% | .2838 |
| ≥50 | 57.89% | 50% | ||
| Body mass index (mg/m2) | 18.5–24.9 | 15%5 | 9%1 | |
| 25–29.9 | 55%20 | 27%3 | <.001* | |
| >29.9 | 15%5 | 64%7 | ||
| No data | 15%5 | 0% |
Concerning drug schedules, the initial regimen doxorubicin+cyclophosphamide+paclitaxel (AC+T) was used in 33.3% of the nonresponding patients, as was the CMF regimen; 33.3% and 16.67% of the patients were treated with Taxol+Herceptin, and 16.67% were treated with Taxol+Taxotere. The initial regimen of AC+T was used for 75% of the patients who relapsed. In the group of responsive patients, 63.16% used the AC+T scheme.
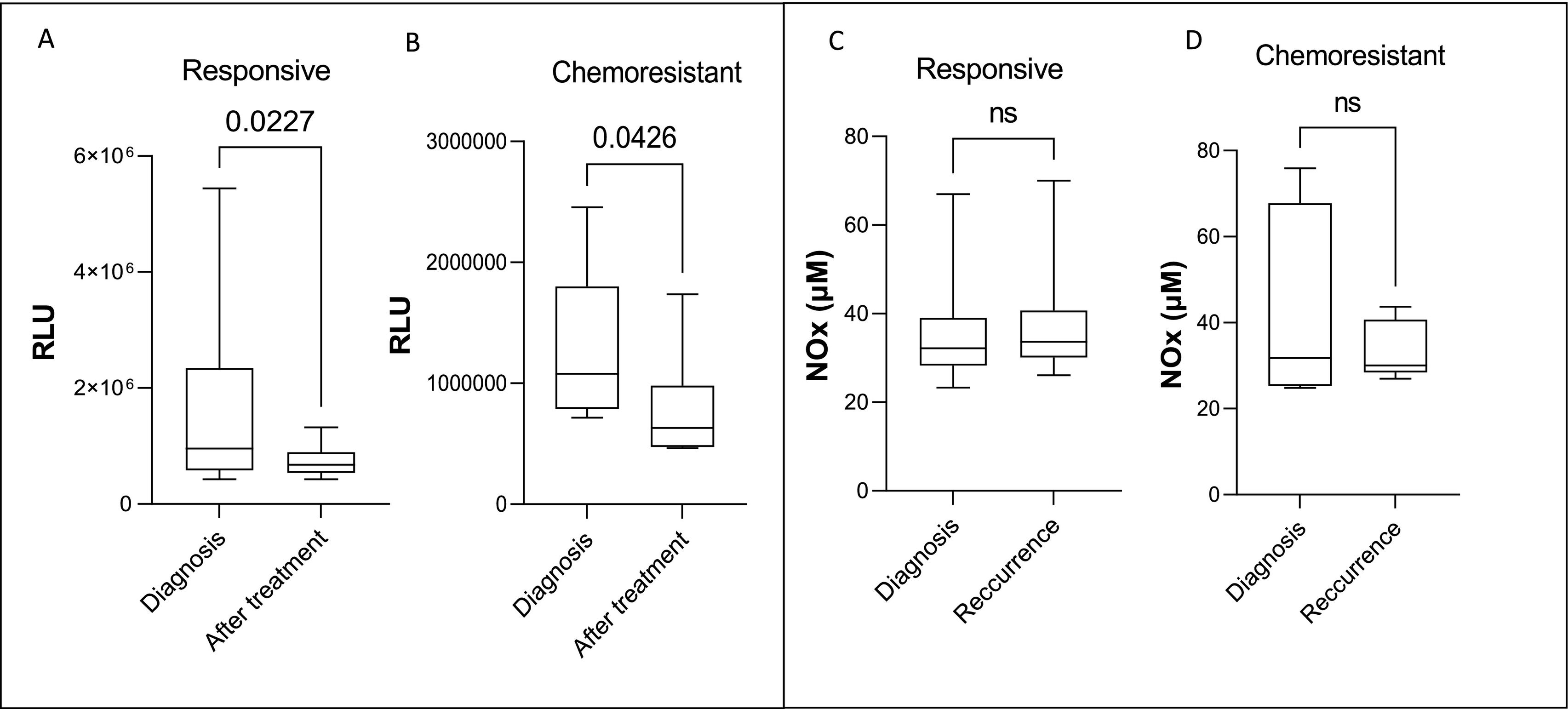
A significant reduction in lipid peroxidation levels was observed in both the responsive and chemoresistant groups when comparing plasma samples at diagnosis vs. samples collected after the end of treatment, as shown in Fig. 1. NOx levels did not vary in any group (Fig. 1C and D).
Systemic lipid peroxidation and Nox levels at diagnosis and after treatment end in breast cancer patients responsive (A and C) and chemoresistant (B and D) to chemotherapy. Plasmatic lipid peroxidation was determined by tert-butyl-induced chemiluminescence and NOX measured by Cadmium–Copper–Griess reaction. Data were integrated and are expressed as relative light units (RLUs). The data are shown as box plots, and the lines in the boxes indicate the medians for each group.
* indicates statistical significance (p<.05). RLU=relative light unit.
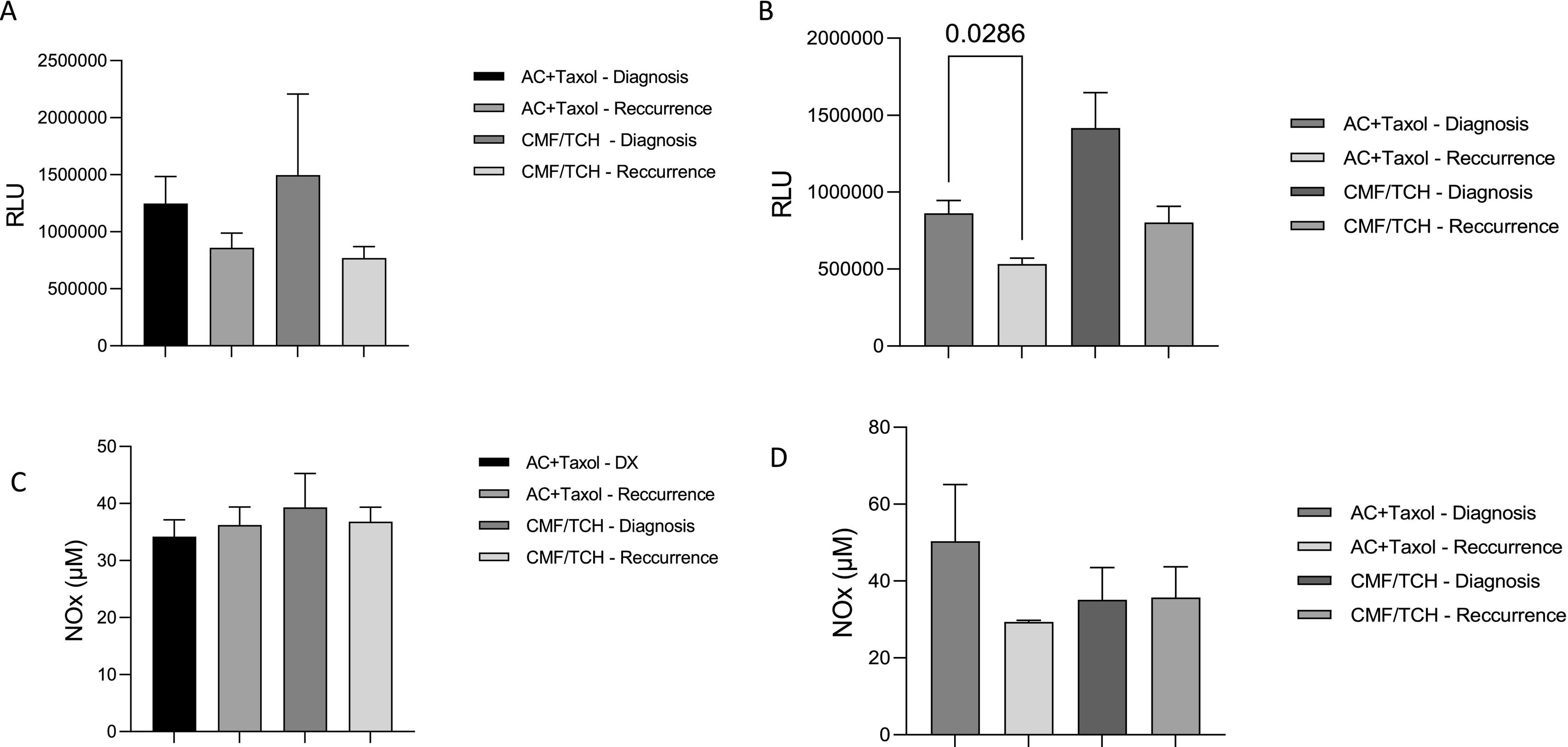
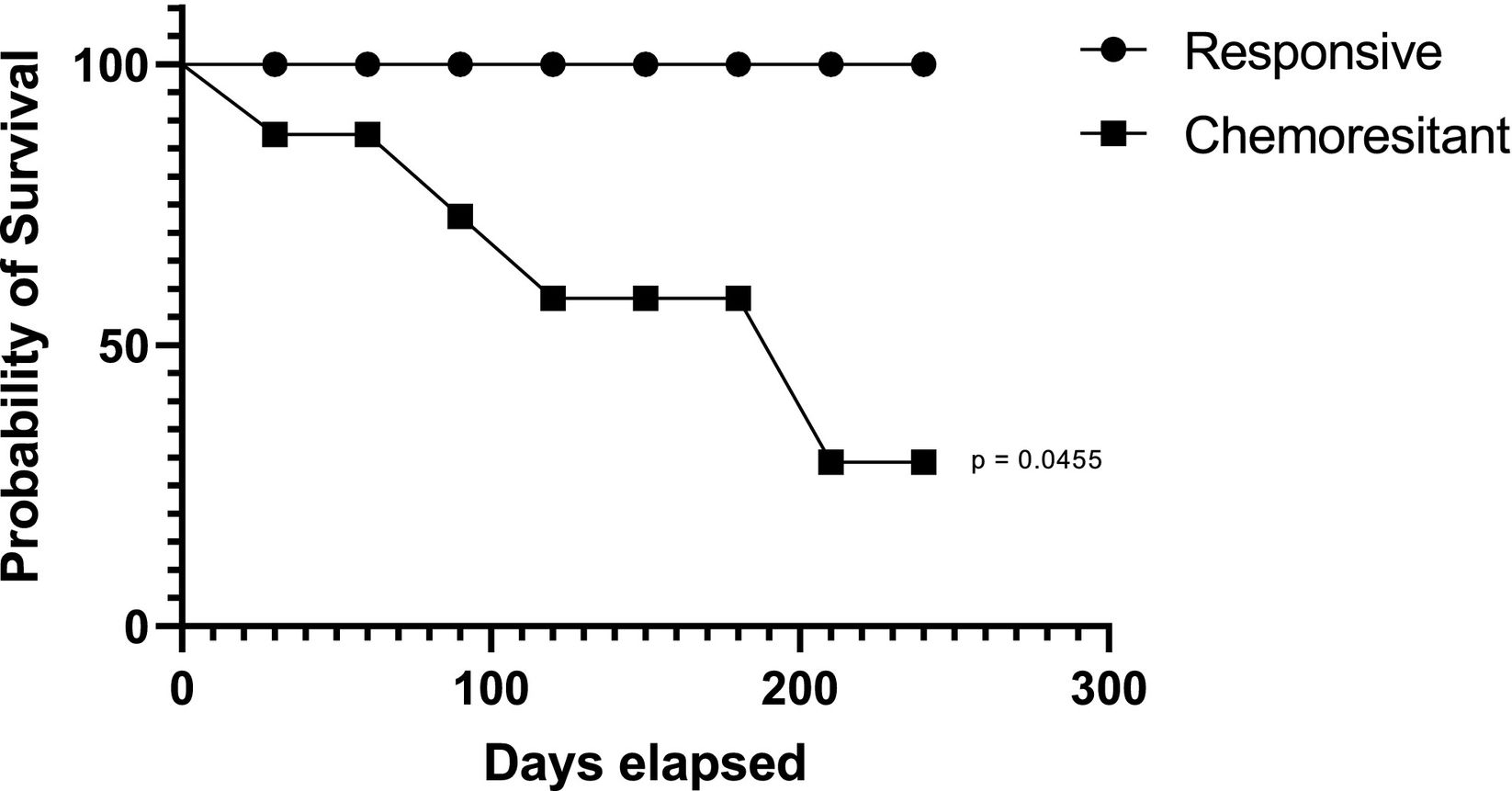
Regarding the impact of the therapeutic scheme on the markers evaluated in responsive patients, it was found that the primary treatment schemes initially implemented did not interfere with the lipid peroxidation or NOx profiles, as shown in Fig. 2A and B. Regarding the impact of the therapeutic regimen on the markers evaluated in chemoresistant patients, patients receiving AC + T had significantly reduced lipid peroxidation compared to patients receiving other regimens. No significant changes in NOx concentrations were detected (Fig. 2 C and D). A Kaplan–Meier survival curve of survival data concerning the patients' chemotherapy response profile, with a follow-up duration of 2 years after surgical resection of primary breast cancer was performed (Fig. 3). Chemoresistant patients had more death occurrence than the responsive ones (Log rank test, p=.0455).
Systemic lipid peroxidation and NOx levels at diagnosis and after treatment end in breast cancer patients responsive to chemotherapy according to their drug schedule. Plasmatic lipid peroxidation was determined by tert-butyl-induced chemiluminescence and NOX measured by Cadmium–Copper–Griess reaction. The data are shown as box plots, and the lines in the boxes indicate the medians for each group.
* indicates statistical significance (p<.05). RLU=relative light unit.
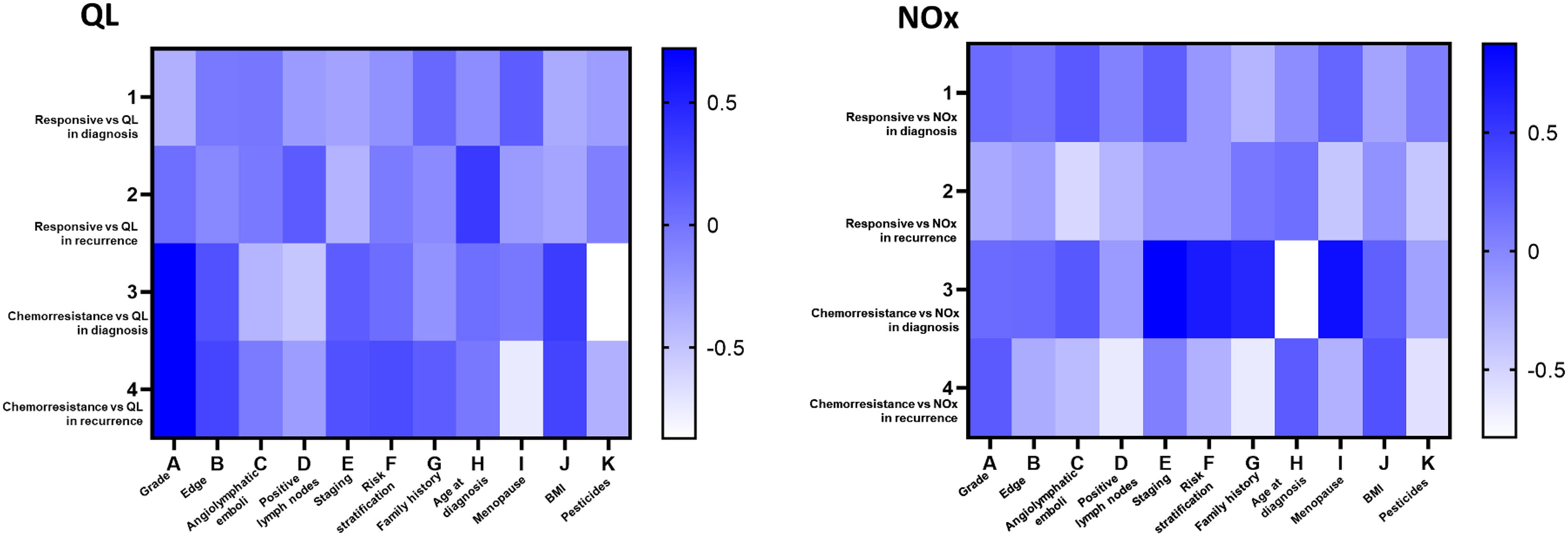
Correlation analysis of chemoresistant patients revealed a significant correlation between tumor grade and BMI (Fig. 4A). For NOx, significant positive strong correlations were observed for disease recurrence and death risk and for menopausal status (Fig. 4B).
DiscussionBreast tumors and their treatment are closely associated with oxidative stress generation.17 Thus, variations in oxidative stress levels are expected to affect disease prevention and treatment response. In this study, we observed changes in plasma lipoperoxidation depending on the treatment responsiveness and the type of drug schedule.
Treating breast cancer involves multiple antineoplastic drugs, which can be used alone or in combination with adjuvant, neoadjuvant, or palliative agents.6 The AC+T combination consists of doxorubicin, cyclophosphamide, and paclitaxel. Doxorubicin is an anthracycline whose metabolic process results in the generation of molecules with strong oxidizing potential and broad-spectrum antitumor effects.18 Cyclophosphamide, in turn, is an alkylating agent that has cytotoxic effects.19 Paclitaxel, in turn, prevents the depolymerization of tubulin, which is necessary for cell replication, by stabilizing the formed microtubules, blocking the process of cell division and playing an antitumor role.20 These drugs are known by generating oxidative stress during cancer treatment,21 and the mechanisms include the generation of reactive species of oxygen and nitrogen22 such as superoxide anion and hydrogen peroxide.23
Changes in systemic oxidative stress are reported in breast cancer patients after AC+T-based chemotherapy.24,25 In such patients, there is augmented oxidative stress characterized by an increase in lipid peroxidation and nitric oxide metabolites immediately after infusion.26 A first analysis of our data showed that breast cancer patients had the same degree of lipid peroxidation at diagnosis and at the end of treatment, independent of their responsiveness. Therefore, we analyzed the impact of specific drug regimens on the lipid peroxidation profiles of both groups. We observed no changes in systemic lipid peroxidation in responsive patients, but the level of this marker was significantly lower in the chemoresistant group.
High levels of lipoperoxides are related to breast cancer and are mainly linked to unfavorable clinical outcomes. Additionally, during treatment, chemotherapeutic drugs act as anticancer agents by triggering lipoperoxide production due to oxidative stress.18,26 In the present study, chemoresistant patients presented a significant decrease in circulating lipoperoxide levels, suggesting a reduction in the production or consumption of these molecules. This is plausible because cancer cells can reprogram their metabolism when they acquire chemoresistance, aiming to increase proliferation and avoid death.27
Lipid peroxidation may have beneficial or detrimental effects on cancer, and some specific lipid peroxidation products can even have anticarcinogenic effects.28 Additionally, under oxidative stress conditions, cancer cells can acquire adaptive mechanisms to counteract the deleterious effects of oxidative stress metabolites, resulting in increased antioxidant activity.29 In this way, antioxidants are potent scavengers of lipoperoxides, reducing their levels. Taken together, these mechanisms could help to understand our results and their relationship with chemoresistance. We further observed moderate correlations between lipoperoxide levels and high-grade tumors, and increased levels correlated positively with high body mass index. A previous study pointed out a correlation between lipoperoxide levels in breast cancer tissue and the occurrence of high-grade breast tumors, as well as its correlation to chemoresistance development, suggesting that breast tissue may be an important source of lipid peroxidation linked to poor prognosis features.15 Considering that fat is the fuel for lipid peroxidation in obese women,30 our findings deserve attention in the context of chemoresistance.
NO axis, especially NO metabolites and nitric oxide synthases (NOSs) have been implicated in breast cancer chemoresistance.31 We found moderate correlations of this marker with poor prognosis parameters, such as advanced disease stage, high BMI, and high risk of death and recurrence, in chemoresistant patients, suggesting a role for the NO axis in this context. Chemoresistant breast cell lineages have increased levels of NOx,32 and inducible NO synthase (iNOS), which is overexpressed in breast tumors, has been successfully used to treat chemorefractory patients, suggesting that the NO axis is involved in this mechanism.33 A recent clinical study demonstrated that chemoresistant breast cancer patients bearing tumors of worse prognosis had greater expression of M2 macrophages, a condition linked to poor clinical disease, while responsive patients had decrease in protumor N2 neutrophils markers, suggesting that patients receiving taxane-based regimens could benefit from NO axis blockage.34 Also, it is known that breast cancer women having higher NOx levels have augmented expression of protumoral molecules such as CTLA-4,35 and are also reported in overweight patients carrying poor prognosis disease36 and high-risk tumors.37 These data corroborate our findings and reinforce the participation of NO axis in breast cancer chemoresistance.
The present study has several limitations, including the need for multiple sample collection points and a modest sample size, which resulted from our strict inclusion criteria. However, it should be noted that the time taken to conduct the study encompassed 7 years to reach several patients, which made up an extremely select group.
In conclusion, our study suggested that circulating lipoperoxide levels, assessed at diagnosis and after the therapeutic regimen, are a valuable marker of response to chemotherapy treatment. Therefore, in the chemoresistant group, the AC+T scheme reduced lipoperoxide levels in these patients, decreasing oxidative stress, a process contrary to what is necessary for therapeutic success and fighting cancer. In turn, by measuring lipid peroxidation at diagnosis and after chemotherapy, lipoperoxide may represent a marker of response failure.
FundingFundação Araucária, Programa de Pesquisa Para o SUS – PPSUS, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Ethics approval and consent to participateAll ethical issues were considered in the study and are reported accordingly in the methods section.
Patients consentThe authors declare that they obtained the patient's consent for publication of the article.
The authors would like to thank all patients who agreed to participate in this work and Francisco Beltrão Cancer Hospital (CEONC) for collaborating with the study. The study was granted by Fundação Araucária (Call number 048/2021-PRPPG/Unioeste and 02/2022), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq grants number 402364/2021-0, 441017/2023-1, and 305335/2021-9).