Breast cancer is a common worldwide healthcare problem. Identifying metastatic lesions is crucial for adequate staging. However, there is no standardized metastatic work-up in early-stage breast cancer patients.
Materials and methodsWe retrospectively analyzed data from patients treated in a tertiary hospital for clinical early-stage breast cancer, to assess the value of alkaline phosphatase (ALP) as a predictor of metastasis and as a prognostic factor.
ResultsWe detected a significant correlation between ALP and metastasis at diagnosis, and found that ALP is both a sensitive and specific marker in screening for metastasis in early-stage breast cancer.
ConclusionALP is a useful marker of metastasis at diagnosis. Further prospective studies are needed to delineate the incidence and impact of missed metastatic patients if metastatic work-up is omitted.
El cáncer de mama es un problema de salud común en todo el mundo. La identificación de lesiones metastásicas es crucial para una estadificación adecuada. Sin embargo, no hay un estudio metastásico estandarizado en pacientes de cáncer de mama precoz.
Materiales y métodosSe analizaron retrospectivamente datos de pacientes tratados en un hospital terciario por cáncer de mama en estadio clínico temprano, para evaluar el valor de la fosfatasa alcalina (ALP) como predictor de metástasis y como factor pronóstico.
ResultadosLos autores detectaron correlación significativa entre ALP y metástasis en el momento del diagnóstico, y demostraron que ALP es un marcador sensible y específico en la detección de metástasis en cáncer de mama precoz.
ConclusiónLa ALP es útil en el diagnóstico de metástasis en el momento de la valoración. Se necesitan más estudios prospectivos para delinear la incidencia y el impacto de los pacientes metastásicos perdidos, si se omite el análisis metastásico.
Breast cancer is the most frequently diagnosed cancer and the leading cause of cancer death among females worldwide.1 In Egypt, breast cancer represents about 32% of all cancers among females.2
The 5-year survival rate for breast cancer had been reported to approach 99% in localized disease. However, approximately a third of breast cancer patients will develop distant non-nodal metastases, and the 5-year survival rate decreases to 27% once distant metastases have developed.3
Metastatic disease is still the leading cause of breast cancer related death, in spite of the progress in adjuvant and neoadjuvant therapies. Moreover, in patients with established metastatic disease, the treatment is palliative, with few breaks and with major adverse effects.4
Breast cancer metastasizes mainly to the bones, lungs, liver and brain hematogenously, in that order.5 Bone metastasis occur in an estimated 65% to 75% of patients with advanced breast carcinoma.6
Various factors were used to predict risk of metastasis in early stage (I and II) breast cancer patients and since a couple of years also multigenomic tests are used in daily practice in most countries. These include; tumor stage, tumor size, histologic grade, lymph node involvement, hormone receptor status, human epidermal growth factor receptor (HER) 2neu status, and age at diagnosis. Of these, estrogen receptor (ER)-positive status has the strongest association with bone metastasis.7 While, age at diagnosis, HER2-enriched, and triple-negative breast cancers tumor status have emerged as risk factors for breast cancer to metastasize to the brain and/or lung.8,9
Another predictor of metastasis is possibly the alkaline phosphatase (ALP). ALP is a serum enzyme whose total levels reflect the combined activity of several isoenzymes found in the liver, bone, kidney, and intestinal lining. The skeletal isoenzyme originates in osteoblasts that release large amounts when bone repair activity occurs, as with bone metastases. Also in cancer patients, ALP is a sensitive indicator of mild biliary obstruction, thus it is supposed to be a very sensitive indicator of liver progression.10
Currently, the national cancer comprehensive network (NCCN) recommends only serum ALP together with liver function tests in the work-up of early breast cancer patients, preserving imaging studies for those with symptoms suggesting metastasis or elevated ALP.11 However, the evidence supporting this practice is limited; also the cutoff level of ALP that warrant further investigations in search for metastasis is not identified. In the FIGO guidelines the indication for bone scan is limited to tumors larger than 5cm, elevation of ALP, palpable axillary lymph nodes, or suspicious clinical manifestations.12
Patients and methodsThe hospital data system was searched for breast cancer cases attending the center in a 2-year period (2014–2015) revealing 1153 cases. After exclusion of patients with DCIS, clinically evident stage III and IV cancers, no ALP recorded, and those with ALP done using different kit, 429 cases were found eligible for the study. In all patients ALP testing was done before surgery. Patients were followed up for metastasis till July 2018. Fractions of alkaline phosphatase were approximated.
According to our laboratory the normal values were; 100/290 in adults of both genders and 180/1200IU/L in children. Test Methodology was Kinetic DGKC using BT3500 (Beckman Coulter, CA, USA).
The data of these patients were analyzed and statistical values were obtained using SPSS version 22 (Inc, Chicago, IL). Continuous variables are presented as mean when symmetrical or median and range when asymmetrical. Categorical variables are presented as proportions. Univariate analysis was done using Chi-Square test, Fisher's exact test (if cell count less than 5), Mann–Whitney test and student t-test. Multivariate analysis was done using binary logistic regression. P value <0.05 was considered significant.
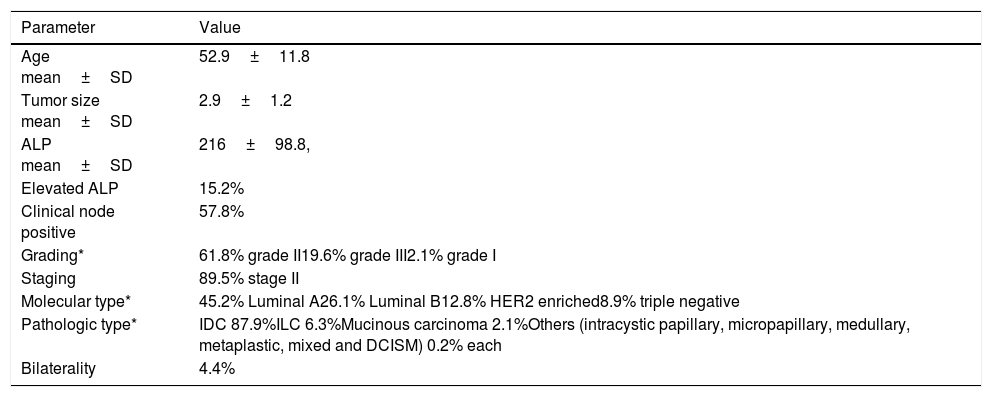
ResultsAll eligible patients were females. Basic epidemiologic data is displayed in Table 1.
Showing basic parameters of the patients included in the study.
| Parameter | Value |
|---|---|
| Age mean±SD | 52.9±11.8 |
| Tumor size mean±SD | 2.9±1.2 |
| ALP mean±SD | 216±98.8, |
| Elevated ALP | 15.2% |
| Clinical node positive | 57.8% |
| Grading* | 61.8% grade II19.6% grade III2.1% grade I |
| Staging | 89.5% stage II |
| Molecular type* | 45.2% Luminal A26.1% Luminal B12.8% HER2 enriched8.9% triple negative |
| Pathologic type* | IDC 87.9%ILC 6.3%Mucinous carcinoma 2.1%Others (intracystic papillary, micropapillary, medullary, metaplastic, mixed and DCISM) 0.2% each |
| Bilaterality | 4.4% |
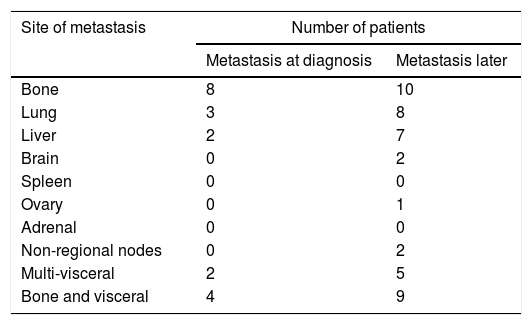
Only 19 (4.4%) of the patients were metastatic at diagnosis with the bone being the commonest site. While another 44 (10.7%) of the patients developed metastasis later suffering 32% with multiple site metastasis (Table 2).
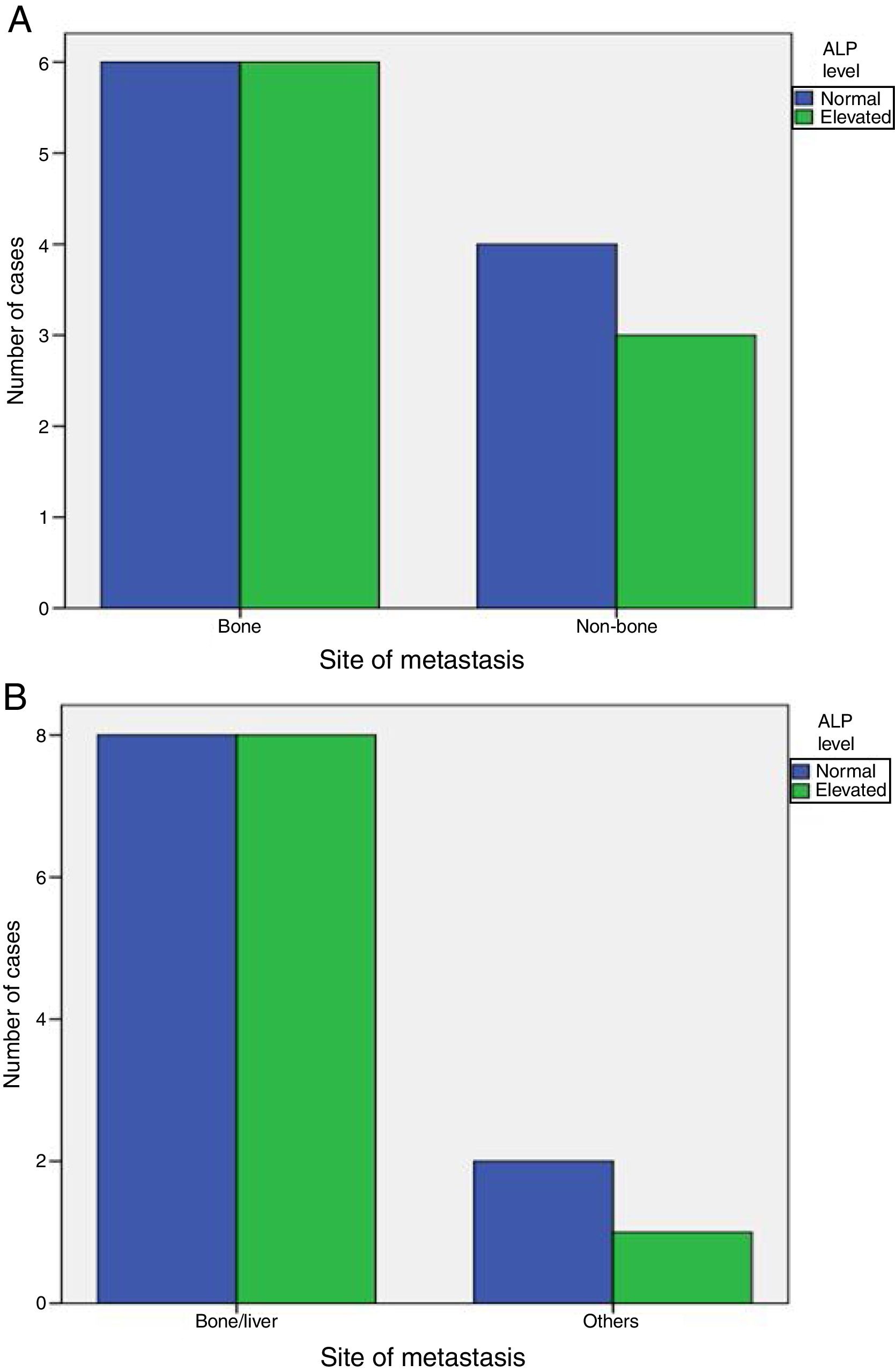
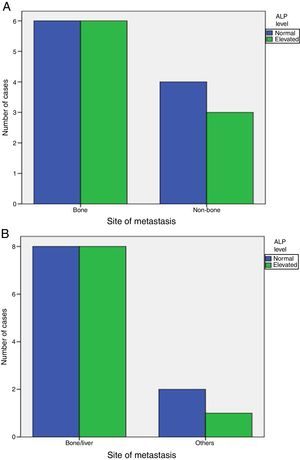
Elevated ALP was significantly associated with metastasis at diagnosis (P-value=0.001), but was not associated with increased incidence of later on metastasis (P-value=1). Also, elevated ALP was similarly associated with bone and visceral alone metastasis (P-value=1) and was elevated in some patients with metastatic sites other than bone and liver (Fig. 1).
Factors predicting metastasis at diagnosis are shown in Table 3, while those predicting later-on metastasis are shown in Table 4.
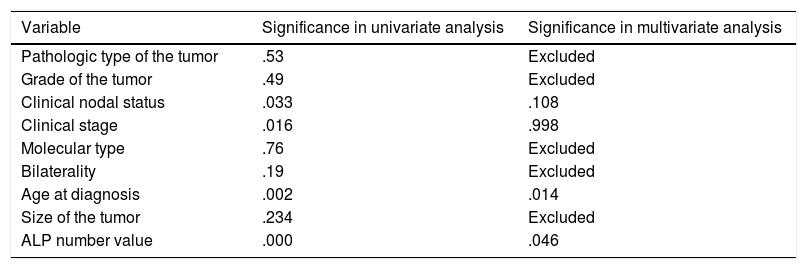
Showing univariate and multivariate analysis of factors predicting metastasis at diagnosis.
| Variable | Significance in univariate analysis | Significance in multivariate analysis |
|---|---|---|
| Pathologic type of the tumor | .53 | Excluded |
| Grade of the tumor | .49 | Excluded |
| Clinical nodal status | .033 | .108 |
| Clinical stage | .016 | .998 |
| Molecular type | .76 | Excluded |
| Bilaterality | .19 | Excluded |
| Age at diagnosis | .002 | .014 |
| Size of the tumor | .234 | Excluded |
| ALP number value | .000 | .046 |
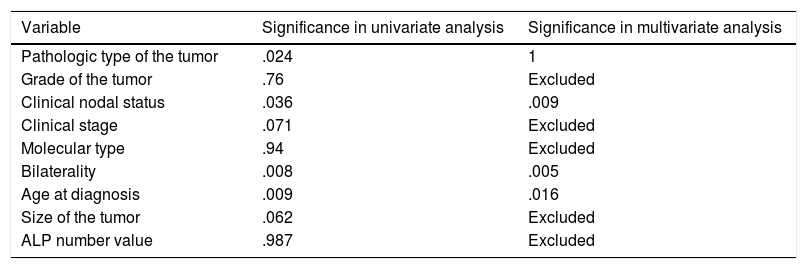
Showing univariate and multivariate analysis of factors predicting risk of later metastasis.
| Variable | Significance in univariate analysis | Significance in multivariate analysis |
|---|---|---|
| Pathologic type of the tumor | .024 | 1 |
| Grade of the tumor | .76 | Excluded |
| Clinical nodal status | .036 | .009 |
| Clinical stage | .071 | Excluded |
| Molecular type | .94 | Excluded |
| Bilaterality | .008 | .005 |
| Age at diagnosis | .009 | .016 |
| Size of the tumor | .062 | Excluded |
| ALP number value | .987 | Excluded |
Logistic regression model using variables that is significantly related to metastasis at diagnosis in univariate analysis namely; nodal status, stage, age, ALP numerical value, was run revealing significant predictors; age (.014) (OR=1.063) (95%CI=1.013–1.116) and ALP value (.046) (OR=1.003) (95%CI=1–1.007) (Table 3). Moreover, another model was run using nodal status, stage and age, but with ALP level (elevated/normal) showed that only age was a significant variable (.012) (OR=1.064) (95%CI=1.014–1.117).
Logistic regression for later-on metastasis revealed significant predictors; age (.016) (OR=.967) (95%CI=.938–.997), nodal status (.009) (OR=2.879) (95%CI=1.307–6.341) and bilaterality (.005) (OR=5.7) (95%CI=1.679–19.357) (Table 4).
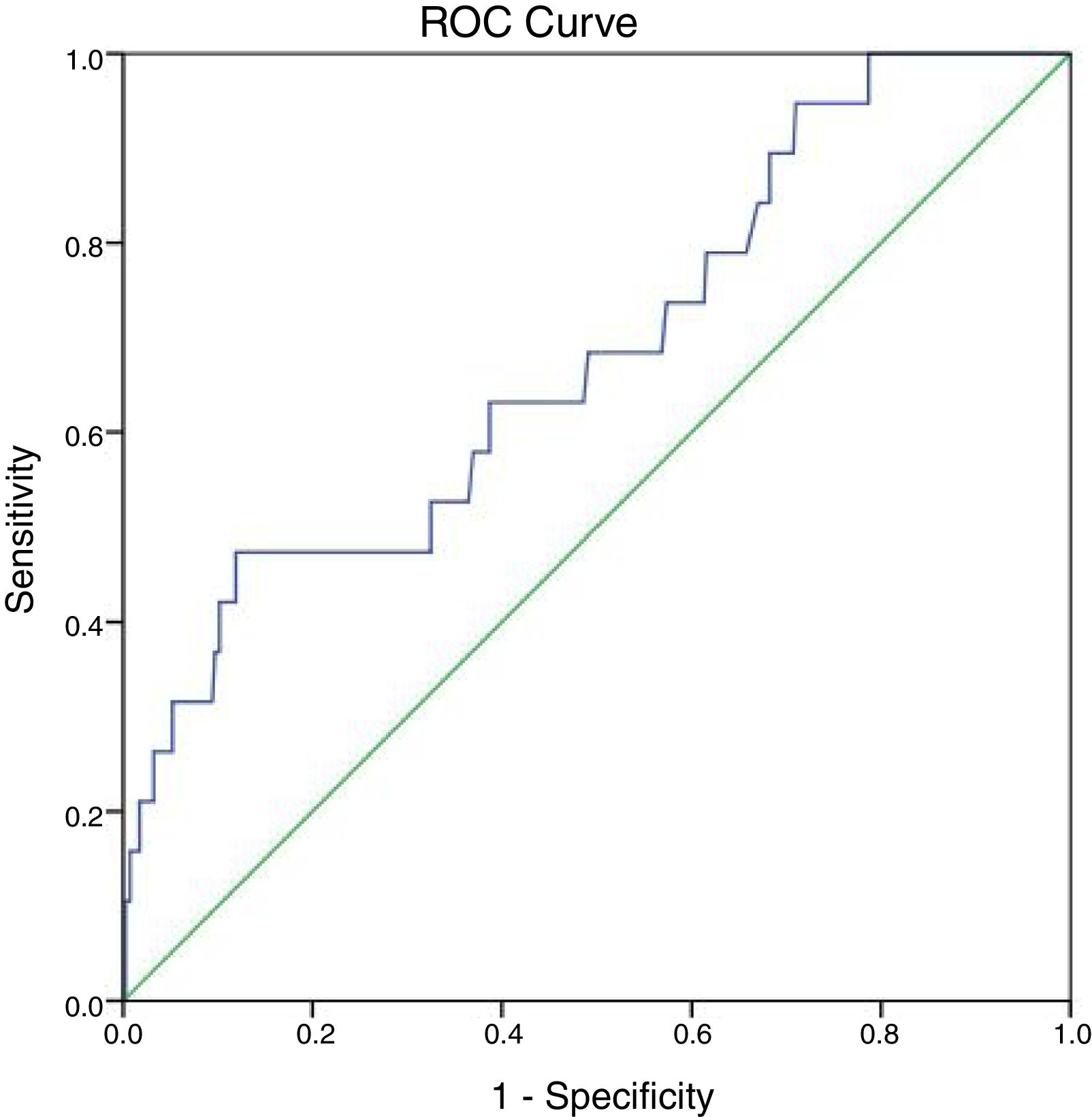
In detection of synchronous distant metastasis, the sensitivity of the ALP test was 47.4%, while the specificity was 86.1%. In addition, the positive predictive value was 13.8%, while the negative predictive value was 97.2%. The overall accuracy of the test was 84.4%.
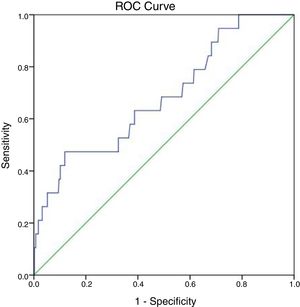
At 147IU/L the sensitivity is 100% but the specificity will drop to 21.3%. The cut off value of ALP test obtained from the ROC curve is 297IU/L with area under the curve 0.683 and P-value=0.007 (95%CI=0.555–0.811) (Fig. 2).
DiscussionAlkaline phosphatase is elevated in patients with cancers that spans throughout the bones or the liver. Cancers that exist in the lung, breast, prostate, colon, thyroid, and further organs can penetrate in the liver or bone. Yet, cancers that are already present in certain organs and tissues can cause ALP elevations even in absence of metastasis. Isoenzymes, which are special forms of ALP generated by these tumors, enlarges the total volume of alkaline phosphatase levels on experimental studies. The Regan isoenzyme is one of the best studied isoenzymes that is linked to several human cancers. Basically, the Regan isozenzyme is located in the placenta and associated with the gonadal and urologic cancers.13 If the reason for ALP elevation is unknown, isoenzyme studies using electrophoresis can confirm the source. Heat stability also distinguishes bone and liver isoenzymes (“bone burns, liver lasts”).
In one study, a significant percentage of patients with stage I/II breast cancer underwent unnecessary chest computed tomography (CT) as part of their initial work-up. Nearly a third of these patients were found to have pulmonary nodules, but only 1.3% were ever diagnosed with lung metastases.14 In another study, approximately 16% of patients with a diagnosis of stage II breast cancer in 2005 underwent preoperative CT, a rise from 7% in 1992.15 At Washington University up to 46% of patients underwent staging with CT, bone scanning, or positron emission tomography (PET) between 1998 and 2012.16 In a study from Canada, in 2012, approximately 35% of patients with stage II breast cancer underwent chest CT for staging and 40% had abdominal/pelvis CT.17 Moreover, no scientific support exists for performing initial staging bone scan (BS) in patients with early breast cancer with lesions up to 2cm in diameter or with clinical stage IIA (T2N0M0) disease because the result is not going to modify treatment. According to some scholars, BS is probably necessary prior to the treatment in patients with clinical stage IIB cancers because a change in stage entails therapeutic modifications.18 In the current study, almost all patients (about 90%) underwent routinely a CT scan of chest and abdomen, and a BS either at diagnosis or before starting medical treatment. The rest was investigated by abdominal ultrasound and CXR/bone survey with referral to further investigations if any suspicion. However, metastasis at diagnosis was low occurring in only 4.4% of our cases.
Ritzke et al. found that the sensitivity of ALP in bone metastases detection was 39%, of electrophoresis (total alkaline phosphatase and specific isoenzymes) was 48%, and of enzymes and tumor markers together was 85%. For liver metastases even though the positive predictive value was only 6%, the sensitivity of ALP and of electrophoresis was 58% and 94%, respectively. The determination of ALP isoenzymes is a non-invasive, in-expensive, reproducible and rapid method to detect progressive disease in breast cancer.19 At the current laboratory range of ALP (290 in our lab), the sensitivity was 47.4% while the specificity was 86.1%, and the overall accuracy was 84.4%. The cutoff level was slightly higher than the normal range at 297 (102.4% of normal level), and the maximum sensitivity was achieved at 147 (50.7% of the high normal level). But, we did not examine the isoenzymes.
In another study, the risk of recurrence was increased by 4% for those with abnormal ALP (HR=1.04; P=0.82), and they found that ALP better predicted liver recurrence.10 In our study, total ALP was found to be a significant predictor of metastasis at the time of diagnosis, but a bad prognostic marker for later on distant metastasis. In multivariate analysis, ALP value was directly correlated with increased incidence of detection of metastasis at diagnosis (either skeletal or visceral) on radiology.
In this study, increasing age was also a risk for metastasis at diagnosis where every 10 years elder increase the risk by 6.4%. On the other hand, every increase of age by 2.5 years was 3.3% protective of later on metastasis i.e. younger patients were at lower risk of synchronous metastasis and at a higher risk of metachronous metastasis. Node positive patients carry nearly triple and those with bilateral breast cancer are associated with nearly 6-fold increase in risk of later metastasis.
This study was limited by being retrospective affecting the quality and completeness of the data registered. Also, we did not analyze the isoenzymes of ALP, rather used total ALP only as recommended in NCCN guidelines. We encourage, researchers to further investigate the value of ALP in early breast cancer in prospective studies, to further delineate the role of isoenzymes in the metastatic work up of these patients, and to determine the fate of missed metastatic cases if adopting no work up approach.
ConclusionsEarly breast cancer only rarely metastasizes and as such extensive radiologic investigations for metastasis may not be cost-effective. ALP is a good predictor of metastasis in these cases with moderate sensitivity and high specificity. Younger age, node positive and bilateral breast cancer cases are indicators of increased risk of metachronous metastasis, while the prognostic role of ALP could not be proved.
Confidentiality of dataThe authors declare that they have followed the protocols of their Institution regarding the publication of patient data.
Conflict of interestsThe authors declare that they have no conflict of interest.