The inflammatory response and immune microenvironment has always been a field of interest in the study of solid tumors. In breast cancer it has been known for some time that the presence of a significant inflammatory infiltrate has been related to a better prognosis and, recently, its predictive value for response to treatment has also been identified. This has become a new parameter that has been incorporated into anatomic pathology reports. Furthermore, the introduction of immunotherapy in triple-negative breast cancer has led to the emergence of new biomarkers in order to identify candidate patients, the most important of which is the immunohistochemical determination of PD-L1. At present there are different antibody clones and scoring methods for this staining. Therefore, it is essential to know which one is indicated for each of the treatments currently available, since they are not interchangeable.
La respuesta inflamatoria y el microambiente inmunitario siempre ha sido un campo de interés en el estudio de los tumores sólidos. En el cáncer de mama se conoce hace tiempo que la presencia de un importante infiltrado inflamatorio se ha relacionado con un mejor pronóstico y recientemente se ha identificado además su valor predictivo de respuesta al tratamiento, convirtiéndose en un nuevo parámetro que se ha incorporado a los informes de Anatomía Patológica. Por otro lado, la introducción de la inmunoterapia en el cáncer de mama triple negativo ha supuesto la aparición de nuevos biomarcadores que permitan identificar a las pacientes candidatas, destacando entre todos la determinación inmunohistoquímica de PD-L1. En la actualidad existen diferentes clonas de anticuerpos y métodos de valoración de esta tinción, por lo que es imprescindible conocer el indicado para cada uno de los tratamientos disponibles en la actualidad, puesto que no son intercambiables entre sí.
For many years now, treatment with immune checkpoint inhibitors has been a reality in the therapy of oncological diseases such as non-small cell lung carcinoma, squamous cell carcinoma of the head and neck and urothelial carcinomas.1-3 However, it has been in recent years that immunotherapy has forged ahead in the treatment of breast cancer, considered a tumor with low T-lymphocyte infiltration and low mutational load.4 Results from the IMpassion 130 and KEYNOTE-355 clinical trials confirm the benefit of using therapies that include atezolizumab or pembrolizumab, respectively, for the treatment of advanced or metastatic triple-negative breast cancer.5,6 For both indications, biomarkers have been sought to justify their administration and, among the different candidates studied, the immunohistochemical determination of PD-L1 (programmed death ligand 1) yielded the best results, becoming a requirement for selecting patients who may benefit from these new therapies.
Antibodies available for immunohistochemical determination of PD-L1PD-1 (programmed death 1) is a surface protein that, together with its ligand, PD-L1, is capable of inhibiting the immune response against different antigens, thus, its expression in tumor cells is a mechanism of resistance against different tumors, and a target of multiple immunotherapies. While the expression of these proteins has not always shown a direct correlation with the benefit of targeted therapy,7 PD-L1 immunohistochemical positivity is currently required to select patients with advanced triple-negative breast cancer who may benefit from being treated with atezolizumab or pembrolizumab. However, unlike other currently used biomarkers, there are different clones on the market for PD-L1 which, in turn, have different scoring methods as well as different positivity rates. Currently, four clones are routinely used (SP142, SP263, 22C3 and 28–8) and, although their sensitivity is similar, the different tests used, including development methods or platforms, cause positivities to vary (Table 1)8–10. In that regard, in a study conducted on urothelial carcinomas, the tests for 28–8, 22C3 and SP263 proved to be interchangeable, which was not the case with SP142 and, as a result, the incorrect use of the test meant that it was not possible to choose patients who were candidates for a specific treatment.11 In the case of triple-negative breast cancer, the IMpassion130 trial demonstrated 46% of PD-L1-positive tumors if SP142 was used, while this percentage rose to 81% when using 22C3 or 75% if SP263 was used. However, this new population selected using other PD-L1 clones demonstrated no greater benefit.12
characteristics of antibodies approved by the FDA for immunotherapy indication.
| Antibody | Associated treatment | Scoring method | Cut point | Indication |
|---|---|---|---|---|
| 22C3 (Agilent) | Pembrolizumab |
| ≥10 | advanced or metastatic triple-negative breast cancer |
| SP142 (Ventana) | Atezolizumab |
| ≥1% | advanced or metastatic triple-negative breast cancer |
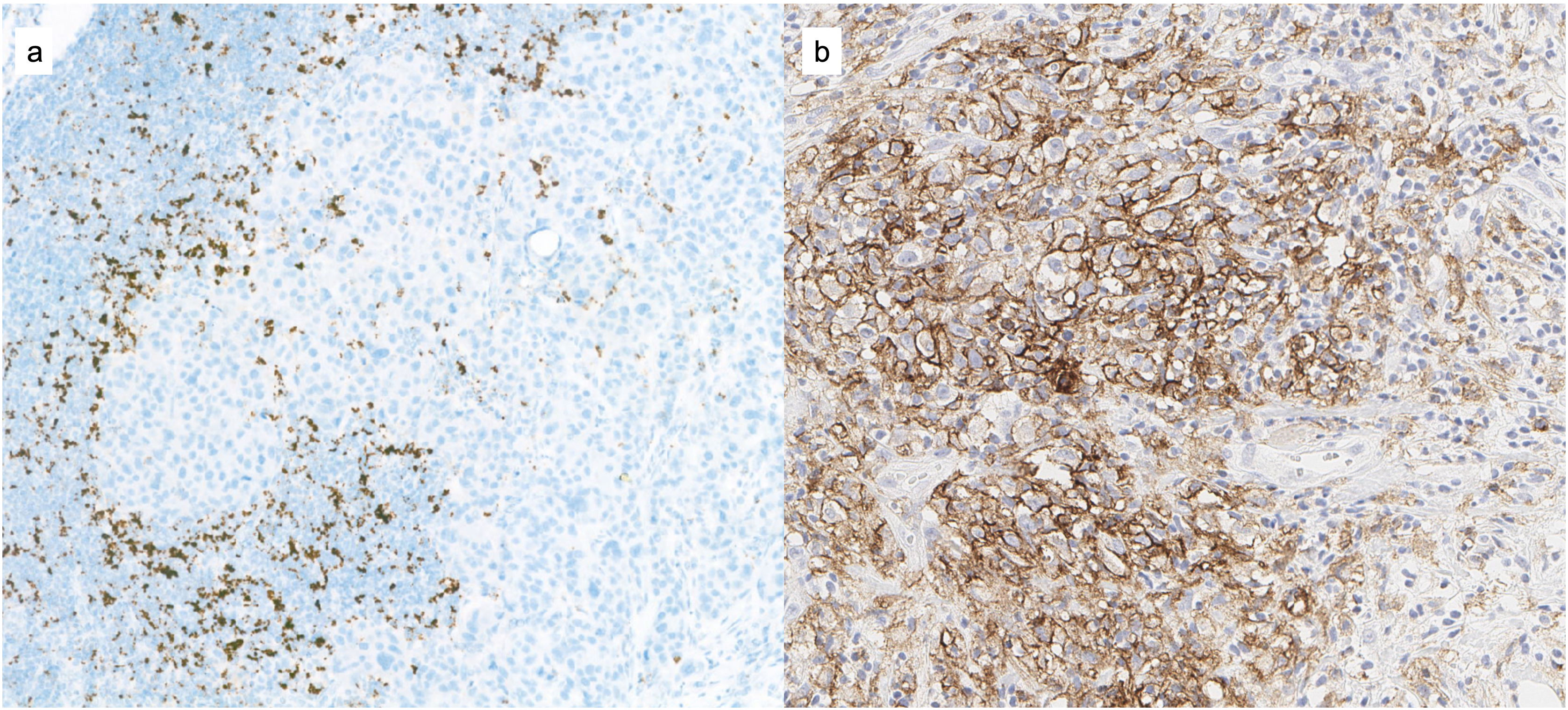
Following the publication of data from the IMpassion130 trial where a 7.5-month increase in overall survival was demonstrated in the group of patients with PD-L1-positive tumors,5 in March 2019 the US Food and Drug Administration (FDA) approved the use of atezolizumab in this subgroup of patients showing PD-L1 positivity. It was defined as such when the immunohistochemical expression with the SP142 antibody (Ventana) of the area represented by positive immune cells (IC) with respect to the total tumor area was ≥1% (IC ≥ 1%). Lymphocytes, histiocytes, dendritic cells or leukocytes are deemed to be IC, provided that they are part of the tumor area and are not found in areas of necrosis or within vascular structures (Fig. 1). The determination can be performed in any tumor sample (primary or metastatic), given that a positive result in any of them makes the patient a candidate for treatment. The indication of atezolizumab in the United States was voluntarily withdrawn in 2021, although it remains in force in European countries.
Determination of PD-L1 for the indication of pembrolizumabThe results of the KEYNOTE-355 clinical trial, which studied the benefit of pembrolizumab in patients with advanced or metastatic triple-negative breast cancer, have also recently been presented, obtaining FDA approval in 2020. As was the case with IMpassion130, the benefit of this therapy was mainly observed in patients with PD-L1 tumor expression, although the biomarker score was different. On the one hand, clone 22C3 (Agilent) was used with a different scoring method, called Combined Positive Score (CPS). This scores the total number of cells with PD-L1 expression (tumor cells, lymphocytes and histiocytes) out of the total number of viable tumor cells in the area studied (Fig. 1b) and it is considered that the greatest benefit in terms of progression-free survival, with a mean follow-up of 17.5 months for the group receiving pembrolizumab (n = 566) versus 15.5 months for the chemotherapy group (n = 281), was observed when CPS ≥ 10.6
Recommendations for PD-L1 determination in breast cancerThese results made it clear that PD-L1 determination should be performed before prescribing treatment with atezolizumab or pembrolizumab in this type of triple-negative tumor. This determination should be performed according to the protocols used in the clinical trials that led to its approval, which includes an IC ≥ 1% using the VENTANA PD-L1 protocol (SP142) for indicating atezolizumab and a CPS ≥ 10 using the PD-L1 IHC 22C3 pharmDx protocol (Agilent) performed with Dako technology for pembrolizumab. However, the availability of both platforms in the same center is not a frequent occurrence, which leaves the option of taking another test available in the institution. As previously mentioned, there are different studies that compare the results between the different antibodies available as well as their reproducibility. Noske et al. demonstrated that, in triple-negative breast cancer, the immune cell (IC) positivity between SP142, 28–8 and 22C3 were similar, while SP263 detected a higher number of positive cases and lower overall concordance with the other platforms.13,14 For this reason, it is still recommended to use the validated test in the clinical trial and to avoid interchanging antibodies or platforms. For Laboratory Developed TestS (LDTs), rigorous prior validation is essential before incorporating it into the diagnostic routine. Regarding inter-observer agreement, the general results of the different studies published show high percentages of agreement, mainly in the scoring of SP142.15
General recommendations for determining PD-L1 and indicating immunotherapy have recently been published highlighting —in addition to the technical aspects previously discussed— other points that should be taken into account in its assessment: always determine PD-L1 before any diagnosis of advanced or metastatic triple-negative breast carcinoma and do not perform it in cases of early stages, avoid determining PD-L1 in liver samples (due to the scarce inflammatory component present) and do not use decalcified samples or cytological material (as they have not been validated).16,17
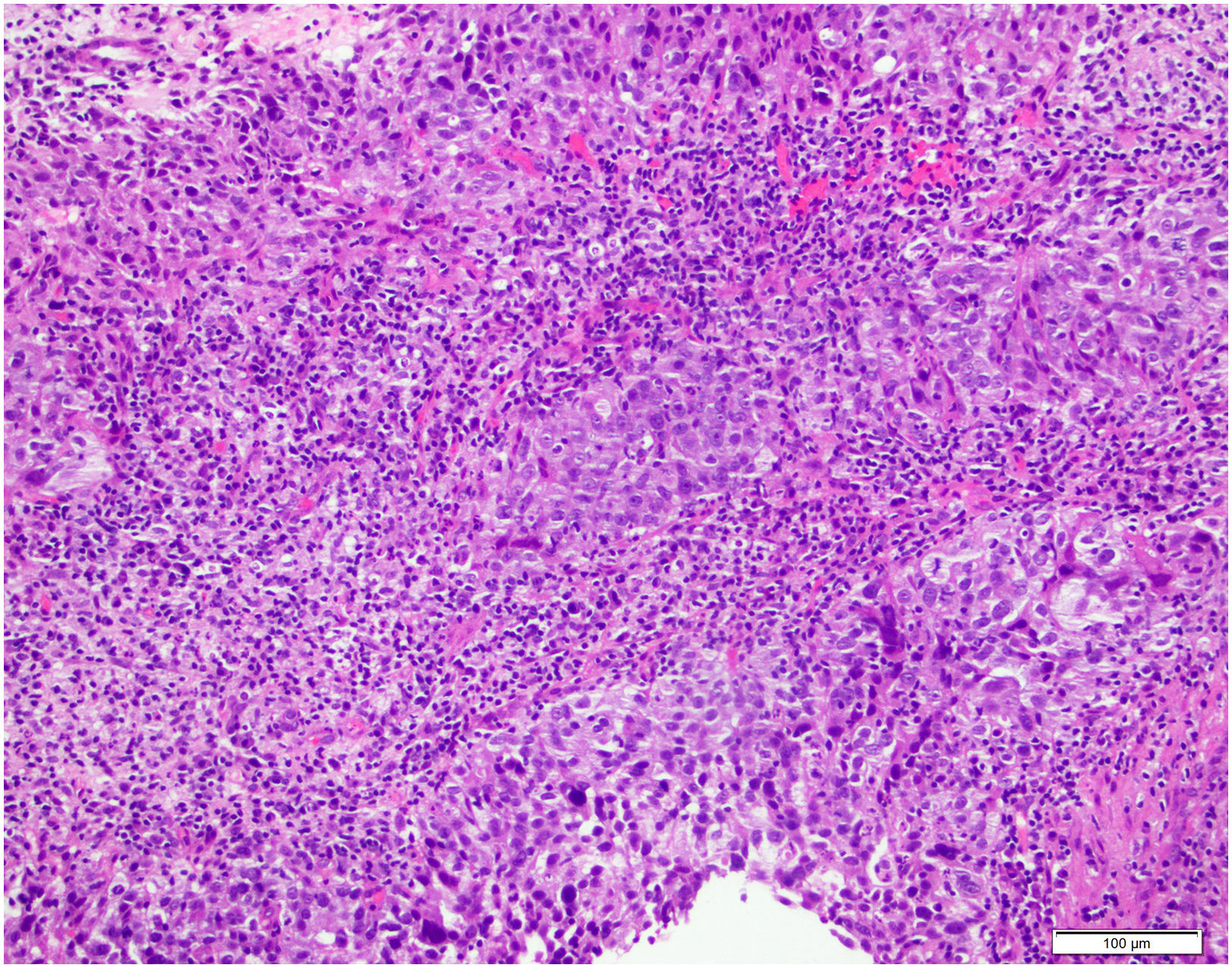
TILs in breast cancerTILs as a biomarker for immunotherapy treatmentIntratumoral stromal tumor-infiltrating lymphocytes (TILs) are mononuclear immune cells, mainly lymphocytes and plasma cells, that infiltrate tumor tissue and can be found in the tumor stroma (sTIL) or in carcinoma nests without intermediate stroma (iTIL) (Fig. 2). Immune cells have been implicated in the control of cancer development and progression, but they may also help to create an immunosuppressive microenvironment that favors tumor growth. They can be found in most solid cancers such as breast (BC), colon, cervical or lung cancer.18,19
In recent years, the determination of TILs has gained clinical relevance as an immunological biomarker.20 Several systematic reviews and meta-analyses have shown that high levels of TILs are associated with increased disease-free survival (DFS) and overall survival (OS) in triple-negative (TN) and HER2-positive (HER2+) subtypes, with no significant benefit observed in estrogen receptor-positive breast carcinomas.21,22 Furthermore, TILs have been found to be an independent predictor of response to neoadjuvant chemotherapy in BC.23
TILs in neoadjuvant therapyNeoadjuvant therapy prior to surgery is the treatment of choice in advanced BC and is increasingly used in early stages.23 The clinicopathological factors routinely used to predict prognosis may not be sufficient to distinguish between those patients who are more likely to achieve complete response (CR) with chemotherapy and those patients who are candidates for alternative therapies.24
Obtaining a CR after adjuvant treatment varies according to tumor subtype, being more frequent in tumors with a high proliferative index (TN and HER2+), and the association between failure to obtain this CR and a worse prognosis is more evident in these subtypes.25 In several trials in patients with TN and HER2+ BC, a robust lineal relationship has been observed between an increase in the number of TILs over time and improved relapse-free survival (RFS).26–29 In the BIG02–98 trial (n = 2009) the results showed that a 10% increase in TILs in TNBC was associated with a decreased risk of recurrence and a decreased risk of death. It also showed that this increase in TILs is associated with greater benefit from anthracycline-only therapy in HER2+ BC patients.26 In the NEAT/BR9601 trial, CD8+ T cells were observed to be associated with a significant reduction in the risk of death in TN BC and HER2+ BC. In estrogen receptor-negative tumors, the benefit of anthracyclines was the greatest in patients with tumors with CD8+ cells.30 The FinHER trial describes the association between higher levels of TILs and greater benefit from trastuzumab.27 Another trial showed that higher levels of TILs in residual disease were significantly associated with better RFS and better OS.31
Scoring TILsThe international working group on immuno-oncology biomarkers has established guidelines for evaluating TILs.19,32 First of all, the tumor area where we will evaluate the TILs must be defined. TILs should be quantified within the border of the invasive tumor, including both the central area of the tumor and the invasive margin, defined as the 1-mm region separating the nests of malignant cells from the nontumor tissue. TILs outside the tumor margins have to be excluded, although TILs immediately adjacent to the invasive margin, peritumoral TILs, can be evaluated when necessary. TILs should also be excluded in artifactual tumor areas with necrosis or regressive hyalinization, as well as in sites of previous biopsies.
Secondly, stromal TILs should be identified, as intratumoral TILs are more difficult to identify on hematoxylin–eosin, with sTILs being more reliable predictors of prognosis and response to treatment. All mononuclear cells, lymphocytes and plasma cells should be scored and neutrophils excluded. Next, and reviewing the slide at low magnification, we classify the inflammatory infiltrate is classified into three groups: group A (0–10% sTILs), group B (10–40% sTILs) and group C (40–90% sTILs). In group B different areas at high magnification must be evaluated. No formal recommendation can currently be given for a clinically relevant TIL threshold or thresholds.
One section (4–5 μm, magnification 200-400x) per patient can be deemed sufficient for practical purposes. However, it is recommended to evaluate additional sections whenever possible and to report the number of sections reviewed, given that the degree of heterogeneity for different tumor types is unknown. A complete assessment of the average number of TILs in the tumor area should be used, without focusing on hotspots.
TILs should be evaluated as a continuous variable, as this may provide more biologically relevant information and allow for more accurate statistical analyses. For the evaluation of the percentage values, the dissociated growth pattern of the lymphocytes must be taken into account. The percentage of stromal TILs is calculated by dividing the area occupied by mononuclear inflammatory cells by the area of tumor stroma.19
TILs in carcinoma in situ (CIS)As in invasive tumors, TILs are present in most carcinomas in situ (CIS) at different levels, however, only a minority have levels greater than 50% (6.5% of cases in the largest studies). Few studies evaluate the prevalence and relevance in ductal carcinoma in situ (DCIS). In the largest study, the highest prevalence (60%) was observed in the HER2 phenotype with TILs levels ≥50%, compared to luminal B-like/HER2 positive (23%), triple negative (7%), luminal B-like (4%) and luminal A-like (3%).33
A high level of TILs is consistently associated with worse histopathologic features, including high nuclear grade, presence of comedo necrosis, high Ki-67, a high Van Nuys Prognostic Index (VNPI), as well as being associated with triple negative and HER2+ phenotypes. They have also been found to be associated with some cases of DCIS with microinvasion. However, to date, no association between TILs and risk of recurrence or a significant association in ipsilateral breast involvement in CIS has been demonstrated.33–35
Studies on TILs in CIS showed that they are most frequently composed of CD3+ cells (CD4 > CD8), followed by CD20+ and FOXP3+ T-regulatory cells.34 The different composition of TILs could be related to tumor behavior, for example: TILs composed of high numbers of CD8+ HLA-DR-T activated cytotoxic T-cells and low numbers of CD115+ macrophages were associated with a lower risk of recurrence, whereas low numbers of CD8+ HLA-DR+ cells had a higher recurrence risk.35 Similarly, a dense chronic inflammatory infiltrate around DCIS could be associated with a high Oncotype DX score.36
These preliminary studies would support the possibility of this active adaptive immune response in DCIS being used as a target for immune therapies.34
Scoring TILs in CISThe International Immuno-Oncology Biomarker Working Group proposes a series of recommendations for evaluating TILs in CIS, in the same way as in invasive tumors, and it can be performed for each type of CIS, whether ductal or lobular, with the same methodology, whether in scenarios of pure CIS, associated with micro-invasion or with an infiltrating component, without distinguishing between TILs in CIS with different degrees of differentiation.20 In any case, the final TILs score in CIS should not contain the TILs score in the invasive component, which is reported separately.
These recommendations are for the evaluation of TILs in hematoxylin and eosin-stained specimens in formalin-fixed/paraffin-embedded tissue sections. All sections containing CIS should be evaluated. If several CIS lesions are found, an evaluation of the TILs in each lesion should be performed and the mean value should be given and not concentrated only in hotspots.
Only stromal TILs should be reported, excluding intratumoral TILs. It is recommended to define the stromal compartment as the area of specialized stroma surrounding the CIS lesions (the “periductal” area). If a DCIS is surrounded by a fibrous rim with TILs immediately adjacent to it, these should be evaluated. If the specialized stromal area is not easily distinguishable, an arbitrary stromal area extending over 2 high-power fields (HPF, x40) can be used, taking into account any type of circumferential TIL infiltration (minimal, partial, subtotal or total).
TILs that are in continuity between invasive lesions and in situ lesions, without clear distinction whether they are associated with the invasive component or the in situ lesion, should be evaluated within the 2 HPF area of the in situ component while the remaining TILs are evaluated as part of the invasive component.
TILs around normal lobules, wherever they are found, TILs in tumor areas with crush artifacts, necrosis, regressive hyalinization, as well as at the site of previous biopsy should be excluded from the assessment.
The recommendation for reporting this score should be as a % of the surface area of the stroma area occupied by TILs as a parameter (0–100%), reporting the results in as much detail as you are comfortable with, keeping in mind that lymphocytes typically do not form solid cellular aggregates and, therefore, the designation “100% stromal TILs” would still allow for some empty space between individual lymphocytes.
At present no formal recommendation is given about clinically relevant TIL limits. From the group's point of view, it is currently more important to determine a valid assessment methodology than limits for clinical use, which could be determined once a robust method is available.23
Computational scoring of TILsVisual scoring of TILs has its limitations, including inter-rater variability, the time required for routine diagnóstico, and limitations of visual perception, with the risk of creating bias in the assessment. This is why computational scoring becomes a possible tool for this purpose, enabling a quantitative and objective assessment, addressing the limitations of perception and mitigating the high clinical demand, and even extending the assessment of TILs to other fields.
Research in the use of machine learning algorithms to analyze histological specimens, as well as the development of deep-learning-based pattern recognition algorithms, have shown promising results in challenging tasks. Their success has been transferred to digital pathology, excelling in tasks such as mitosis detection, lymph node metastasis identification, tissue segmentation and computational evaluation of TILs, although these methods are, for the time being, experimental.
For the development of these algorithms it is important to take into account several factors: to accurately include the inflammatory component, including plasma cells that may escape the algorithm because of their larger cytoplasm, and to properly exclude normal tissue, as small or perpendicularly cut stromal cells and even prominent nucleoli, apoptotic bodies, necrosis, neutrophils and some tumor cells can be misclassified and are frequently confused in the scoring of TILs.
These new technologies could be implemented in two ways: in a purely assistive manner, using algorithms to guide the site in which to conduct TILs assessment, providing a quantitative estimate of TIL density within the regions of interest identified by the pathologist, thus reducing ambiguity in visual estimation, and/or calculating an overall estimated TIL score, improving workflow efficiency; or in an automated and unsupervised manner, achieving cost reduction and expediting the evaluation of TILs, although the latter is more used in the research context, given that in a clinical setting it is necessary to go through exhaustive quality standards as they are not supervised.37
FundingNo funding has been used for this review.
Ethical committee approvalThe characteristics of the review exempt it from ethics committee approval.
Conflict of interestThe authors declare no conflict of interest.