The Spanish Guideline on the Management of Asthma, better known by its acronym in Spanish GEMA, has been available for more than 20 years. Twenty-one scientific societies or related groups both from Spain and internationally have participated in the preparation and development of the updated edition of GEMA, which in fact has been currently positioned as the reference guide on asthma in the Spanish language worldwide.
Its objective is to prevent and improve the clinical situation of people with asthma by increasing the knowledge of healthcare professionals involved in their care. Its purpose is to convert scientific evidence into simple and easy-to-follow practical recommendations. Therefore, it is not a monograph that brings together all the scientific knowledge about the disease, but rather a brief document with the essentials, designed to be applied quickly in routine clinical practice. The guidelines are necessarily multidisciplinary, developed to be useful and an indispensable tool for physicians of different specialties, as well as nurses and pharmacists.
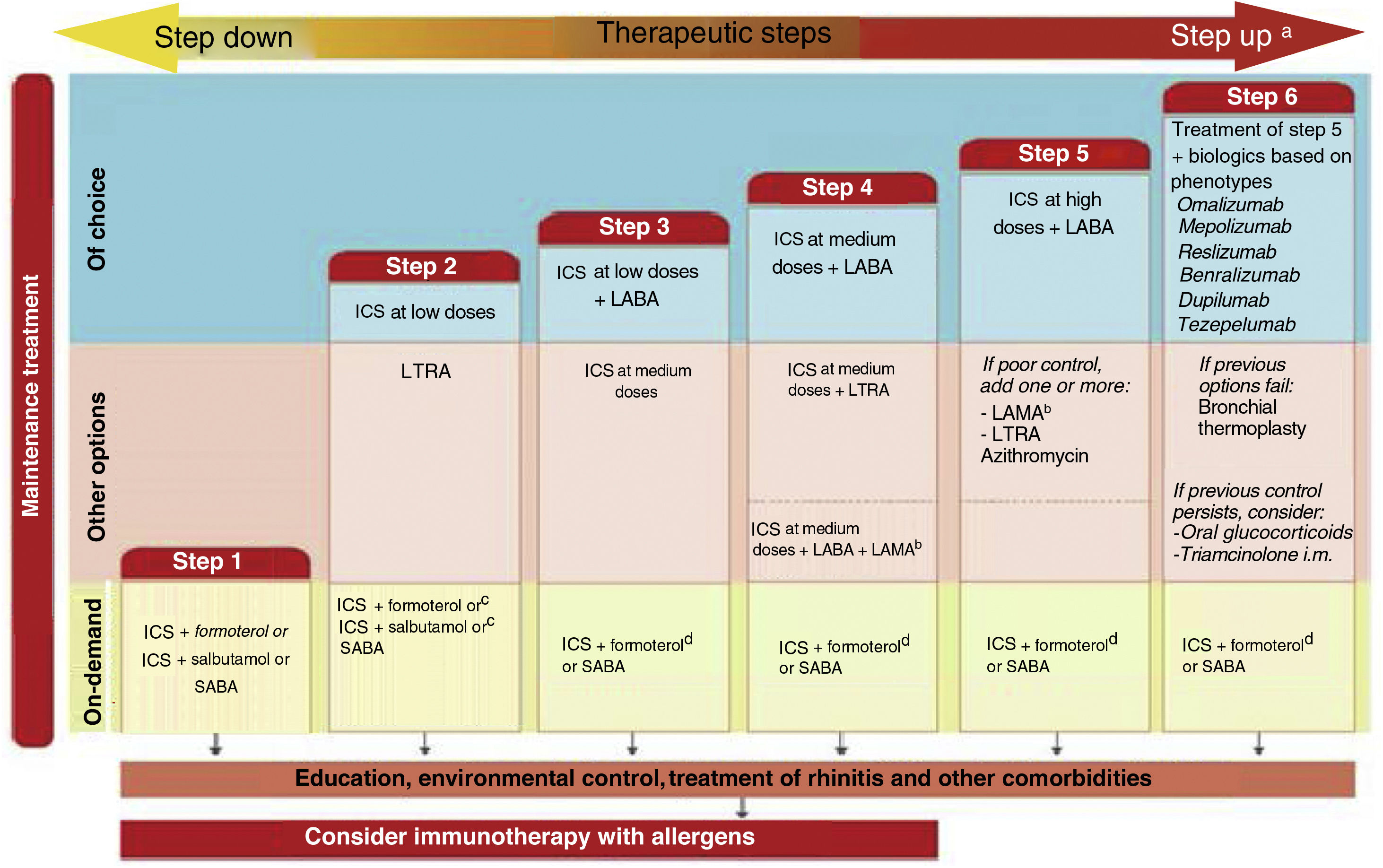
Probably the most outstanding aspects of the guide are the recommendations to: establish the diagnosis of asthma using a sequential algorithm based on objective diagnostic tests; the follow-up of patients, preferably based on the strategy of achieving and maintaining control of the disease; treatment according to the level of severity of asthma, using six steps from least to greatest need of pharmaceutical drugs, and the treatment algorithm for the indication of biologics in patients with severe uncontrolled asthma based on phenotypes. And now, in addition to that, there is a novelty for easy use and follow-up through a computer application based on the chatbot-type conversational artificial intelligence (ia-GEMA).
La Guía Española para el Manejo del Asma, mejor conocida por su acrónimo en español, GEMA, está a nuestra disposición desde hace más de veinte años. Veintiuna sociedades científicas o grupos relacionados, tanto de España como de otros países, han participado en la preparación y desarrollo de la edición actualizada de GEMA que, de hecho, se ha posicionado en la actualidad a nivel mundial como la guía de referencia sobre asma en lengua española.
Su objetivo es prevenir y mejorar la situación clínica de las personas con asma, aumentando el conocimiento de los profesionales sanitarios involucrados en su cuidado. Su propósito es convertir la evidencia científica en recomendaciones prácticas sencillas y fáciles de seguir. Por lo tanto, no se trata de una monografía que reúna todo el conocimiento científico sobre la enfermedad, sino más bien de un documento conciso con lo esencial, diseñado para ser aplicado rápidamente en la práctica clínica de rutina. Las recomendaciones son necesariamente multidisciplinares, están desarrolladas para ser útiles y una herramienta indispensable para médicos de diferentes especialidades, así como para profesionales de enfermería y farmacia.
Seguramente, los aspectos más destacados de la guía son las recomendaciones para: establecer el diagnóstico del asma utilizando un algoritmo secuencial basado en pruebas diagnósticas objetivas; el seguimiento de los pacientes, preferentemente basado en la estrategia de lograr y mantener el control de la enfermedad; el tratamiento según el nivel de gravedad del asma utilizando seis escalones, desde la menor hasta la mayor necesidad de medicamentos, y el algoritmo de tratamiento basado en fenotipos para la indicación de biológicos en pacientes con asma grave no controlada. A esto se suma ahora una novedad para su fácil uso y seguimiento a través de una aplicación informática basada en la inteligencia artificial conversacional de tipo chatbot (ia-GEMA).
Once again, I have the privilege and satisfaction of presenting the 2023 update of the Spanish Guideline for the Management of Asthma (GEMA), GEMA 5.3. Furthermore, this is a special edition as we celebrate the 20th anniversary of the acronym “GEMA.” Although we consider that the guideline was born in 1997 with the SEPAR-semFYC consensus on asthma (GEMA 1.0), it is worth noting that it was the first consensus conducted by SEPAR with another society. GEMA is now an international multidisciplinary guideline in which 17 scientific societies from Spain, Latin America (ALAT), and Portugal (SPP) participate. Twenty years later, it is a source of great pride for us to mention that GEMA is the world's reference guideline in Spanish for this disease. It is a global guide for a globalized world.
As in previous editions, in this one we have followed the same procedure to carry out the usual annual update, which basically consisted of incorporating new and relevant bibliographic references published in 2022. For this purpose, “our” four experts in reviewing the scientific literature, Drs. Astrid Crespo (Pulmonology), Miguel Ángel Lobo (Family Medicine), Álvaro Gimeno (Pediatrics), and Manuel Rial (Allergology), reviewed the articles published during that period in the main indexed journals of international literature. This review selected the 40 most appropriate citations for the update, which were used to edit the proGEMA5.3 text. This bibliographic selection, although not exclusively, was primarily used by the members of the GEMA Executive Committee to discuss and decide on the novelties for this new edition.
The main changes, edited in blue for easy identification, can be grouped into the following six conceptual areas:
Remission in asthma. A fashion subject of this year could not have been overlooked in the guide. However, all of this is provisional because, along with the Asthma Forum, SEPAR is sponsoring a consensus on the topic that involves over 120 asthma specialists, and its outcome will be binding for the future GEMA 5.4.
Diagnosis of asthma in adults and children. The publication of the recent ERS/ATS consensus on spirometry required the addition as a complementary criterion of bronchodilation in adults of the new ≥ 10% of the predicted FEV1 or FVC value, besides the classic criterion of ≥ 12% and 200mL of FEV1 after inhalation of the bronchodilator.
Treatment of asthma. Theophyllines are removed from the therapeutic armamentarium. The consequences of excessive use of SABA (SABINA studies) are described. Triple therapy in a single inhaler of fluticasone/vilanterol/umeclidinium, is incorporated into the treatment of severe asthma, which has been approved by regulatory agencies in Hispanic America but not by European agencies.
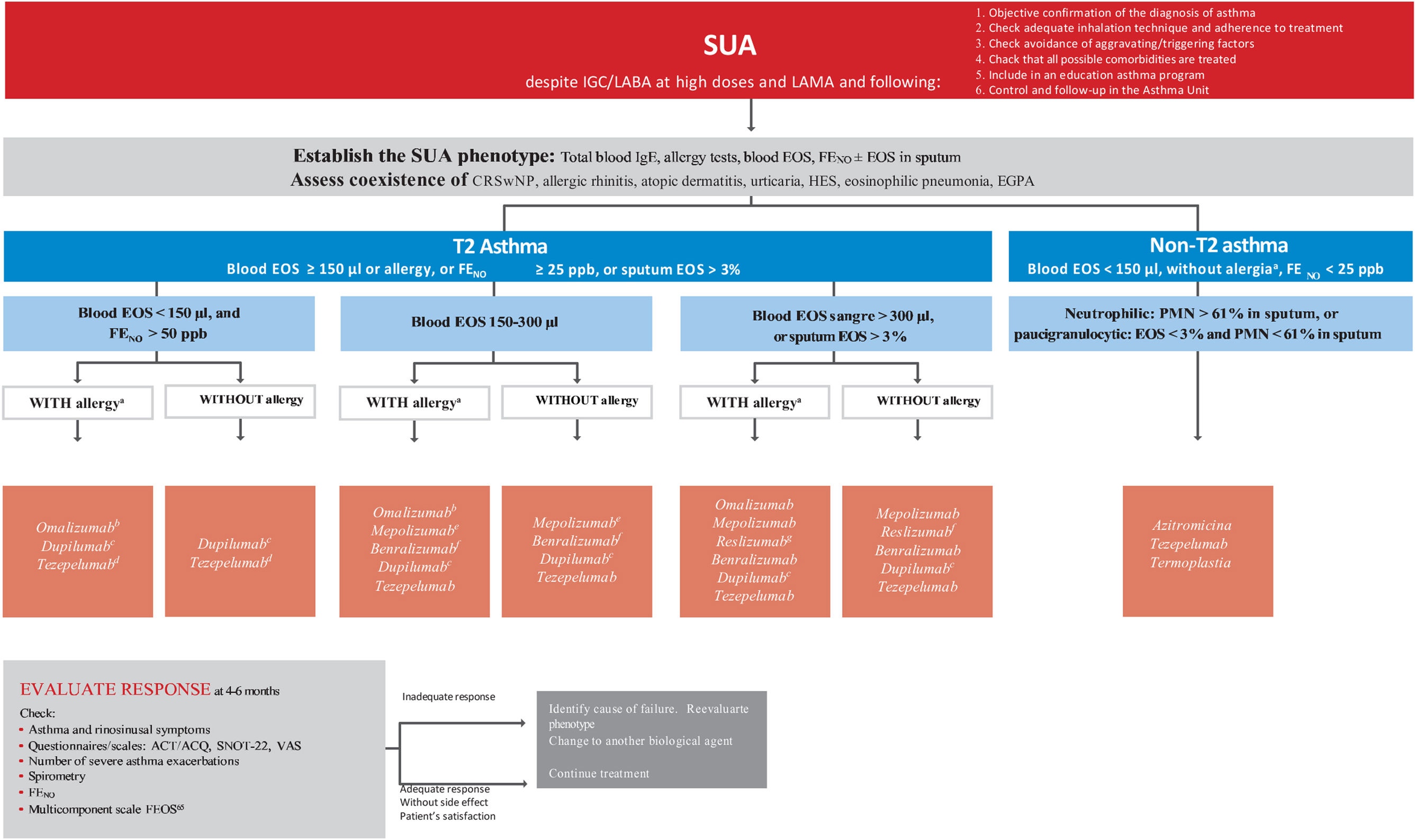
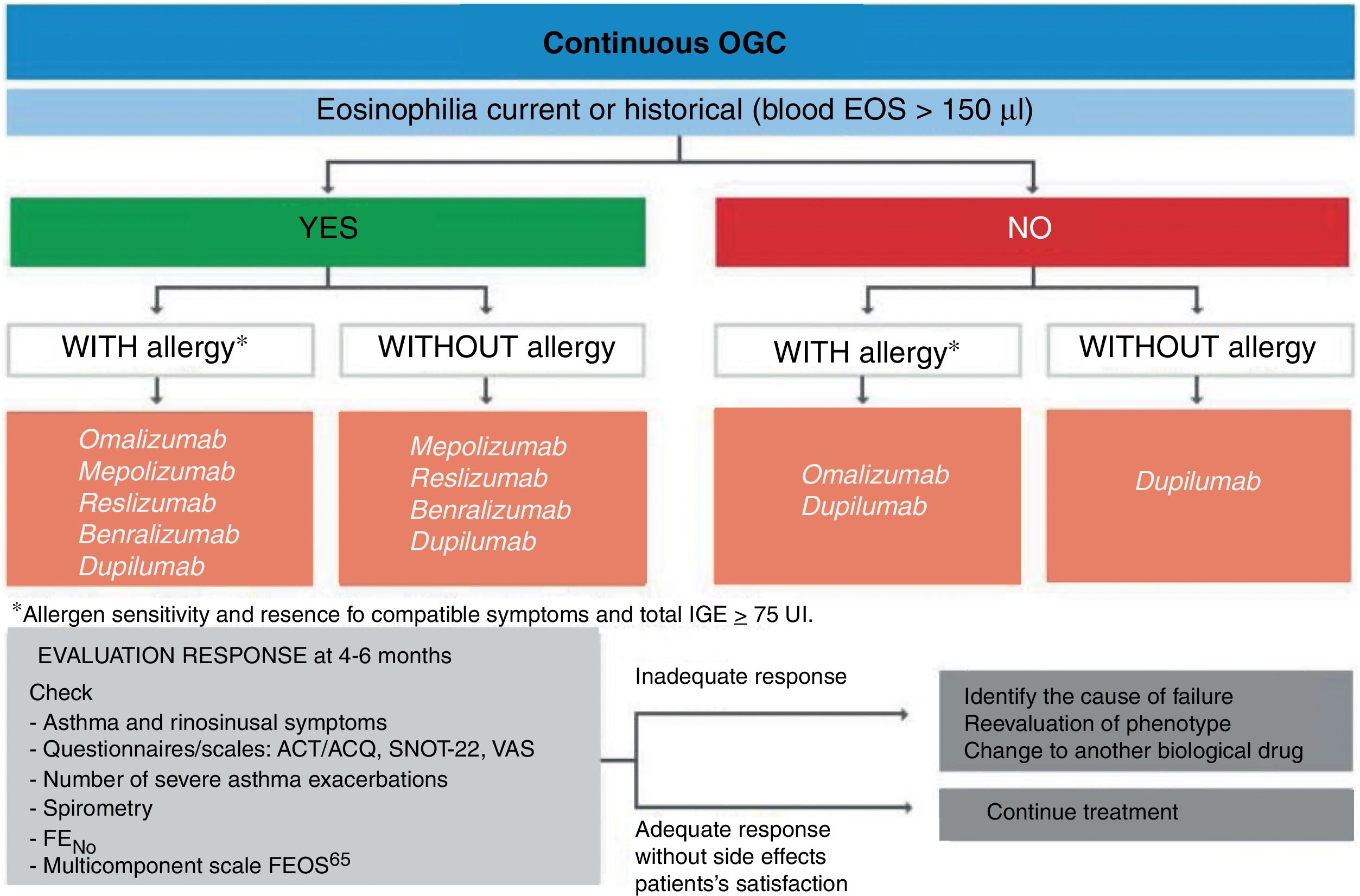
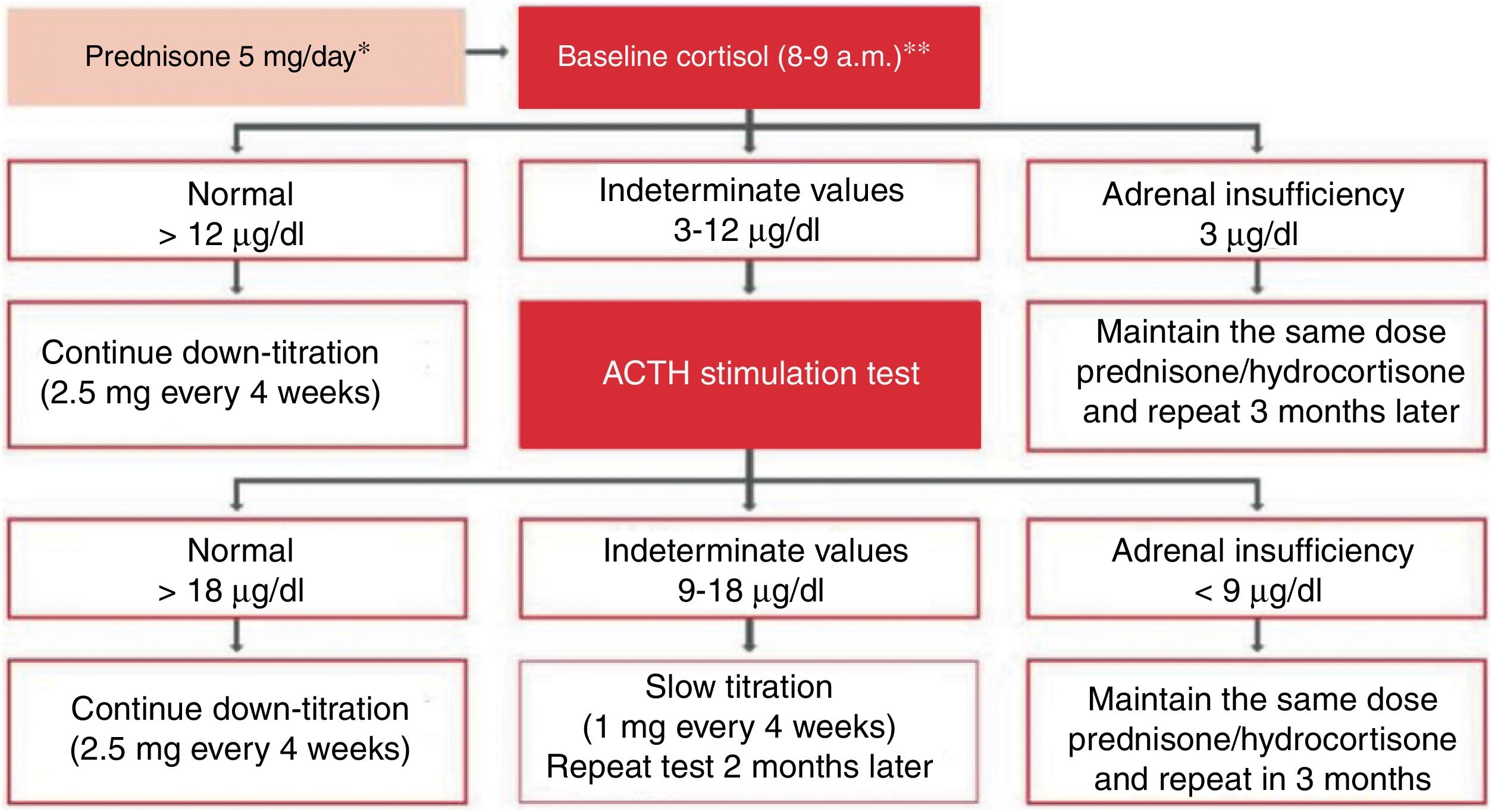
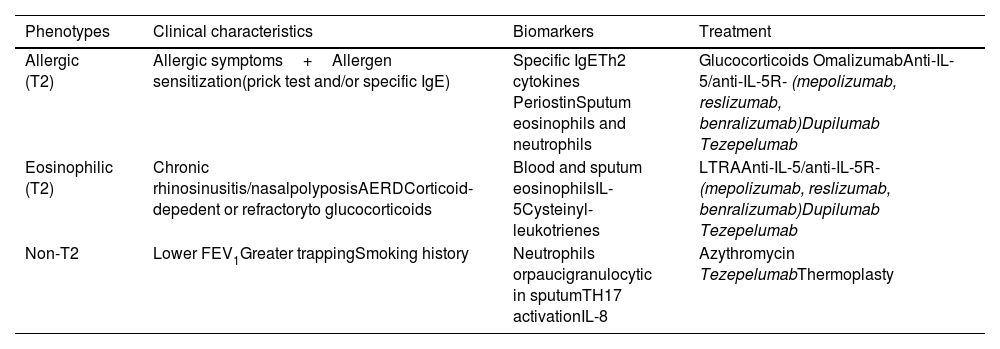
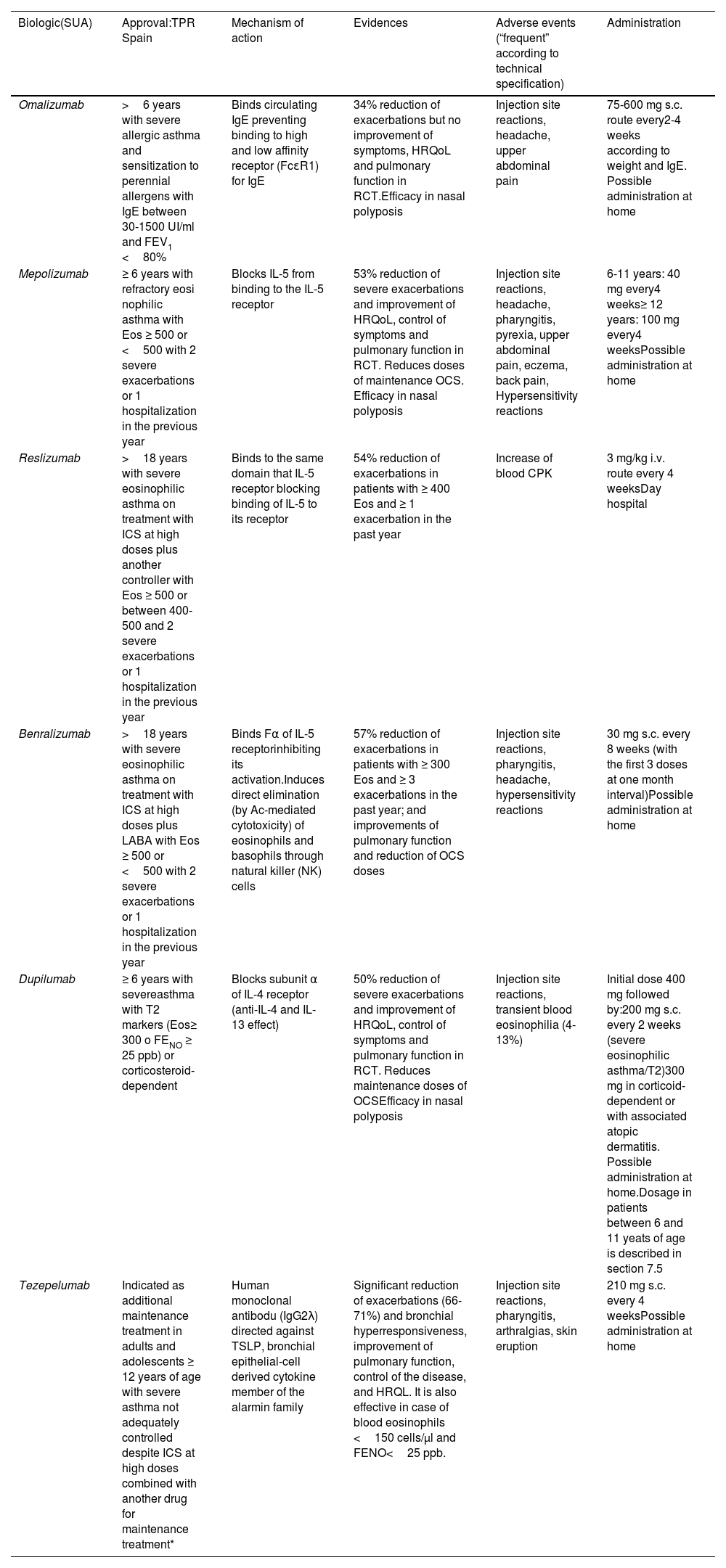
Treatment of severe uncontrolled asthma. A new definition is introduced, which includes the use of a third drug (LAMA) at high doses of ICS/LABA to establish it. New algorithms (with a new design) for the treatment of severe uncontrolled asthma are provided, including a specific one for corticosteroid-dependent asthma. Tezepelumab is included in the therapeutic algorithm, and benralizumab in the treatment of T2 asthma with blood eosinophils between 150-300/μL.
New recommended questionnaires. The Asthma Impairment and Risk Questionnaire (AIRQ) to determine the current level of control and future risk, a weighted scoring system is used, taking into account FEV1 (forced expiratory volume in 1 second), exacerbations, oral corticosteroid use, and asthma symptoms. This scoring system helps assess the response to biological drugs in asthma (FEOS). The Sino-Nasal Outcome Test 22 (SNOT-22) is used to assess the impact and quality of life caused by rhinosinusitis.
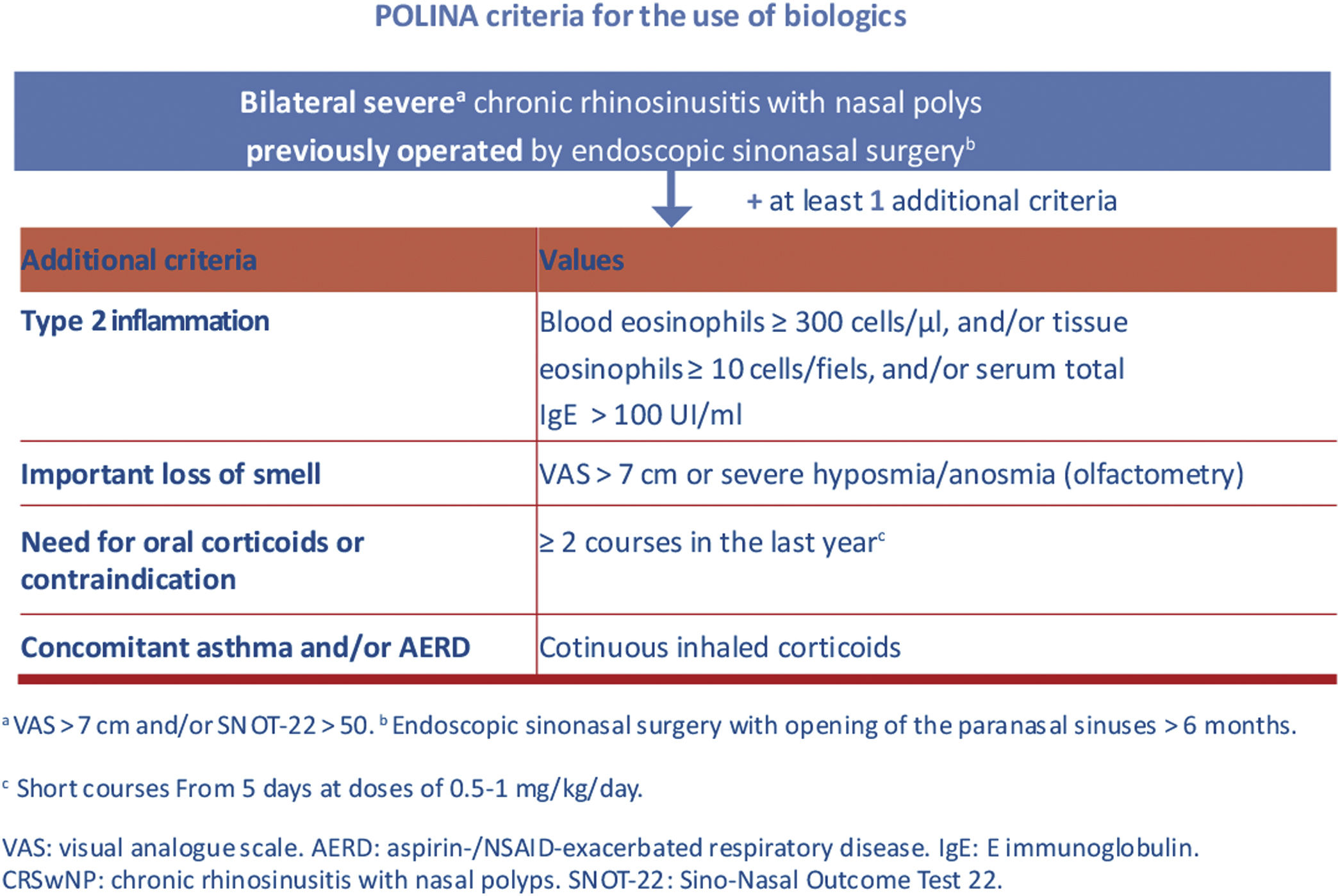
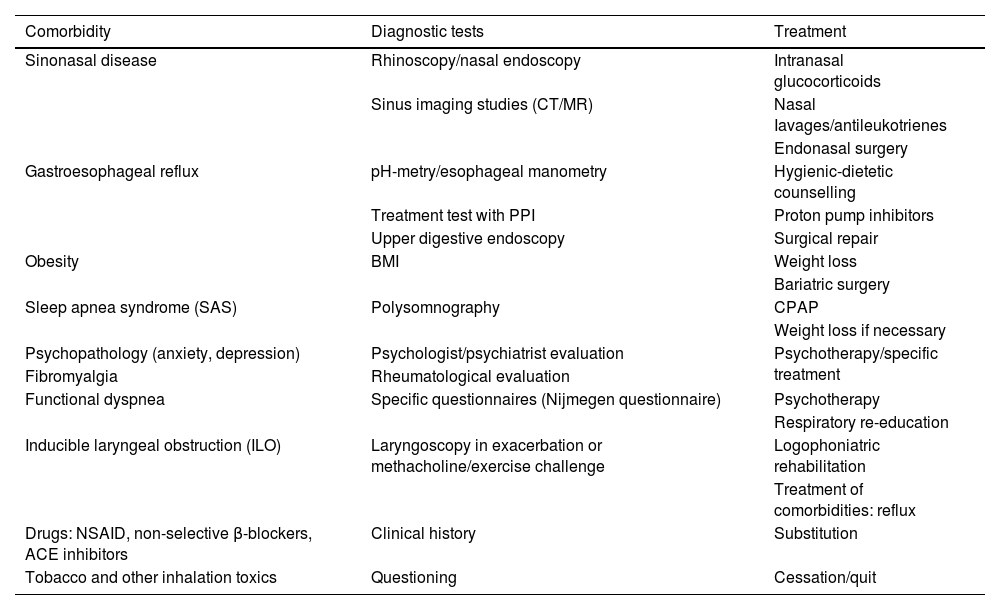
Finally, it is important to highlight some of the most relevant contributions from the recent POLINA consensus (chronic rhinosinusitis and nasal polyposis), such as the proposed stepped treatment approach based on severity and control, as well as the criteria for prescribing biological drugs.
However, without diminishing the importance of these changes, which are undoubtedly very relevant, perhaps the most notable innovation in this edition is the incorporation of a new tool called iaGEMA. It is a computer application that includes artificial intelligence software with which one can interact (chatbot-like). The application is addressed to the healthcare professional and will be capable of providing GEMA recommendations in response to real clinical questions regarding the care of asthma of patients. We believe that this will be the first guide that incorporates this technology, and we anticipate that the future of guideline implementation will involve technologies like the one offered by this new iaGEMA application.
Finally, on behalf of the members of the GEMA Executive Committee, the true “engine” behind the guide, it is a great personal satisfaction to have been able, once again, to fulfill the commitment of updating the guide. I would like to express my gratitude to all of them, as well as to the four expert reviewers and the staff at Luzan5, for their hard work and invaluable support, which have been crucial for successfully achieving the present GEMA 5.3 edition.
Dr. Vicente Plaza Moral
On behalf of the Executive Committee of GEMA 5.3
ObjectiveThe main objective of the present guideline is to improve the control and quality of life of patients with asthma by enhancing the technical expertise of healthcare professionals in charge of them, particularly in aspects related to prevention and diagnosis-therapeutic evaluation of the disease.
GEMA, however, is a platform that brings together a series of complementary actions, all designed to reach the aforementioned objective, among which this document acquires a special relevance: an evidence-based clinical practice guideline. Further documents will complete the GEMA “family” (e.g., pocket-size GEMA, GEMA for patients, GEMA for educators, etc.).
Specifically, the current document (clinical practice guideline) as well as the whole strategy conforming the GEMA 5.3 platform, is addressed to professions in the setting of Family and Community Medicine; Primary Care Pediatrics; Pneumology, Allergology, Pediatric Pneumology and Allergology; Otorhinolaryngology; Pharmacology; Hospital and Primary Care Pharmacy; General and Specialized in Respiratory Diseases Nursing, educators, teachers, patients, and patients’ relatives and caregivers.
MethodSearching for evidence. Based on the previous (complete) edition of GEMA,1 published in 2015, and following the recommendations for Updating Clinical Practice Guidelines in the National Health System,2 the members of the Executive Committee undertook a systematic search of the literature to select and evaluate articles on asthma published from 2015 to 2020 (Pro-GEMA Project). After reviewing high impact factor journals of Pneumology, Allergology, Pediatrics, Primary Care, Internal Medicine and Otorhinolaryngology, which were also classified within the two first quartiles of their specialty field, a total of 120 documents were selected (abstracts available at http://www.progema-gemasma.com/foco.html) that were considered of interest for updating this guideline. All these documents were provided to the authors for evaluation. Furthermore, authors were encouraged to perform their own literature searches for specific topics. To this purpose, the procedure normally established to develop clinical practice guidelines was followed.3 Also, the reference lists of the main international practice guidelines4,5 were reviewed in order to identify the most relevant systematic reviews and clinical trials. These guidelines were searched in specialized databases (National Guideline Clearinghouse, National Library of Guidelines) and the TRIP medical literature meta-search engine database. Databases from the Centre for Reviews and Dissemination (DARE y HTA database) and The Cochrane Library were reviewed in order to identifying systematic reviews and evaluations of additional technologies. The search was completed with an update of the systematic reviews from the date of search and relevant studies included in the main electronic databases of original studies (MEDLINE, CENTRAL and EMBASE).
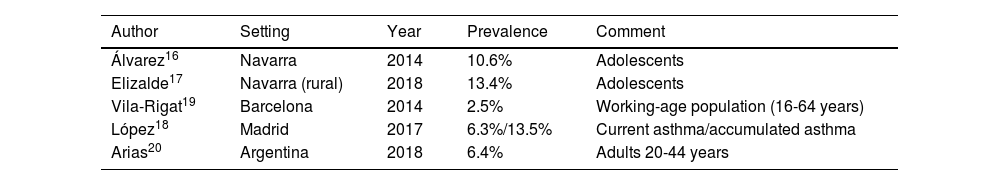
Classification of evidence. To assess the quality of evidence, an alphabetic classification was used (table 1) that classifies the information into four categories (A, B, C, D) reflecting the grade of confidence in the results obtained in the available studies. Category A would correspond to a high quality evidence and D to a very low quality. For category A. confidence in the results is high and the potential modification of available findings by further studies is unlikely. In contrast, for lower categories, C or D, the confidence level will be low or very low, and there is a high probability that further studies will modify the results, or even the direction of the effect. However, it must be remember that this system is very useful to categorize the evidence regarding therapeutic efficacy of drugs or other therapeutic actions, but the effect of other interventions may be underestimated. This can explain why evidence from studies aimed at determining the appropriateness of some diagnostic procedures has often been assigned a level of evidence C.
Classification of the quality of evidence.
| Categories of evidence | |
|---|---|
| A | SR of RCTs with or without MA; and RCTs with low risk of bias. Evidence based on a substantial number of well-designed studies with consistent results. |
| B | SR of RCTs with or without MA; and RCTs with moderate risk of bias. Evidence obtained from a limited number of studies and/or inconsistent results. |
| C | Evidence obtained from non-randomized, observational or uncontrolled studies. |
| D | Clinical experience or scientific literature that cannot be included in category C. |
SR: systematic reviews; RCTs: randomized controlled trials; MA: meta-analysis.
Taking into account the recent emergence of new approaches used to classify the quality of evidence based on aspects other than the study design,6,7 some of the characteristics of the GRADE framework were used,8 although the GRADE system was not applied in full.
Classification of recommendations. To classify the relevance and consistency of clinical recommendations, the same method used in the previous editions of GEMA was followed, in which recommendations were categorized in two levels: robust recommendations (R1), that is, those to be associated with more benefits than risks according to the opinion of the group of authors, and weak recommendations (R2), that is, those in which some uncertainty exists as to whether its application might entail more benefits than risks. To carry out this distribution in R1 o R2, the quality of information was weighed (based on the above-mentioned classification), along with the balance between risks and benefits of interventions, the costs (according to the available specialized literature), and the patients’ values and preferences (through the participation of FENAER members).
The categorization of the recommendation level was established by consensus, first of the authors (see below for the working method used) and finally by the agreement of reviewers (through the Delphi method), whose opinions were binding for the final version of all recommendations.
Drafting text and building consensus of recommendations. The writing process was based on a pyramidal consensus system going from a multidisciplinary thematic mini-consensus by chapter to a large final consensus among all authors and reviewers. Based on the document of the previous edition and the new references on asthma published between 2015 and 2020, a group of authors and coordinators made up by experts from the participating scientific societies drew up the new chapter sections they were assigned (including the classification of evidence and recommendations). The authors submitted their texts to each chapter coordinators who were members of the GEMA Executive Committee. After unifying and reviewing the texts, the chapter coordinator submitted the draft to the authors of each chapter in order to reach the first partial consensus. After implementation of changes, all chapters were brought together in one single document which, in turn, was sent to all authors and coordinators for telematics discussion (and for face-to-face group discussion, when necessary) and approval. The resulting document was submitted to experts in the methodology of clinical practice guidelines from the INPECS (Institute for Clinical and Healthcare Excellence), who made a critical review of the methodology and writing of both the text and the recommendations. Finally, after these modifications and improvements, recommendations were revised and agreed on (through the Delphi method) by a group of experts in asthma from the participating societies. Recommendations not achieving a certain consensus level were removed from the final document.
Method followed for bibliographic updating of GEMA 5.3. Four asthma experts, Drs. Astrid Crespo (Pulmonology), Miguel Ángel Lobo (Family Medicine), Álvaro Gimeno (Pediatrics), and Manuel Rial (Allergology), reviewed the articles published on the disease since the previous GEMA update (GEMA 5.2). They focused on journals with high impact factors, many of which are ranked in the first quartiles of the specialties of Pulmonology, Allergology, Pediatrics, Family Medicine, and Internal Medicine. As in previous instances, this selection of articles was predominantly (although not exclusively) used by the members of the GEMA Executive Committee to discuss and decide on the majority of novelties to be included in the new GEMA 5.3.
Editorial independenceThe GEMA5.0 project was funded by pharmaceutical companies listed on the back cover of the document. The viewpoints of these funding bodies did not influence the content of the guide.
The authors of this guide declare that in the past two years, they have received honoraria for their participation in meetings, congresses, or research projects organized by the following pharmaceutical companies: ALK, AstraZeneca, Bial, Boehringer-Ingelheim, Chiesi, Esteve, GlaxoSmithKline, Leti, Menarini, MSD, Mundipharma, Novartis, Orion, Pfizer, Sanofi, Teva, and Zambón.
1Introduction1.1DefinitionAsthma is a syndrome that includes various clinical phenotypes that share similar clinical manifestations, probably of different etiologies. Classically, it is defined as a chronic inflammatory disease of the respiratory tract involving various cells and mediators of inflammation. It is partially influenced by genetic factors and is characterized by bronchial hyperresponsiveness and a variable degree of airflow obstruction that is totally or partially reversible by either the action of drugs or spontaneously.9 As a chronic disease included in the current different strategies for the care of patients with chronic conditions, the objective of asthma management is to achieve and maintain control of the disease and prevention of future risks, particularly exacerbations, which can be life-threatening and generate a burden for the society.10
1.2PrevalenceAsthma prevalence is highly variable worldwide, ranging from 2% in Tartu (Estonia) to 11.9% in Melbourne (Australia). Similarly, the prevalence of wheezing (over the last 12 months) varies from 4.1% in Mumbai (India) to 32% in Dublin (Ireland).11,12
According to the 2015 Global Burden of Disease study, the prevalence of asthma has increased worldwide by 12.6% from 1990 to 2015. On the contrary, the age-standardized mortality rate has decreased by almost 59% in the same period.13 This increase in prevalence mainly affects middle-aged individuals and women and can be explained by a rise in allergic asthma, with stabilization of non-allergic asthma.14
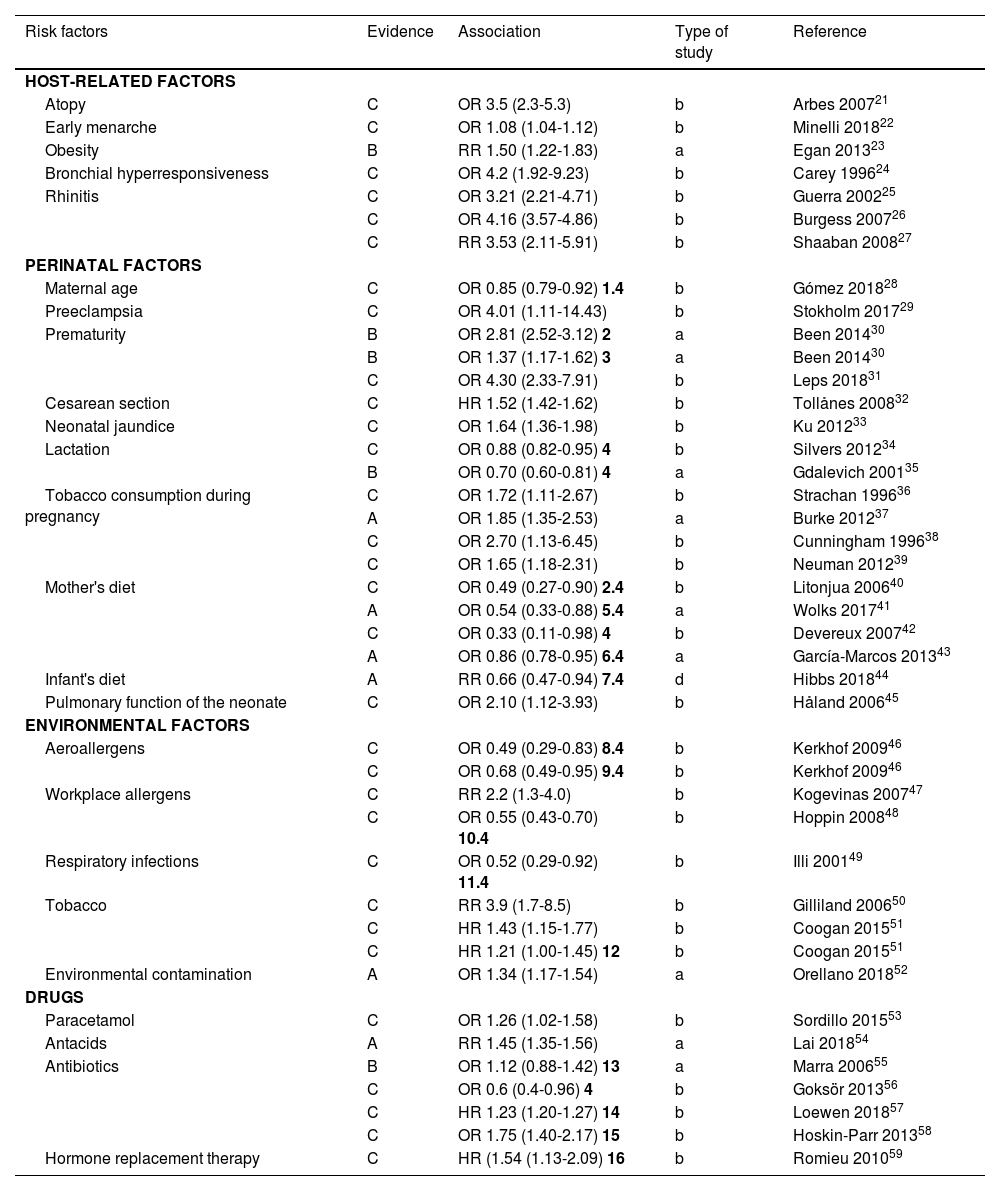
The European Respiratory Health Study in Spain reported prevalence rates of 4.7% in Albacete, 3.5% in Barcelona, 1.1% in Galdakao, 1% in Huelva, and 1.7% in Oviedo.15 Other recent studies report highly variable prevalences based on different variables, such as age (adolescents), ranging from 10.6%16 to 13.4%;17 the method used (self-reported by the patient), 13.5%;18 or the study setting (work environment), 2.5%.19
In Spain, a study carried out in Navarre showed a prevalence of 10.6% in adolescents.16
In another study conducted in rural areas of Navarre, a prevalence of asthma of 13.4% was found among adolescents. The prevalence was slightly higher in females (13.7% compared to 10.9% in males), with rhinitis, wheezing (especially associated with physical activity), and dry cough as related symptoms.17
A study carried out in Argentina showed a prevalence of asthma in adults (between 20 and 44 years of age) of 6.4%20 (table 2).
Prevalence of asthma in adults and adolescents.
| Author | Setting | Year | Prevalence | Comment |
|---|---|---|---|---|
| Álvarez16 | Navarra | 2014 | 10.6% | Adolescents |
| Elizalde17 | Navarra (rural) | 2018 | 13.4% | Adolescents |
| Vila-Rigat19 | Barcelona | 2014 | 2.5% | Working-age population (16-64 years) |
| López18 | Madrid | 2017 | 6.3%/13.5% | Current asthma/accumulated asthma |
| Arias20 | Argentina | 2018 | 6.4% | Adults 20-44 years |
Risk factors for the development of asthma syndrome should be distinguished from triggers of asthma symptoms or asthma exacerbations.
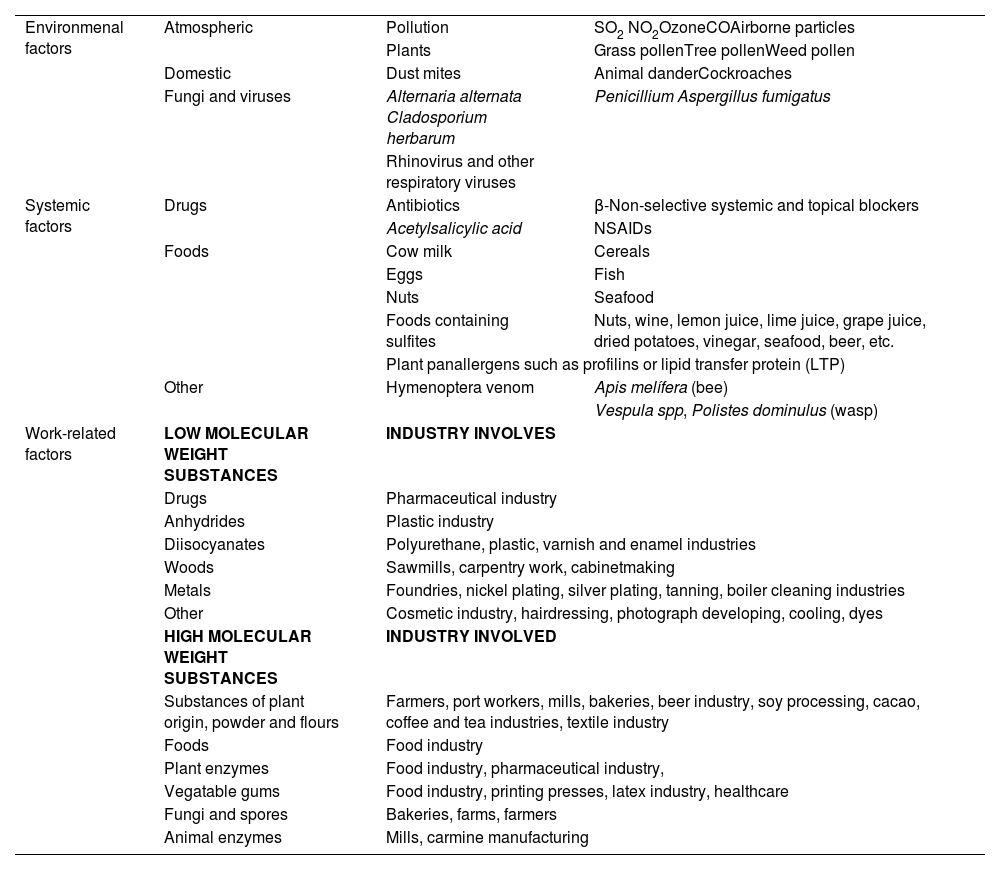
In relation to factors associated with the development of asthma, those better known or with a higher degree of association are shown in table 3. Many host-related factors are perinatal, while environmental factors vary greatly and can impact on patients of different age groups.
Factors associated with the development of asthma.
| Risk factors | Evidence | Association | Type of study | Reference |
|---|---|---|---|---|
| HOST-RELATED FACTORS | ||||
| Atopy | C | OR 3.5 (2.3-5.3) | b | Arbes 200721 |
| Early menarche | C | OR 1.08 (1.04-1.12) | b | Minelli 201822 |
| Obesity | B | RR 1.50 (1.22-1.83) | a | Egan 201323 |
| Bronchial hyperresponsiveness | C | OR 4.2 (1.92-9.23) | b | Carey 199624 |
| Rhinitis | C | OR 3.21 (2.21-4.71) | b | Guerra 200225 |
| C | OR 4.16 (3.57-4.86) | b | Burgess 200726 | |
| C | RR 3.53 (2.11-5.91) | b | Shaaban 200827 | |
| PERINATAL FACTORS | ||||
| Maternal age | C | OR 0.85 (0.79-0.92) 1.4 | b | Gómez 201828 |
| Preeclampsia | C | OR 4.01 (1.11-14.43) | b | Stokholm 201729 |
| Prematurity | B | OR 2.81 (2.52-3.12) 2 | a | Been 201430 |
| B | OR 1.37 (1.17-1.62) 3 | a | Been 201430 | |
| C | OR 4.30 (2.33-7.91) | b | Leps 201831 | |
| Cesarean section | C | HR 1.52 (1.42-1.62) | b | Tollånes 200832 |
| Neonatal jaundice | C | OR 1.64 (1.36-1.98) | b | Ku 201233 |
| Lactation | C | OR 0.88 (0.82-0.95) 4 | b | Silvers 201234 |
| B | OR 0.70 (0.60-0.81) 4 | a | Gdalevich 200135 | |
| Tobacco consumption during pregnancy | C | OR 1.72 (1.11-2.67) | b | Strachan 199636 |
| A | OR 1.85 (1.35-2.53) | a | Burke 201237 | |
| C | OR 2.70 (1.13-6.45) | b | Cunningham 199638 | |
| C | OR 1.65 (1.18-2.31) | b | Neuman 201239 | |
| Mother's diet | C | OR 0.49 (0.27-0.90) 2.4 | b | Litonjua 200640 |
| A | OR 0.54 (0.33-0.88) 5.4 | a | Wolks 201741 | |
| C | OR 0.33 (0.11-0.98) 4 | b | Devereux 200742 | |
| A | OR 0.86 (0.78-0.95) 6.4 | a | García-Marcos 201343 | |
| Infant's diet | A | RR 0.66 (0.47-0.94) 7.4 | d | Hibbs 201844 |
| Pulmonary function of the neonate | C | OR 2.10 (1.12-3.93) | b | Håland 200645 |
| ENVIRONMENTAL FACTORS | ||||
| Aeroallergens | C | OR 0.49 (0.29-0.83) 8.4 | b | Kerkhof 200946 |
| C | OR 0.68 (0.49-0.95) 9.4 | b | Kerkhof 200946 | |
| Workplace allergens | C | RR 2.2 (1.3-4.0) | b | Kogevinas 200747 |
| C | OR 0.55 (0.43-0.70) 10.4 | b | Hoppin 200848 | |
| Respiratory infections | C | OR 0.52 (0.29-0.92) 11.4 | b | Illi 200149 |
| Tobacco | C | RR 3.9 (1.7-8.5) | b | Gilliland 200650 |
| C | HR 1.43 (1.15-1.77) | b | Coogan 201551 | |
| C | HR 1.21 (1.00-1.45) 12 | b | Coogan 201551 | |
| Environmental contamination | A | OR 1.34 (1.17-1.54) | a | Orellano 201852 |
| DRUGS | ||||
| Paracetamol | C | OR 1.26 (1.02-1.58) | b | Sordillo 201553 |
| Antacids | A | RR 1.45 (1.35-1.56) | a | Lai 201854 |
| Antibiotics | B | OR 1.12 (0.88-1.42) 13 | a | Marra 200655 |
| C | OR 0.6 (0.4-0.96) 4 | b | Goksör 201356 | |
| C | HR 1.23 (1.20-1.27) 14 | b | Loewen 201857 | |
| C | OR 1.75 (1.40-2.17) 15 | b | Hoskin-Parr 201358 | |
| Hormone replacement therapy | C | HR (1.54 (1.13-2.09) 16 | b | Romieu 201059 |
HR: hazard ratio; OR: odds ratio. Type of study: a meta-analysis-systematic review, b prospective epidemiological study, c retrospective epidemiological study, d clinical trial.
Comments: 1 female sex, 2 very preterm, 3 moderate preterm, 4 protective factor, 5 level of vitamin D at the beginning of pregnancy, 6 Mediterranean diet, 7 vitamin D supplement, 8 dog exposure, 9 cat exposure, 10 living on a farm, 11 non-respiratory viral infection, 12 passive smoking, 13 no association, 14 prenatal exposure, 15 postnatal exposure, 16 with estrogens only.
On the other hand, the most common triggers of asthma symptoms or exacerbations are presented in table 4. It is important to be aware of them because they can lead to serious situations and, therefore, should be avoided.
Triggers of asthma symptoms and exacerbations.
| Environmenal factors | Atmospheric | Pollution | SO2 NO2OzoneCOAirborne particles |
| Plants | Grass pollenTree pollenWeed pollen | ||
| Domestic | Dust mites | Animal danderCockroaches | |
| Fungi and viruses | Alternaria alternata Cladosporium herbarum | Penicillium Aspergillus fumigatus | |
| Rhinovirus and other respiratory viruses | |||
| Systemic factors | Drugs | Antibiotics | β-Non-selective systemic and topical blockers |
| Acetylsalicylic acid | NSAIDs | ||
| Foods | Cow milk | Cereals | |
| Eggs | Fish | ||
| Nuts | Seafood | ||
| Foods containing sulfites | Nuts, wine, lemon juice, lime juice, grape juice, dried potatoes, vinegar, seafood, beer, etc. | ||
| Plant panallergens such as profilins or lipid transfer protein (LTP) | |||
| Other | Hymenoptera venom | Apis melífera (bee) | |
| Vespula spp, Polistes dominulus (wasp) | |||
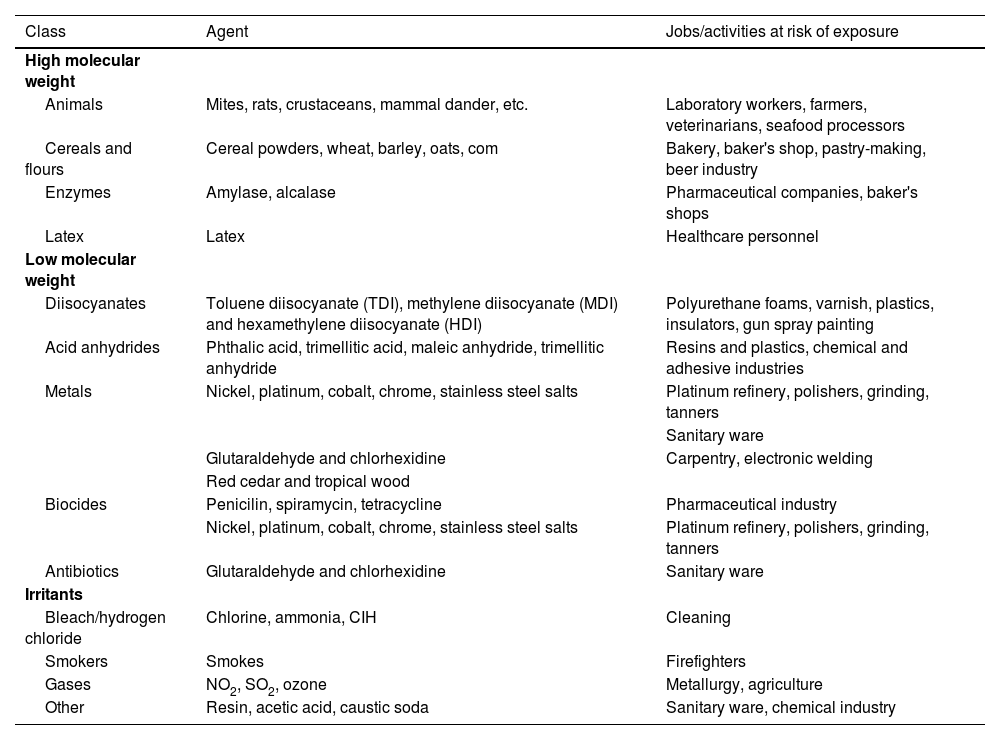
| Work-related factors | LOW MOLECULAR WEIGHT SUBSTANCES | INDUSTRY INVOLVES | |
| Drugs | Pharmaceutical industry | ||
| Anhydrides | Plastic industry | ||
| Diisocyanates | Polyurethane, plastic, varnish and enamel industries | ||
| Woods | Sawmills, carpentry work, cabinetmaking | ||
| Metals | Foundries, nickel plating, silver plating, tanning, boiler cleaning industries | ||
| Other | Cosmetic industry, hairdressing, photograph developing, cooling, dyes | ||
| HIGH MOLECULAR WEIGHT SUBSTANCES | INDUSTRY INVOLVED | ||
| Substances of plant origin, powder and flours | Farmers, port workers, mills, bakeries, beer industry, soy processing, cacao, coffee and tea industries, textile industry | ||
| Foods | Food industry | ||
| Plant enzymes | Food industry, pharmaceutical industry, | ||
| Vegatable gums | Food industry, printing presses, latex industry, healthcare | ||
| Fungi and spores | Bakeries, farms, farmers | ||
| Animal enzymes | Mills, carmine manufacturing | ||
Genetic factors are gaining increasing relevance as research progresses. Current studies indicate their involvement in the onset of asthma, the phenotypic expression of the disease, the individual response to triggers of asthma symptoms or exacerbations, and very especially in the response to new therapies in cases of severe asthma.60
Finally, it should be emphasized the growing evidence of the importance of environmental pollution, both indoors, from biomass combustion, and outdoors, from the combustion of fossil fuel-derived products.61,62 This environmental pollution acts as a contributing factor in the onset of asthma and as a trigger for asthma symptoms or exacerbations. Furthermore, it contributes to increased morbidity and mortality of asthma, as well as the incidence of other chronic respiratory diseases, cardiovascular diseases, and various types of cancer.63
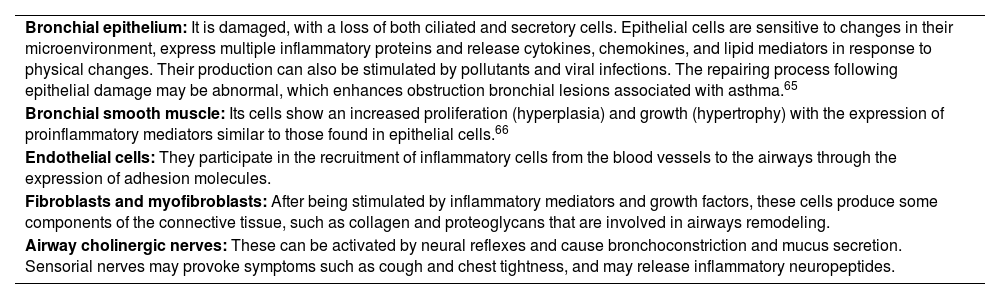
1.4PathogenesisInflammation affects the entire respiratory tract, including the nasal mucosa, and is present even when symptoms are episodic. However, the relationship between the severity of asthma and the intensity of inflammation has not been consistently established.64 The epithelium initiates the response to inhaled substances, secreting cytokines such as Thymic Stromal Lymphopoietin (TSLP), IL-33 y IL-25, which are crucial for activation of the type 2 innate immune system (table 5).67,68
Cells and structural elements of the airways involved in asthma.
| Bronchial epithelium: It is damaged, with a loss of both ciliated and secretory cells. Epithelial cells are sensitive to changes in their microenvironment, express multiple inflammatory proteins and release cytokines, chemokines, and lipid mediators in response to physical changes. Their production can also be stimulated by pollutants and viral infections. The repairing process following epithelial damage may be abnormal, which enhances obstruction bronchial lesions associated with asthma.65 |
| Bronchial smooth muscle: Its cells show an increased proliferation (hyperplasia) and growth (hypertrophy) with the expression of proinflammatory mediators similar to those found in epithelial cells.66 |
| Endothelial cells: They participate in the recruitment of inflammatory cells from the blood vessels to the airways through the expression of adhesion molecules. |
| Fibroblasts and myofibroblasts: After being stimulated by inflammatory mediators and growth factors, these cells produce some components of the connective tissue, such as collagen and proteoglycans that are involved in airways remodeling. |
| Airway cholinergic nerves: These can be activated by neural reflexes and cause bronchoconstriction and mucus secretion. Sensorial nerves may provoke symptoms such as cough and chest tightness, and may release inflammatory neuropeptides. |
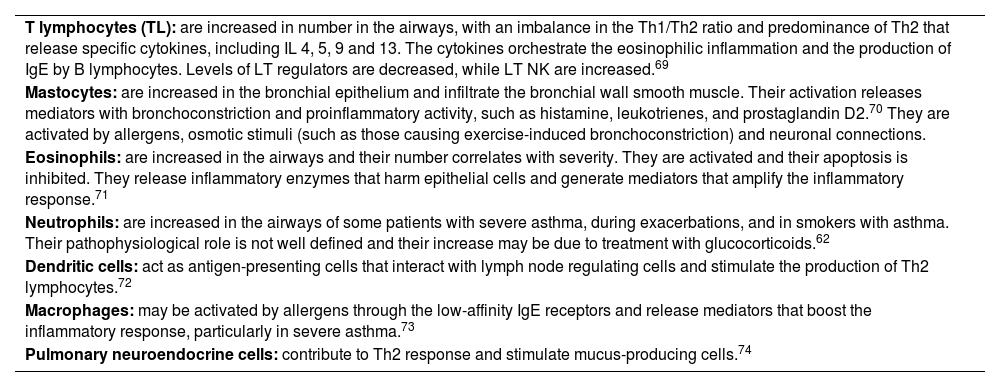
Once activated, type 2 innate lymphoid cells secrete type 2 pro-inflammatory cytokines, such as IL-4, IL-5 and IL-13, which assume the role of starting and maintaining T2 response (table 6).
Inflammatory cells involved in asthma.
| T lymphocytes (TL): are increased in number in the airways, with an imbalance in the Th1/Th2 ratio and predominance of Th2 that release specific cytokines, including IL 4, 5, 9 and 13. The cytokines orchestrate the eosinophilic inflammation and the production of IgE by B lymphocytes. Levels of LT regulators are decreased, while LT NK are increased.69 |
| Mastocytes: are increased in the bronchial epithelium and infiltrate the bronchial wall smooth muscle. Their activation releases mediators with bronchoconstriction and proinflammatory activity, such as histamine, leukotrienes, and prostaglandin D2.70 They are activated by allergens, osmotic stimuli (such as those causing exercise-induced bronchoconstriction) and neuronal connections. |
| Eosinophils: are increased in the airways and their number correlates with severity. They are activated and their apoptosis is inhibited. They release inflammatory enzymes that harm epithelial cells and generate mediators that amplify the inflammatory response.71 |
| Neutrophils: are increased in the airways of some patients with severe asthma, during exacerbations, and in smokers with asthma. Their pathophysiological role is not well defined and their increase may be due to treatment with glucocorticoids.62 |
| Dendritic cells: act as antigen-presenting cells that interact with lymph node regulating cells and stimulate the production of Th2 lymphocytes.72 |
| Macrophages: may be activated by allergens through the low-affinity IgE receptors and release mediators that boost the inflammatory response, particularly in severe asthma.73 |
| Pulmonary neuroendocrine cells: contribute to Th2 response and stimulate mucus-producing cells.74 |
On the other hand, Dendritic cells promote the development of T-helper (Th2) lymphocytes, which secrete the previously mentioned type 2 cytokines. Recent studies have shown that not all patients develop Th2 inflammation, but there are also other molecules such as IL-17 and IF-γ that are involved in the so-called Th2-low asthma.
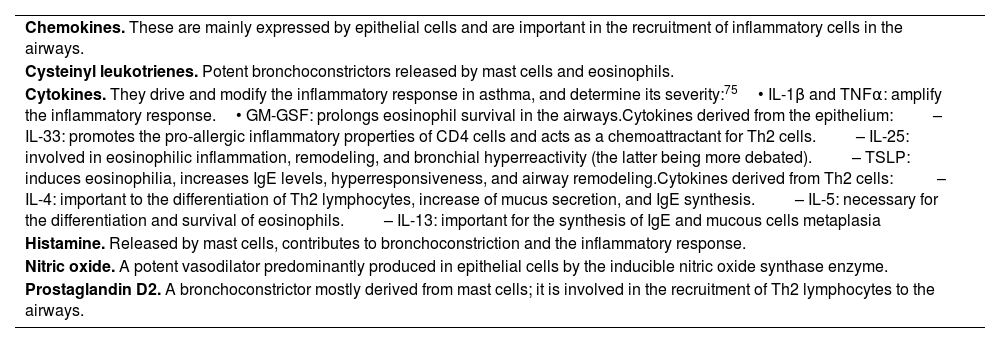
Molecules involved in this inflammatory process are summarised in table 7.
Relevant molecules involved in the asthma inflammatory process.
| Chemokines. These are mainly expressed by epithelial cells and are important in the recruitment of inflammatory cells in the airways. |
| Cysteinyl leukotrienes. Potent bronchoconstrictors released by mast cells and eosinophils. |
| Cytokines. They drive and modify the inflammatory response in asthma, and determine its severity:75• IL-1β and TNFα: amplify the inflammatory response.• GM-GSF: prolongs eosinophil survival in the airways.Cytokines derived from the epithelium:– IL-33: promotes the pro-allergic inflammatory properties of CD4 cells and acts as a chemoattractant for Th2 cells.– IL-25: involved in eosinophilic inflammation, remodeling, and bronchial hyperreactivity (the latter being more debated).– TSLP: induces eosinophilia, increases IgE levels, hyperresponsiveness, and airway remodeling.Cytokines derived from Th2 cells: – IL-4: important to the differentiation of Th2 lymphocytes, increase of mucus secretion, and IgE synthesis.– IL-5: necessary for the differentiation and survival of eosinophils.– IL-13: important for the synthesis of IgE and mucous cells metaplasia |
| Histamine. Released by mast cells, contributes to bronchoconstriction and the inflammatory response. |
| Nitric oxide. A potent vasodilator predominantly produced in epithelial cells by the inducible nitric oxide synthase enzyme. |
| Prostaglandin D2. A bronchoconstrictor mostly derived from mast cells; it is involved in the recruitment of Th2 lymphocytes to the airways. |
GM-GSF: Granulocyte-macrophage colony-stimulating factor; TNF: Tumor necrosis factor.
Patients with asthma may present a phenomenon, known as airway remodeling, which include: thickening of the reticular layer of the basal membrane, subepithelial fibrosis, hypertrophy and hyperplasia of the bronchial smooth muscle, vascular proliferation and dilatation, mucosal gland hyperplasia and mucus hypersecretion, all of which are associated with a progressive deterioration of pulmonary function.69 Some of these changes are related to the severity of the disease and may lead to a bronchial obstruction, which is occasionally irreversible.76
These changes may result from a repairing response to chronic inflammation or may occur independently of the inflammatory process.77
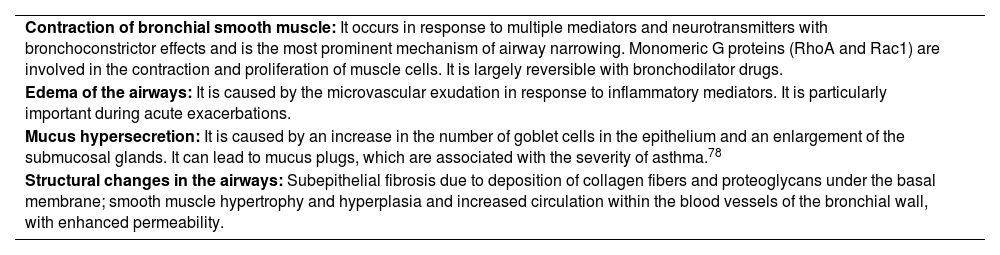
Narrowing of the airways is common end result of the pathophysiological changes and the origin of most symptoms. This limitation of airflow and the symptoms it triggers can spontaneously resolve or respond to medication (reversibility) and may even be absent for some time in a particular patient. Table 8 shows the different mechanisms that contribute to the onset of obstruction.
Mechanisms of airway obstruction in asthma.
| Contraction of bronchial smooth muscle: It occurs in response to multiple mediators and neurotransmitters with bronchoconstrictor effects and is the most prominent mechanism of airway narrowing. Monomeric G proteins (RhoA and Rac1) are involved in the contraction and proliferation of muscle cells. It is largely reversible with bronchodilator drugs. |
| Edema of the airways: It is caused by the microvascular exudation in response to inflammatory mediators. It is particularly important during acute exacerbations. |
| Mucus hypersecretion: It is caused by an increase in the number of goblet cells in the epithelium and an enlargement of the submucosal glands. It can lead to mucus plugs, which are associated with the severity of asthma.78 |
| Structural changes in the airways: Subepithelial fibrosis due to deposition of collagen fibers and proteoglycans under the basal membrane; smooth muscle hypertrophy and hyperplasia and increased circulation within the blood vessels of the bronchial wall, with enhanced permeability. |
Various triggering agents may cause a significant airway narrowing, thus leading to an asthma exacerbation. The most severe episodes usually occur in association with viral infections of the upper respiratory tract (mainly rhinovirus and respiratory syncytial virus) or exposure to allergens.79 Also, exacerbations may be caused by non-steroidal anti-inflammatory drugs (NSAIDs) in patients with hypersensitivity to these drugs, physical exercise, cold air and certain non-specific irritants.80–82 The intensity of the response to these stimuli is related to the underlying inflammation.
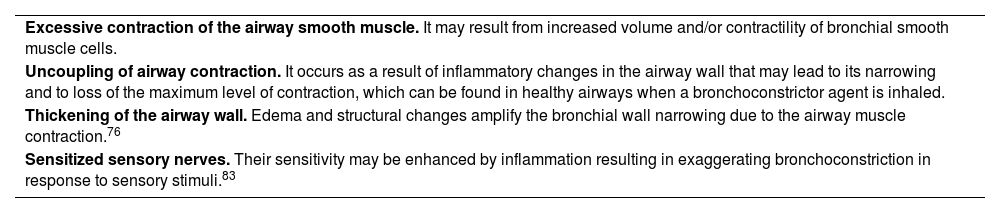
Bronchial hyperresponsiveness (BHR) is an additional pathophysiological characteristic of asthma, which leads to airway narrowing in response to stimuli that are harmless to people without asthma. BHR is linked to airway inflammation and repair, and is partially or totally reversible with therapy. Mechanisms involved in BHR are shown in table 9. The degree of BHR is partially correlated with the clinical severity of asthma and the inflammation markers.84 Anti-inflammatory therapy improves asthma control and attenuates BHR, but does not completely suppress it.85
Mechanisms of bronchial hyperresponsiveness.
| Excessive contraction of the airway smooth muscle. It may result from increased volume and/or contractility of bronchial smooth muscle cells. |
| Uncoupling of airway contraction. It occurs as a result of inflammatory changes in the airway wall that may lead to its narrowing and to loss of the maximum level of contraction, which can be found in healthy airways when a bronchoconstrictor agent is inhaled. |
| Thickening of the airway wall. Edema and structural changes amplify the bronchial wall narrowing due to the airway muscle contraction.76 |
| Sensitized sensory nerves. Their sensitivity may be enhanced by inflammation resulting in exaggerating bronchoconstriction in response to sensory stimuli.83 |
Variability is another important feature of asthma. It is defined as the variation or fluctuation of both symptoms and pulmonary function over time, even during the same day, beyond physiological circadian changes.
1.5Childhood asthmaAsthma is one of the most prevalent chronic diseases in childhood. According to the International Study of Asthma and Allergies in Childhood (ISAAC), the prevalence in Spain is 10%, which is similar to the prevalence in the European Union. It is more common in coastal areas and among males in the 6-7-year age group.86–89
It is estimated that more than half of adults with asthma already had the disease during childhood.90
In the first three years of life, definition, diagnostic criteria, and even the classification of asthma are complex and controversial,91 which make difficult to determine the prevalence of asthma at these ages.92,93
This is because typical symptoms (coughing, wheezing, and difficulty breathing) are common in children under 3 years of age without asthma and also for the impossibility to assess lung function routinely.
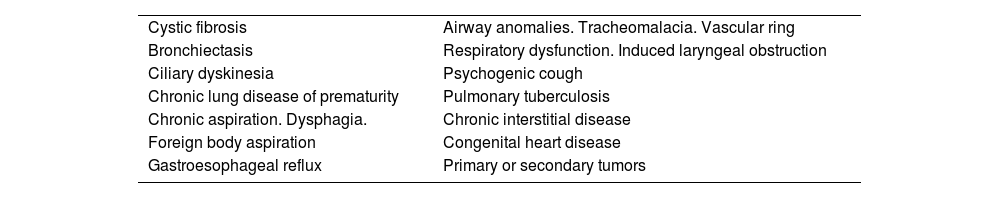
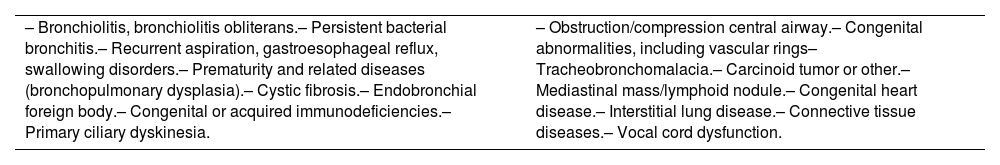
The definitive diagnosis of asthma requires the exclusion of other diseases that can present with similar signs and symptoms (table 10).94–97 In fact, some of these conditions may be associated with asthma.98
Differential diagnosis of childhood asthma.
| Cystic fibrosis | Airway anomalies. Tracheomalacia. Vascular ring |
| Bronchiectasis | Respiratory dysfunction. Induced laryngeal obstruction |
| Ciliary dyskinesia | Psychogenic cough |
| Chronic lung disease of prematurity | Pulmonary tuberculosis |
| Chronic aspiration. Dysphagia. | Chronic interstitial disease |
| Foreign body aspiration | Congenital heart disease |
| Gastroesophageal reflux | Primary or secondary tumors |
The presence of personal and family atopy is the most important risk factor for the subsequent development of asthma. Other factors include age at onset, severity and frequency of episodes, male gender, and severe bronchiolitis (RSV, rhinovirus).98–100
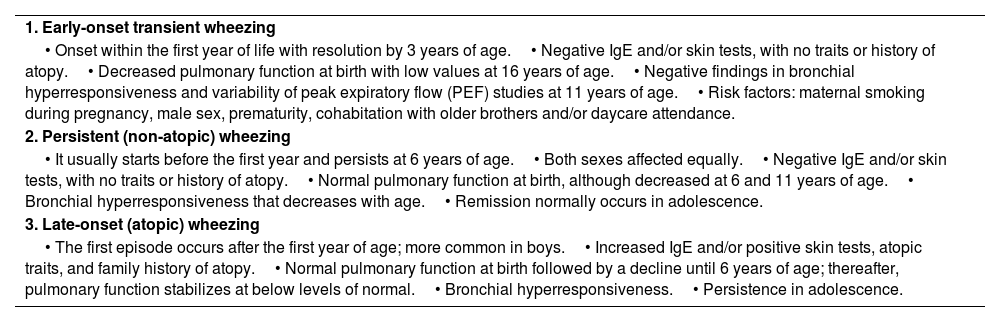
After the first description of phenotypes in childhood asthma reported in the study of Tucson (table 11),101 a number of prospective clinical studies (cohorts of children followed since birth)102–104 or complex biostatistical studies (cluster of populations without previous hypothesis)105 have been published, all of them trying to identify different phenotypes of childhood asthma. The clinical value of these studies is controversial.103
F Traditional phenotypes in wheezing children from the Tucson study based on their long-term time.
| 1. Early-onset transient wheezing |
| • Onset within the first year of life with resolution by 3 years of age.• Negative IgE and/or skin tests, with no traits or history of atopy.• Decreased pulmonary function at birth with low values at 16 years of age.• Negative findings in bronchial hyperresponsiveness and variability of peak expiratory flow (PEF) studies at 11 years of age.• Risk factors: maternal smoking during pregnancy, male sex, prematurity, cohabitation with older brothers and/or daycare attendance. |
| 2. Persistent (non-atopic) wheezing |
| • It usually starts before the first year and persists at 6 years of age.• Both sexes affected equally.• Negative IgE and/or skin tests, with no traits or history of atopy.• Normal pulmonary function at birth, although decreased at 6 and 11 years of age.• Bronchial hyperresponsiveness that decreases with age.• Remission normally occurs in adolescence. |
| 3. Late-onset (atopic) wheezing |
| • The first episode occurs after the first year of age; more common in boys.• Increased IgE and/or positive skin tests, atopic traits, and family history of atopy.• Normal pulmonary function at birth followed by a decline until 6 years of age; thereafter, pulmonary function stabilizes at below levels of normal.• Bronchial hyperresponsiveness.• Persistence in adolescence. |
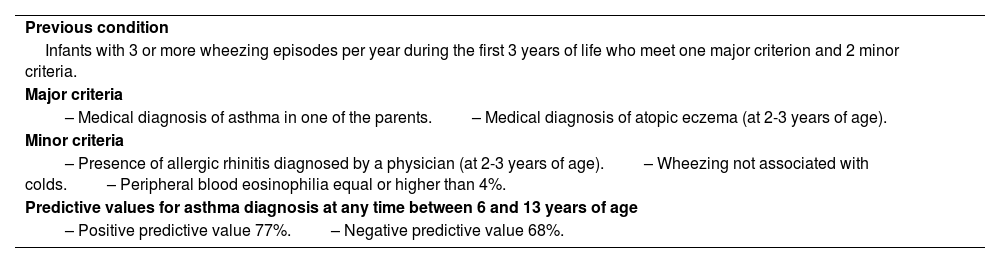
Based on the findings from these studies, some tools or models have been developed to predict the future risk in children with asthma but a few of these instruments have been validated. The best known instrument is the Asthma Predictive Index (table 12), which was developed from the Tucson cohort study.106
Asthma Predictive Index.
| Previous condition |
| Infants with 3 or more wheezing episodes per year during the first 3 years of life who meet one major criterion and 2 minor criteria. |
| Major criteria |
| – Medical diagnosis of asthma in one of the parents.– Medical diagnosis of atopic eczema (at 2-3 years of age). |
| Minor criteria |
| – Presence of allergic rhinitis diagnosed by a physician (at 2-3 years of age).– Wheezing not associated with colds.– Peripheral blood eosinophilia equal or higher than 4%. |
| Predictive values for asthma diagnosis at any time between 6 and 13 years of age |
| – Positive predictive value 77%.– Negative predictive value 68%. |
Although other indexes or modifications of the Asthma Predictive Index have been developed, this one continues to be the most useful, because of its simplicity, having been more validated, and better positive likelihood ratio.107
The diagnosis of asthma in children under 3 years of age must be probabilistic, a probability that increases in the presence of atopy. The term asthma should not be avoided when there are more than 3 episodes a year, or severe episodes, of coughing, wheezing, and difficulty breathing, with a good response to maintenance treatment with inhaled glucocorticoids and worsening of symptoms upon withdrawal of this medication.
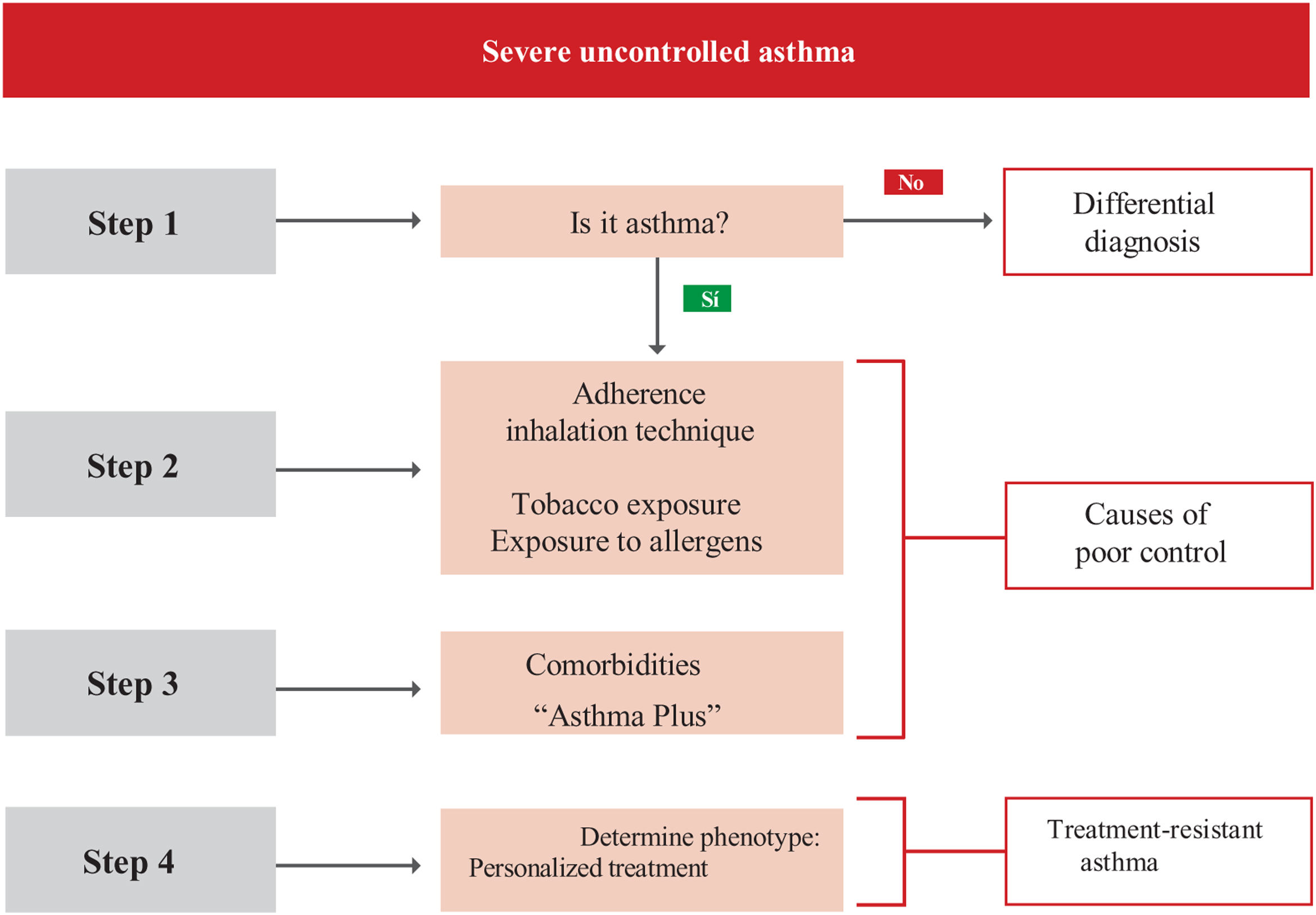
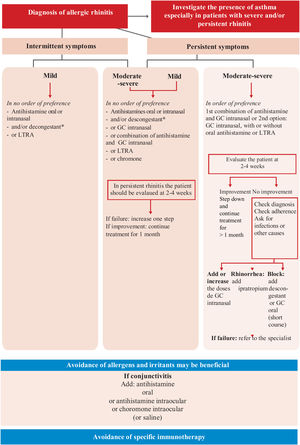
2Diagnosis2.1Clinical featuresThe diagnosis of asthma should be considered in the presence of clinical suspicion based on signs and symptoms, such as wheezing (the most typical symptom),108 dyspnea or breathing difficulty, cough, and chest tightness. These are named “guide symptoms”,109,110 which are usually variable regarding intensity and the time of appearance, occurring mainly at night or in the early morning and are caused by different triggers (viral infections, allergens, tobacco smoke, exercise, emotions, etc.). Seasonal variations, along with a family and personal history of atopy are important aspects to be considered.111–114
Usually, several signs or symptoms appear together; when they occur as single manifestations, they are usually poor predictive of asthma.111,115,116 None of these symptoms and signs are specific to asthma,117 hence the need to include some objective diagnostic test, usually respiratory function tests.
The patient's clinical history should also include other aspects, such as the onset of symptoms, the presence of chronic rhinosinusitis with or without polyposis, rhinitis, dermatitis, and a family history of asthma or atopy,112 all of which increase the probability to establish a diagnosis of asthma. Table 13 shows the key questions for the identification of patients with suspected asthma.109,110
Key questions for the diagnostic suspicion of asthma.118
| – Have you ever had “whistling” in the chest?– Have you had cough especially at night?– Have you had cough, wheezing, breathing difficulty in certain periods of the year or when in contact with animals, plants, tobacco or at the workplace?– Have you had cough, “whistling”, breathing difficulty after a moderate or intense physical exercise? | – Have you had colds lasting more than 10 days or “going down into the chest”?– Have you used inhaled medications that relieve your symptoms?– Do you have any kind of allergy? Do you have any relatives with asthma or allergy? |
On physical examination, wheezing on auscultation of the chest is most characteristic finding, and sometimes nasal obstruction on anterior rhinoscopy, as well as dermatitis or eczema. However, a normal physical examination does not exclude a diagnosis of asthma.
If the onset of the disease presents with acute symptoms, a brief medical history and physical examination will be performed, and treatment will be initiated. Objective diagnostic tests will be conducted once the symptoms are under control.115
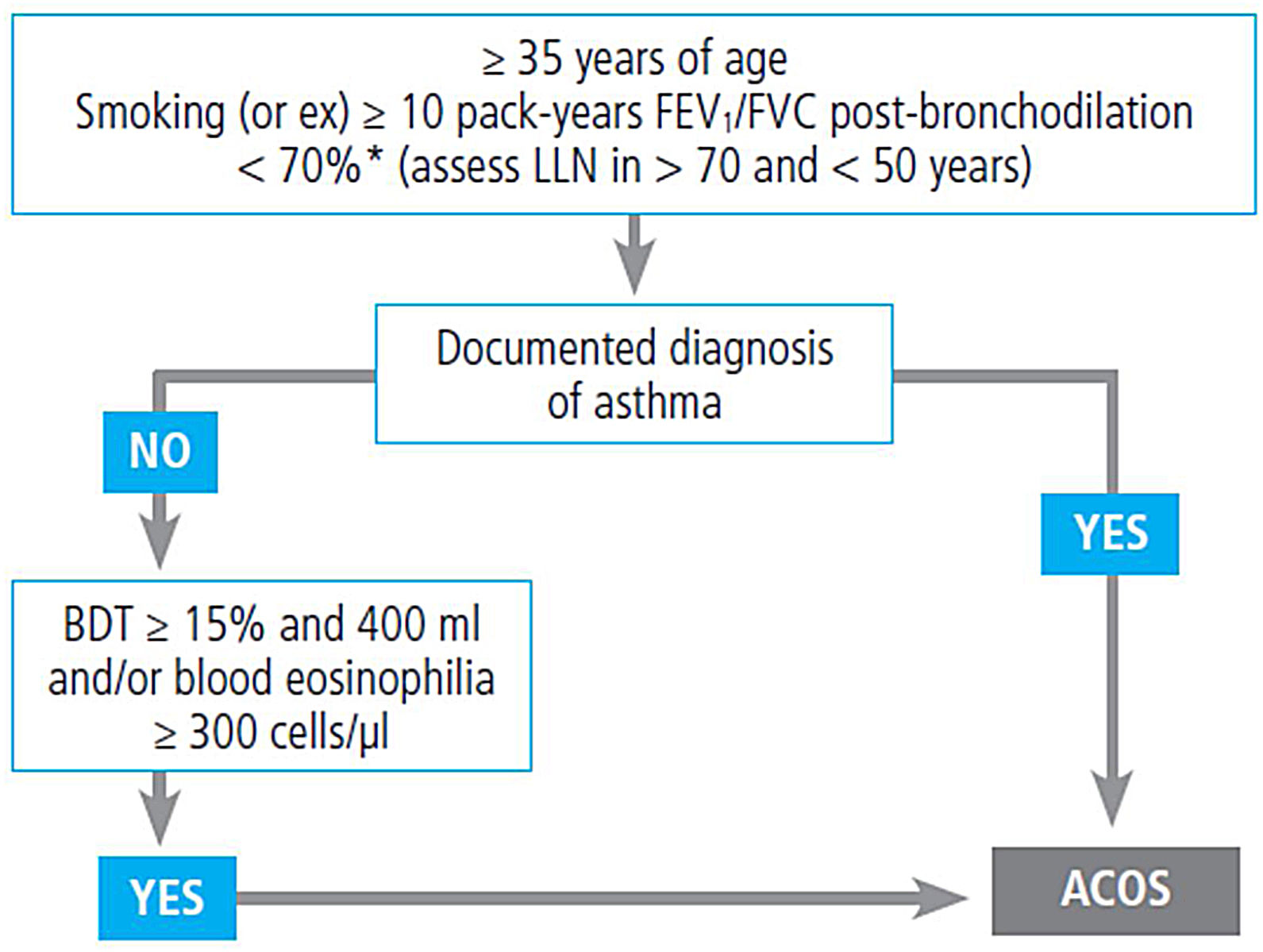
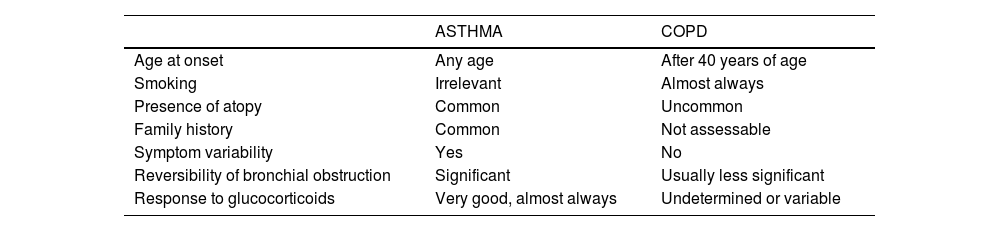
If asthma is suspected, a differential diagnosis with other diseases, particularly chronic obstructive pulmonary disease (COPD) should be made, as shown in table 14.
Differential diagnosis of asthma in adults.
| ASTHMA | COPD | |
|---|---|---|
| Age at onset | Any age | After 40 years of age |
| Smoking | Irrelevant | Almost always |
| Presence of atopy | Common | Uncommon |
| Family history | Common | Not assessable |
| Symptom variability | Yes | No |
| Reversibility of bronchial obstruction | Significant | Usually less significant |
| Response to glucocorticoids | Very good, almost always | Undetermined or variable |
| Other possible diseases | Characteristic symptoms | |
|---|---|---|
| Age between 15 and 40 years | – Inducible laryngeal obstruction– Hyperventilation– Inhaled foreign body– Cystic fibrosis– Bronchiectasis– Congenital heart disease– Pulmonary thromboembolism | – Dyspnea, inspiratory stridor– Fainting, paresthesia– Sudden onset of symptoms– Excessive cough and mucus– Recurrent infections– Heart murmurs– Sudden onset of dyspnea, tachypnea, chest pain |
| Age older than 40 years of age | – Inducible laryngeal obstruction– Hyperventilation– Bronchiectasis– Parenchymal lung disease– Heart failure– Pulmonary thromboembolism | – Dyspnea, inspiratory stridor– Fainting, paresthesia– Recurrent infections– Exertional dyspnea, non-productive cough– Exertional dyspnea, nighttime symptoms– Sudden onset of dyspnea, tachypnea |
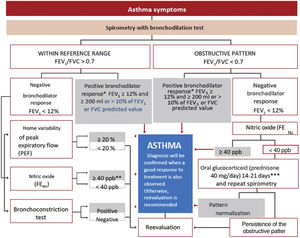
The diagnosis of asthma is established when in a patient with suspected symptoms of the disease, a pulmonary function test (preferably spirometry) objectively demonstrates an alteration compatible with asthma.119
The main functional abnormalities of asthma are airflow obstruction, reversibility, variability, and bronchial hyperresponsiveness.
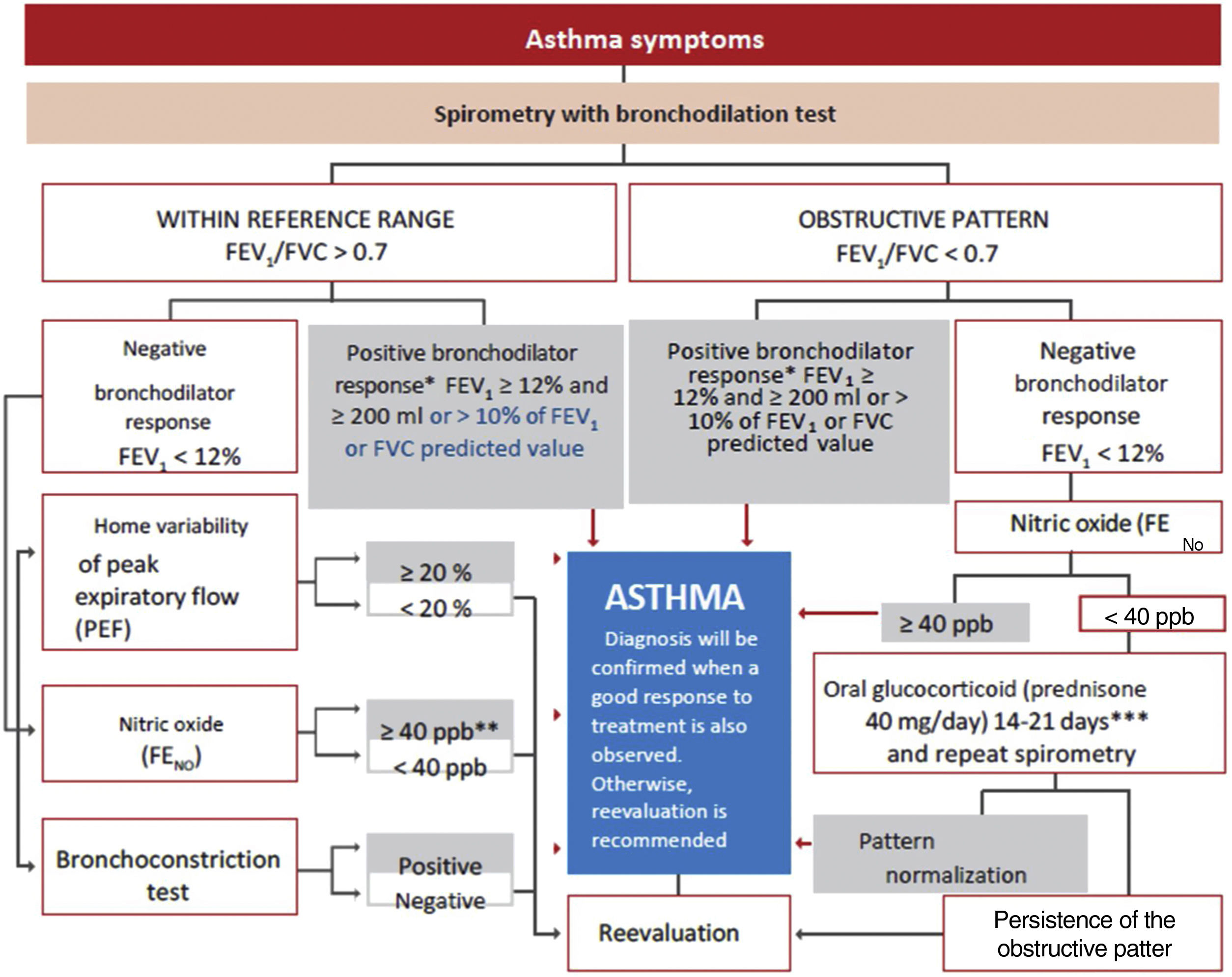
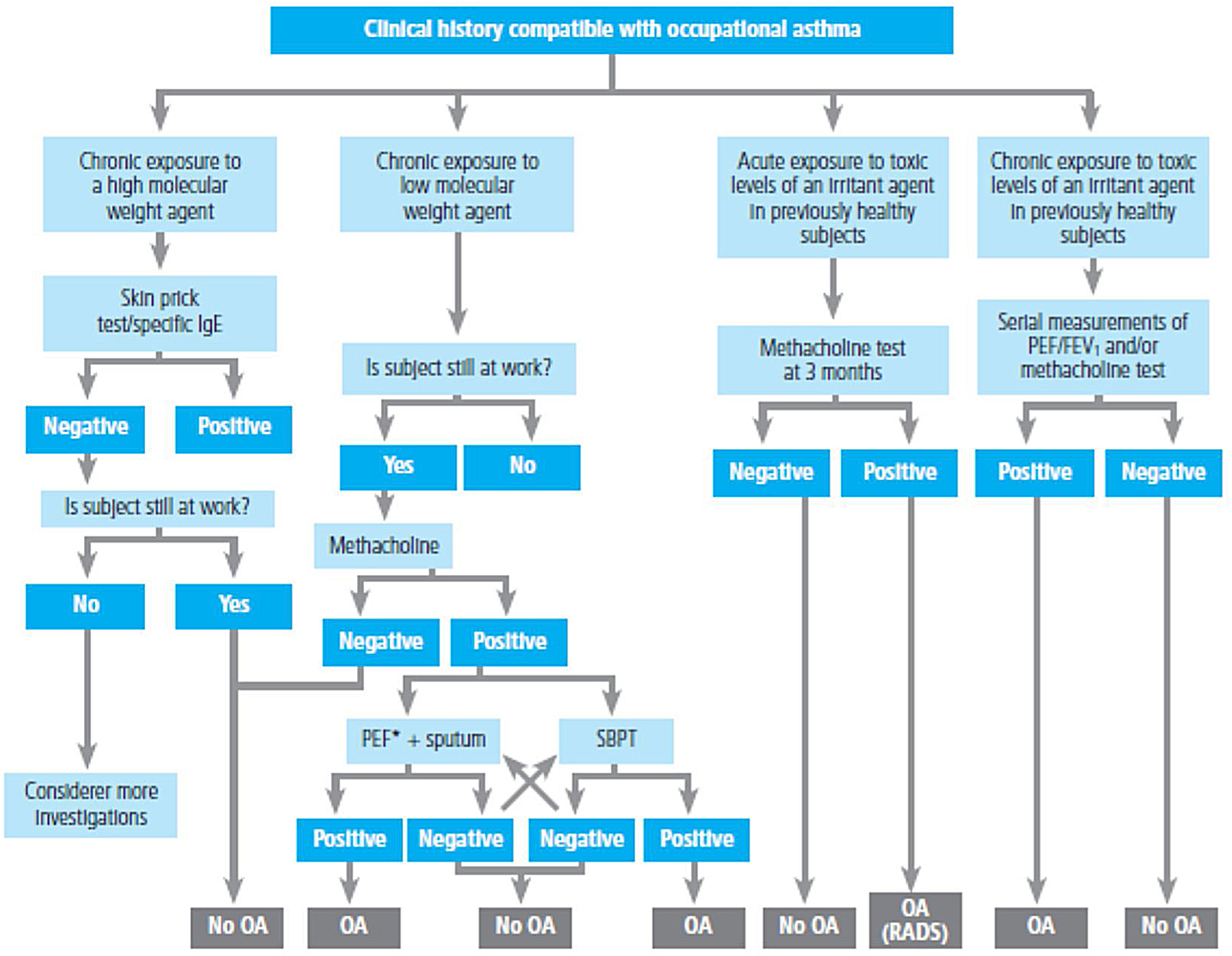
Spirometry is the first-choice diagnostic test, as shown in the algorithm of the diagnostic process (Figure 1). The main parameters to be determined are forced expiratory volume in one second (FEV1) and forced vital capacity (FVC). Reference values should be adjusted to the age and ethnic group/race of each patient. Airway obstruction is defined as a FEV1/FVC ratio below the lower limit of reference values, which has been arbitrarily set at 0.7120 However, this criterion may lead to an overestimation of airway obstruction in patients of advanced age.121 For this reason, it is recommended to use international reference values that are suitable for all ages and allow expressing the results as deviations from the mean (Z-score), establishing the lower limit of normality (LLN) at -1.64.122,123
Diagnostic algorithm.
**In children, a 12% increase is sufficient to consider this test as positive, even if <200ml. **In case of a negative bronchoconstriction test, a diagnosis of eosinophilic bronchitis should be considered. ***Alternatively, inhaled glucocorticoids at very high doses, 1500 – 2000μg of fluticasone propionate, 3 or 4 times a day for 2-8 weeks may be used.
A reduced FEV1 value confirms the obstruction, helps to establish its severity, and indicates a greater risk of exacerbations.124 On the other hand, many patients with asthma may show spirometric values close to the reference range or even a non-obstructive (restrictive) pattern due to air trapping.
For the bronchodilation test, the administration of 4 successive/puffs of 100μg of salbutamol, or its equivalent, using a pressurized inhaler with spacer and repeating spirometry after 15minutes is recommended. A response is considered to be positive (or significant bronchodilation) when there is a ≥ 12% and a ≥ 200ml increase in FEV1 from baseline (table 15)121 or >10% of the theoretical value of FEV1 or FVC.123 An alternative criterion for bronchodilation is an increase of the peak expiratory flow (PEF) of >20%.125 Reversibility can also be identified as an improvement in FEV108 or PEF after 2 weeks of treatment with systemic glucocorticoids (prednisone 40mg/day or equivalent) or 2-8 weeks of inhaled glucocorticoids (1500-2000mg/day of fluticasone propionate or equivalent).126 Although reversibility of bronchial obstruction is a typical characteristic of asthma, it is not present in all patients.
Reversibility and daily variability criteria recommended for the diagnosis of asthma.
| Reversibility | Post-Bd FEV1−pre-Bd FEV1≥200 mlandPost-Bd FEV1−pre-Bd FEV1pre-Bd FEV1×100≥12% |
| Daily variability | Maximum PEF−minimum PEFMaximum PEF×100Variability ≥ 20% during ≥ 3 days per week, in a 2-week recording |
FEV1: forced expiratory volume in one second; PEF: peak expiratory flow; Bd: bronchodilation
Variability, or excessive fluctuation of pulmonary function over time, is important for the diagnosis and control of asthma. The most widely recommended daily variability index is the PEF amplitude in relation to the averaged mean over at least 1-2 weeks (table 15) and recorded before the use of medication.127 A PEF variability ≥ 20% is diagnostic of asthma.128
Bronchial hyperresponsiveness is the terms used to define an excessive narrowing of the bronchial lumen in response to physical or chemical stimuli that usually only cause a small or negligible reduction of the airways.129 The identification of this exaggerated response to a bronchonstrictor by means of a non-specific challenge test may be useful in patients with clinical suspicion of asthma and normal pulmonary function. Direct agents, such as methacholine or histamine, or indirect agents, such as adenosine monophosphate, mannitol or hypertonic saline solution can be used.130 Indirect agents show a better relationship with inflammation and a higher sensitivity to the effect of glucocorticoids.131 In addition, mannitol offers the advantage of being administered via a dry power inhaler.132
The analysis of bronchial hyperresponsiveness is performed in terms of sensitivity or threshold, determining the dose or concentration that produces a 20% decrease in FEV1 compared to the post-diluent value.129,133 Recently, it has been recommended, in the case of methacholine, to use the cumulative dose of methacholine that reduces FEV1 by 20% (PD20) compared to the value obtained after diluent administration.134 This type of bronchial provocation has high sensitivity but limited specificity,135 making it more useful for excluding rather than confirming the diagnosis of asthma. Bronchial hyperresponsiveness is also present in other conditions such as allergic rhinitis, COPD, bronchiectasis, cystic fibrosis, or heart failure. The mannitol test is considered to be positive when a 15% fall in FEV1 from baseline (PD15) occurs or when there is an incremental decrease of FEV1 of ≥ 10% between two consecutive doses.129 This test is more useful to confirm the diagnosis of asthma (particularly in cases of exercise-induced bronchoconstriction) because its specificity is >95%, although its sensitivity is 60%.
Fractional exhaled nitric oxide (FENO) is a non-invasive measure of bronchial inflammation associated with the allergic-T2 phenotype (see section 7.3) and is partially related to eosinophilic inflammation. Although both FeNO and eosinophils are part of the T2 inflammatory cascade, these two biomarkers are regulated by different inflammatory pathways. The determination procedure of FENO has been standardized,136 and the recently recommended cutoff point is >40 ppb in adults who are not taking glucocorticoids.115,137 It achieves high sensitivity and specificity for the diagnosis of asthma in non-smoking patients not using inhaled glucocorticoids,138 especially when associated with reduced FEV1.139 However, a normal FENO value does not exclude the diagnosis of asthma, particularly in non-atopic individuals.140
2.3Pulmonary function in childrenAlthough most children with asthma have FEV1 values within the reference range,140,141 respiratory function tests are essential for establishing the diagnosis of asthma.142 They contribute decisively to the diagnosis, although their normality does not exclude the diagnosis of asthma and, for this reason it should be performed periodically. However, they do not sufficiently discriminate the level of severity.143
With the appropriate method, reliable forced spirometry can be obtained in children from the age of three. Above the age of 5-6, the functional diagnosis of asthma is similar to that in adults. In children, FEV1/FVC ratio correlates better with asthma severity than FEV1.131,144 The availability of international reference values suitable for all ages,122all ages equations, allows to express the results as deviations from the mean (z-score), establishing the LLN at -1.64. In children, obstruction is defined by an FEV1/FVC ratio<LLN (lower limit of normality).
A bronchodilation test is considered positive when the increase in FEV1 as compared with baseline value is equal or higher than 12% or 9% in relation to the predicted value.145,146 The ERS/ATS proposes for the general population a change of FEV1 greater than 10% of the predicted value.123
As children can exhale all the air in 2-3seconds, an expiration lasting this amount of time may be considered valid provided its validity can be confirmed by an expert's visual inspection of the correctness of the maneuver.147 Less strict reproducibility criteria are also acceptable: 100ml or 10% of FEV1.148
The FEF25-75% value does not provide any relevant information and therefore does no contribute to clinical decision-making.149
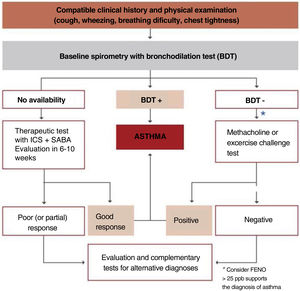
If diagnosis is uncertain, methacholine and exercise challenge tests may be of special interest in children, since exercise challenge test is relatively easy to perform, reproducible and has a high specificity for diagnosing asthma, although its sensitivity is low.150
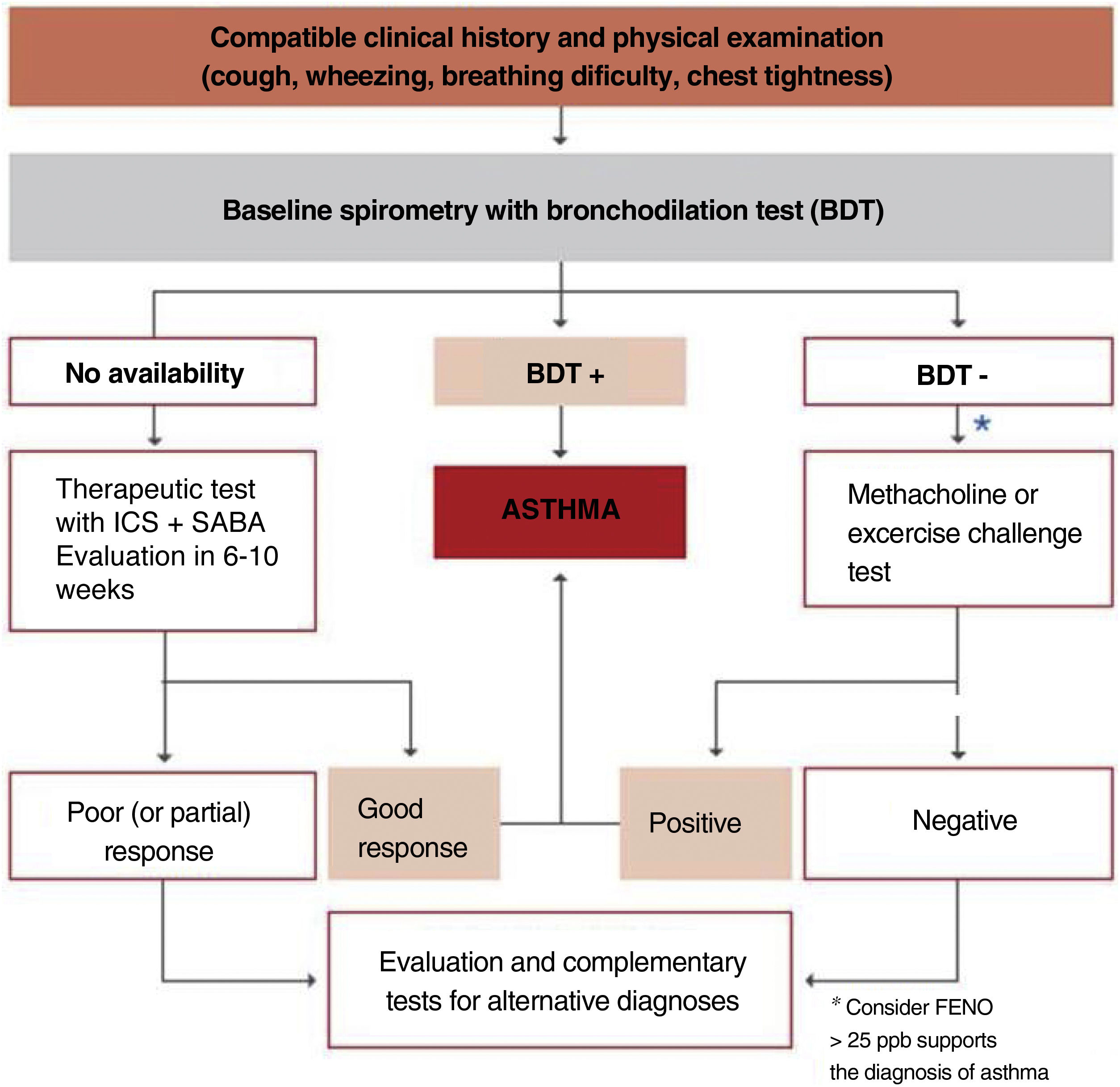
The algorithm shown in fig. 2 is useful to establish the diagnosis of asthma in children.
Between 3 and 5 years of age, it is indispensable to use the adequate methodology and appropriate reference values and do not extrapolate values of older children.151–153 Since these children may occasionally have expiration times lower than 1 second, the most useful value would be FEV0.5 or FEV0.75 rather than FEV1.154 In this age segment, the normal FEV1/FVC value would be greater than 90%.
As for the use of the bronchodilator test at this age, the cut-off point for both FEV1 and FEV0.5 or FEV0.75 remains to be determined.155,156 Other tests that may be useful in the management of preschool children with asthma include forced impulse oscillometry (IOS),157–159 the measurement of airway resistance using the interrupter technique (Rint), the tidal flow-volume curve or measurement of airway resistance by plethysmography.
Any of these techniques must be adapted to ATS/ERS guidelines on pulmonary function in preschool children.154 For children under 2 years of age, the rapid thoracoabdominal compression is the most widely used technique.
To perform reliable pulmonary function tests in children, particularly in those younger than 5-6 years of age, it is essential to have nursing staff specifically trained in these techniques as well as laboratories adapted for children.
The measurement of FENO also allows assessing the degree of bronchial inflammation in the child.160 The evaluation of FENO in young children is not relevant for predicting a diagnosis of asthma at school age.161 The diagnostic reliability of FENO in asthma is compromised by the wide confidence intervals of this measurement and the overlapping of FENO values between children without asthma and atopic dermatitis.
Cut-off points above 35 ppb have been suggested to be considered as positive,162,163 but values above 25 ppb in a child with compatible symptoms may support the diagnosis of asthma.143
Regarding its usefulness in the follow-up and adjustment of treatment, its benefits could not have been demonstrated. At follow-up, it is important to know the best value of the patient since therapeutic decisions should be based on variations regarding this optimum value.164 Treatment with inhaled glucocorticoids reduces FENO concentration, so that measurement of FENO may be a predictor of response.165 In some cases (particularly in the most severe ones), upward changes from the optimal value may be indicative of the risk of future exacerbations.166
Although potentially useful as guidance, the available evidence does not confirm its reliability to evaluate adherence to ICS treatment.
FENO can be determined in young children by the multiple breath-exhalation technique, with reference values having been established for the age between 1 and 5 years.118 In this age segment, although some studies have shown an association between high FENO levels and the risk of asthma,167,168 this correlation has not been clearly established.
In general, there is no consistent evidence to recommend the routine use of FENO in the follow-up of children with asthma, and its use should be restricted to the specialized consultation setting.169
Its use for the adjustment of treatment should be complementary to clinical and functional evaluation, and in no case should be considered as a single test.168,170
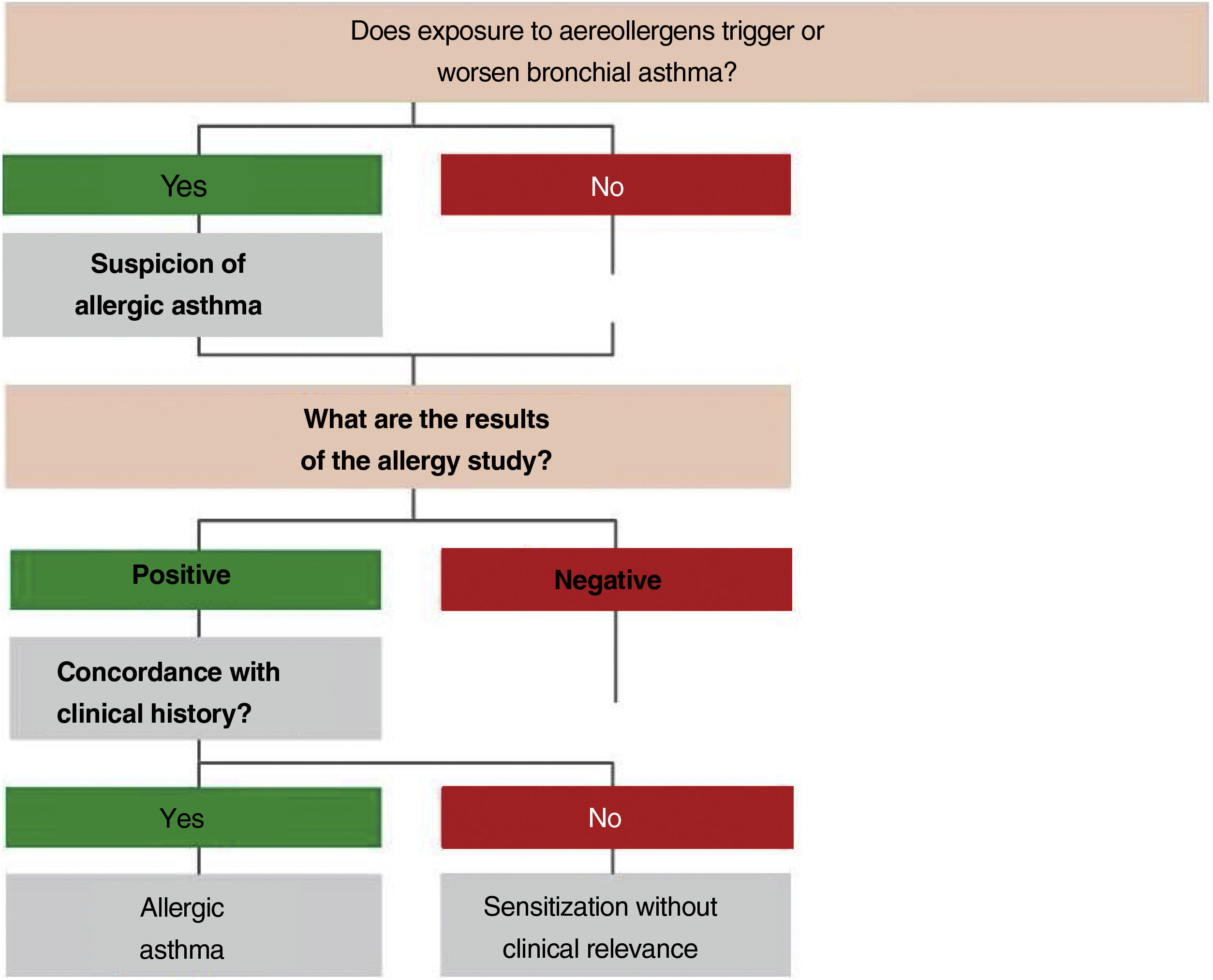
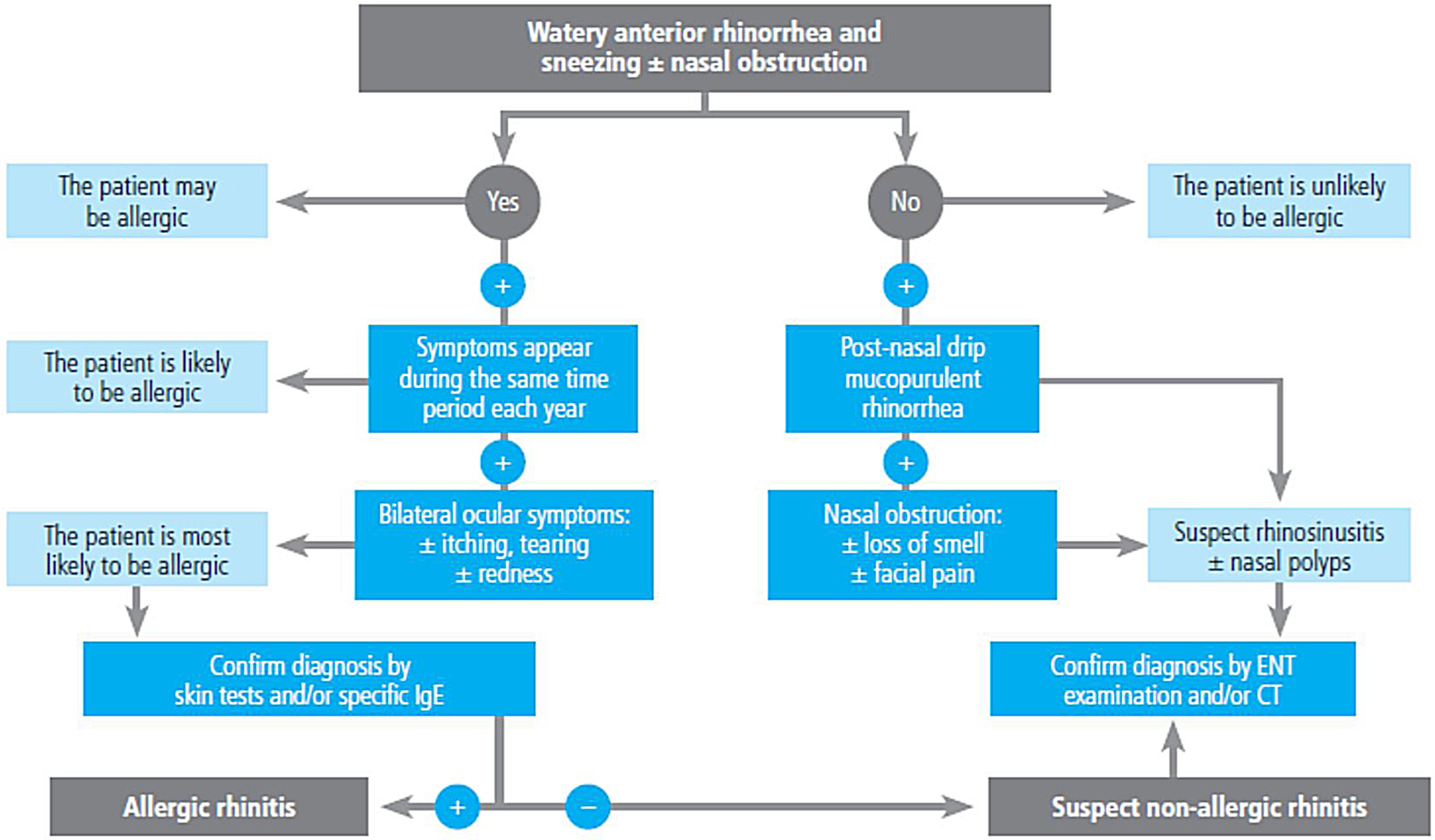
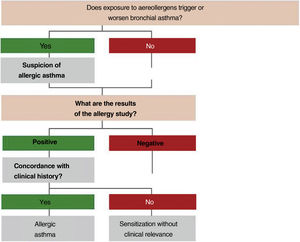
2.4Allergy evaluationThe aim of allergy testing is to determine the presence of sensitization to aeroallergens that may influence the development of the allergic asthma phenotype or to trigger exacerbations. These tests can be performed in any patient with asthma regardless of their age. The anamnesis helps to evaluate personal and family history of atopy (rhinoconjunctivitis, atopic dermatitis, food allergy) and the relationship between symptoms and allergen exposure. To make a diagnosis of allergic asthma, in addition to sensitization to inhaled allergens, it is necessary to demonstrate the clinical relevance of the results obtained171 (fig. 3).
The Intradermal puncture testing orprick test172 with standardized extracts (table 16) is the method of choice for its high sensitivity, low cost and immediately available results. It is necessary to consider the variables affecting the results (drugs, dermographism, etc.) and to have experience in the interpretation of results (false positives by cross-reactivity).173
Standard battery of aeroallergens used in intraepidermal skin tests or prick test.*
| Mites | Dermatophagoides pteronyssinus/farinae Lepidoglyphus destructor, Blomia tropicalis |
| Dander | Cat, dog |
| Pollens | Grasses, Olea europaea, Cupressus spp, Platanus spp, Salsola kali, Parietaria judaica, Artemisia vulgaris |
| Fungi | Alternaria alternata, Aspergillus fumigatus |
The specific IgE against complete aeroallergens, with the same meaning than prick testing, has a lower sensitivity and a higher cost.174 The specific IgE against allergenic components allows distinguishing between primary sensitization and cross-reactivity,175 and in polysensitized patients improves the selection of the composition of specific immunotherapy with allergens.176
The specific bronchial challenge test may be useful when a discrepancy exists between the clinical history and the results of sensitization, although it is not recommended as a routine procedure and should be performed by expert professionals.
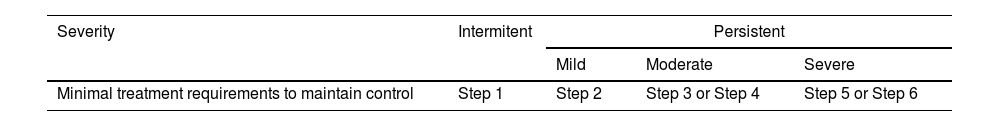
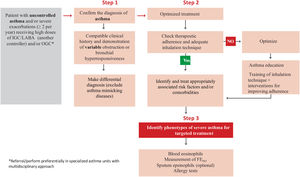
2.5Classification of severity in adultsAsthma has usually been classified according to its severity, although both the definition and assessment of severity has changed over time.113,120,177 Severity is an intrinsic property of asthma that reflects the intensity of its pathophysiological abnormalities.178
The classification of asthma according to clinical and functional parameters has been traditionally divided into four categories: intermittent, mild persistent, moderate persistent and severe persistent.113,120,177
It should be kept in mind that asthma severity involves both the intensity of the process and its response to treatment.179,180 Severity is usually evaluated while the patient is being treated and it is classified according to the need for maintenance therapy to achieve control of symptoms and exacerbations179,180 (table 17).
It is not necessarily a constant characteristic of asthma, as it can vary over time (months or years), so that periodic reassessment of severity is required.
The majority of the asthmatic population suffers from intermittent or mild persistent asthma.181,182 These seemingly non-severe forms of the disease should not underestimate their inflammatory nature.183,184 The absence of symptoms in mild and intermittent asthma requires a correct clinical and functional evaluation of the patient for accurate classification and subsequent adjustment of treatment.
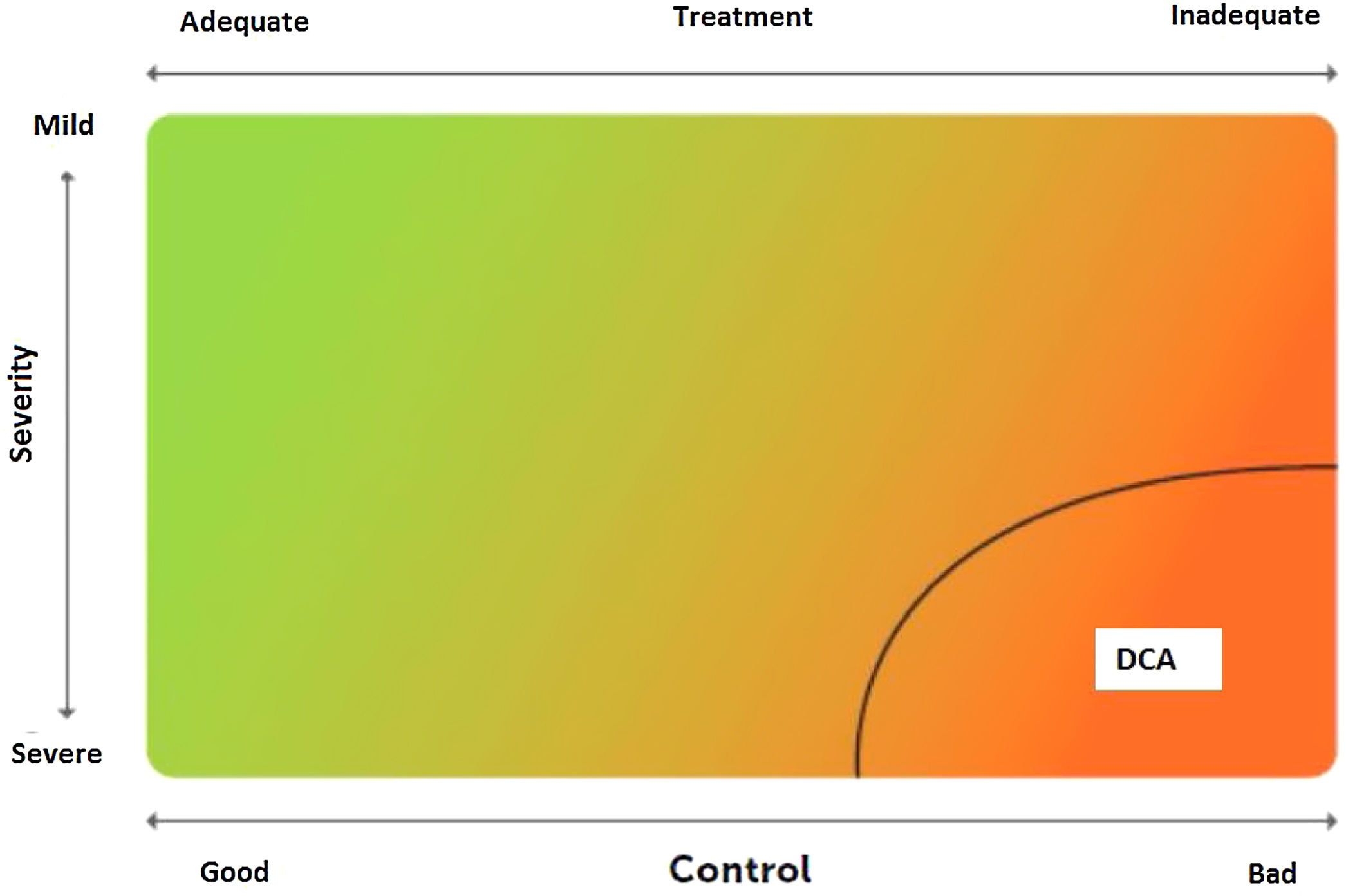
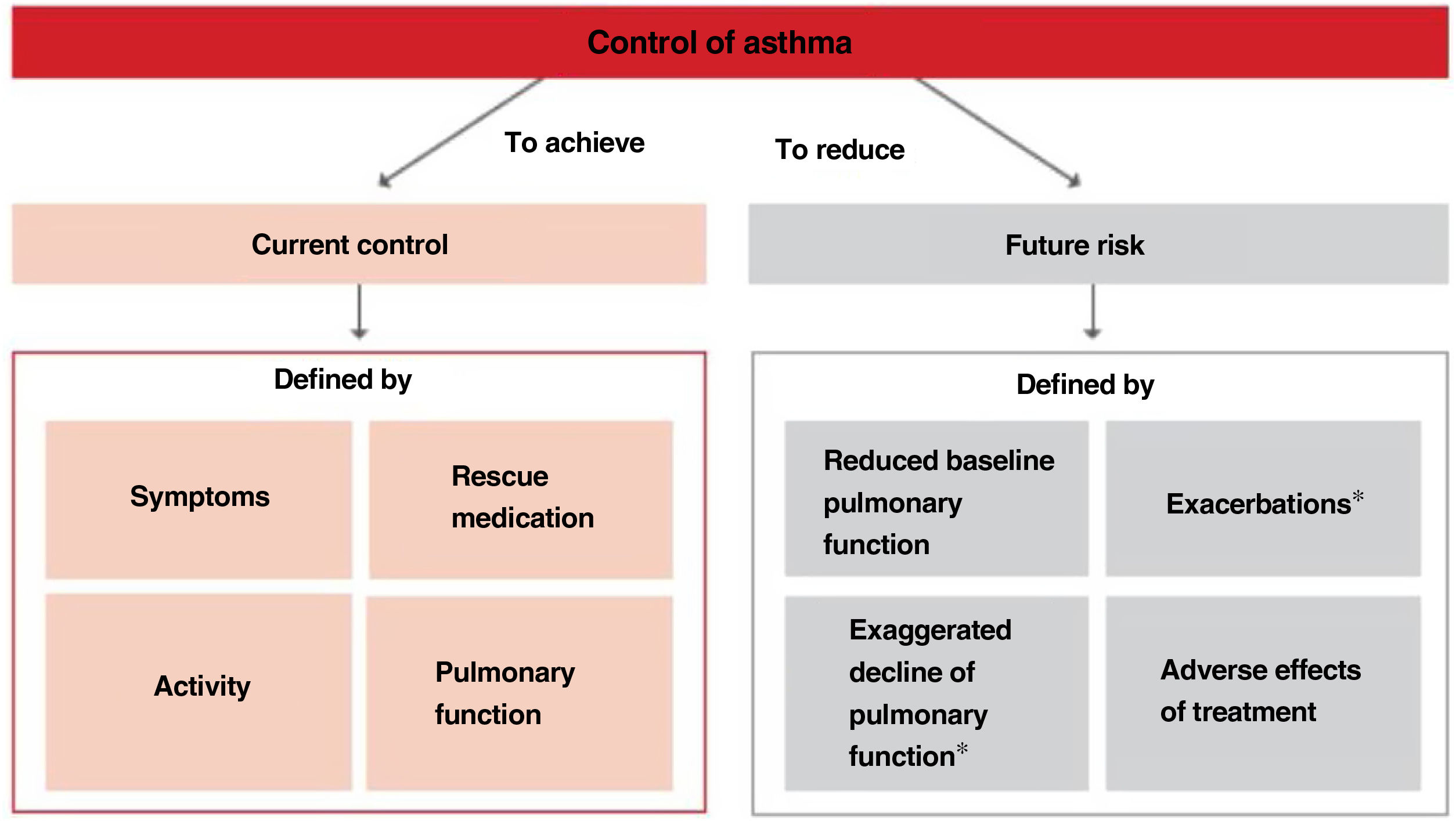
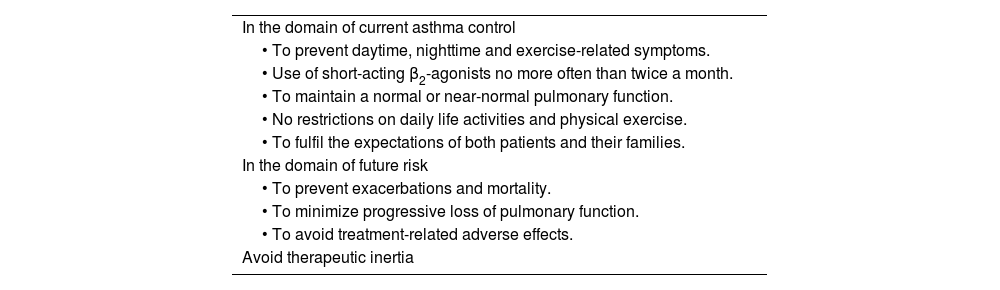
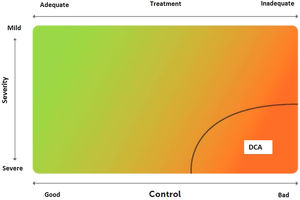
2.6Control and measuring methodsAsthma control is the extent to which disease manifestations can be either absent or maximally reduced by therapeutic interventions, and treatment goals are met,178,180 largely reflecting the adequacy of treatment (fig. 4).
Relationship between severity and control of asthma. The level of control reflects to a large extent the appropriateness of treatment. Some patients suffer from difficult-to-control asthma (DCA).
Modified from Osborne, et al.185.
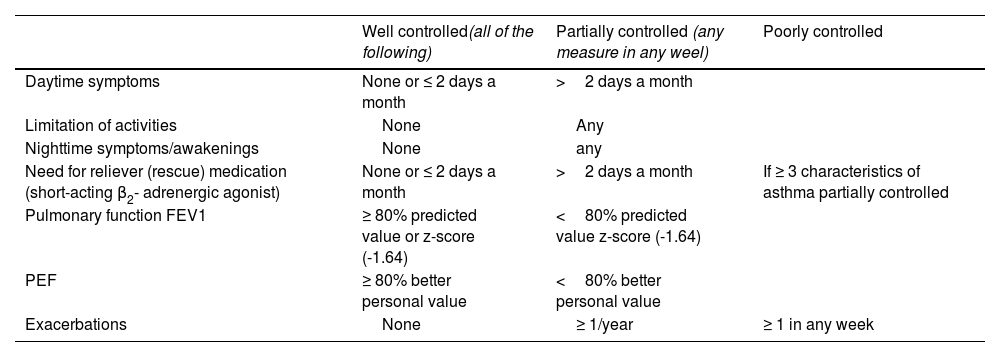
Asthma has been arbitrarily classified according to the degree of disease control in: well-controlled asthma, partially controlled asthma and poorly controlled asthma, based on the criteria shown in table 18.113 Some asthma patients may show a good control of both symptoms and pulmonary function, while simultaneously experiencing frequent exacerbations, whereas some other patients have daily symptoms and very few exacerbations.
Classification of asthma control in adults.
| Well controlled(all of the following) | Partially controlled (any measure in any weel) | Poorly controlled | |
|---|---|---|---|
| Daytime symptoms | None or ≤ 2 days a month | >2 days a month | |
| Limitation of activities | None | Any | |
| Nighttime symptoms/awakenings | None | any | |
| Need for reliever (rescue) medication (short-acting β2- adrenergic agonist) | None or ≤ 2 days a month | >2 days a month | If ≥ 3 characteristics of asthma partially controlled |
| Pulmonary function FEV1 | ≥ 80% predicted value or z-score (-1.64) | <80% predicted value z-score (-1.64) | |
| PEF | ≥ 80% better personal value | <80% better personal value | |
| Exacerbations | None | ≥ 1/year | ≥ 1 in any week |
FEV1: forced expiratory volume in one second; PEF: peak expiratory flow.
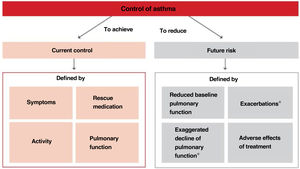
Thus, when trying to minimize the clinical expression of asthma two major aspects should be borne in mind:180 on the one hand, the day-to-day disease manifestations (current control) and, on the other side, its future consequences (future risk), as shown in Figure 5.
Within the current control domain, control would be defined by the presence of daytime and nighttime symptoms; the frequent use of rescue medication for symptomatic relieve; maintenance of pulmonary function within or close to normal limits; the absence of limitations of daily living activities, including family, social, work or school activities, and physical exercise; and finally, the fulfillment of expectations of both patients and their families regarding the quality of care received.
As for the future risk domain, control includes: the absence of exacerbations; the lack of the need of using systemic glucocorticoids, visits to emergency departments and hospitalizations; the prevention of an excessive loss of pulmonary function and the development of a fixed airway obstruction (and an anomalous lung development in the case of children); and finally, the use of an optimal pharmacotherapy with minimum or no adverse effects.
As defined in the control of asthma, a number of procedures should be used for its evaluation.186 The essential tool for assessing asthma control is the continued follow-up medical visit. In this visit, the domains of current control and future risk of exacerbations should be evaluated, together with possible presence of fixed airflow obstruction and treatment-associated adverse effects, and finally and most importantly, the adherence to treatment, including a reminder of the self-management plan and actions to be taken in case of disease decompensation, and trying to reinforce the patient-healthcare professional relationship at each visit.
In order to facilitate and standardize the evaluation of the domain of current control of asthma, different simple questionnaires and easy to be completed by the patient have been developed. The Asthma Control Test (ACT)187,188 and the Asthma Control Questionnaire (ACQ)189,190 have been validated and culturally adapted for use in Spain. Validation of the ACT questionnaire is more detailed for its use in clinical practice with well-defined cut-off points, so that a score equal to or greater than 20 is highly consistent with well-controlled asthma, between 19 and 16 with partially controlled/not well-controlled asthma, and equal to or lower than 15 with poorly controlled asthma.187,188 The minimum clinically relevant difference is 3 points.191 Also, the Spanish version of the ACQ questionnaire has been validated, with cut-off values based on actual clinical practice192,193 with <0.5 for well-controlled asthma, between 0.5 and 0.99 for partially controlled asthma, and ≥ 1 for poorly controlled asthma. However, the reliability of both questionnaires to detect poorly controlled asthma is low,194 and for this reason they should never be used as single tools to evaluate asthma control. To determine the degree of current asthma control and the future risk, the Asthma Impairment and Risk Questionnaire (AIRQ) was developed. The AIRQ is a 10-item instrument that evaluates the presence of symptoms during the previous 2 weeks and the number of exacerbations in the last 12 months;195 the Spanish version has been recently validated.196
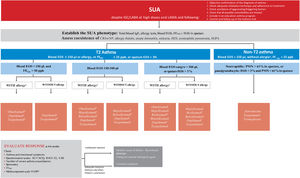
Factors associated with the risk of exacerbations include the presence of uncontrolled asthma symptoms and history of severe exacerbations, but other factors may increase the risk of exacerbations in the absence of uncontrolled asthma or previous severe exacerbations (table 19).
Main risk factors for exacerbations.
| – Absence of current control: ACT <20 or ACQ >1.5.– History of exacerbations: ≥ 1 severe exacerbation in the previous year or history of almost life-threatening asthma.– No use of inhaled steroids: not prescribed, poor adherence or critical errors with the use of inhalers.– Excessive use of rescue medication: ≥ 3 inhalers per year (≥ 2 puffs/day).– Type 2 inflammation: increased peripheral blood/sputum eosinophils, increased FENO.– Pulmonary function: low baseline FEV1, reversibility with the bronchodilator.– Psychosocial problems, low socioeconomic level.– Exposures: tobacco smoke, allergens, pollution.– Comorbidities: obesity, sleep apnea-hypopnea syndrome, chronic rhinosinusitis, gastroesophageal reflux, food allergy, pregnancy. |
Assessment of biomarkers of type 2 inflammation may contribute to stratify the patient's risk, and taking into account that peripheral blood eosinophilia197–199 or sputum eosinophilia200 as well as increased FENO in a patient treated with inhaled glucocorticoids201 are additional factors that increase the risk of exacerbations.
In the patient with severe asthma, adjustment of treatment with inhaled glucocorticoids has been recommended, taking into account results of sputum eosinophils or FENO, since this strategy is associated with a lower risk of exacerbations, although it has no effect on symptoms or pulmonary function.202
Forced spirometry is another tool that can help in the assessment of future asthma control, since a low baseline FEV1 value, in particular <60%,203 and the presence of reversibility204 have been reported as factors that increase the risk of exacerbations.
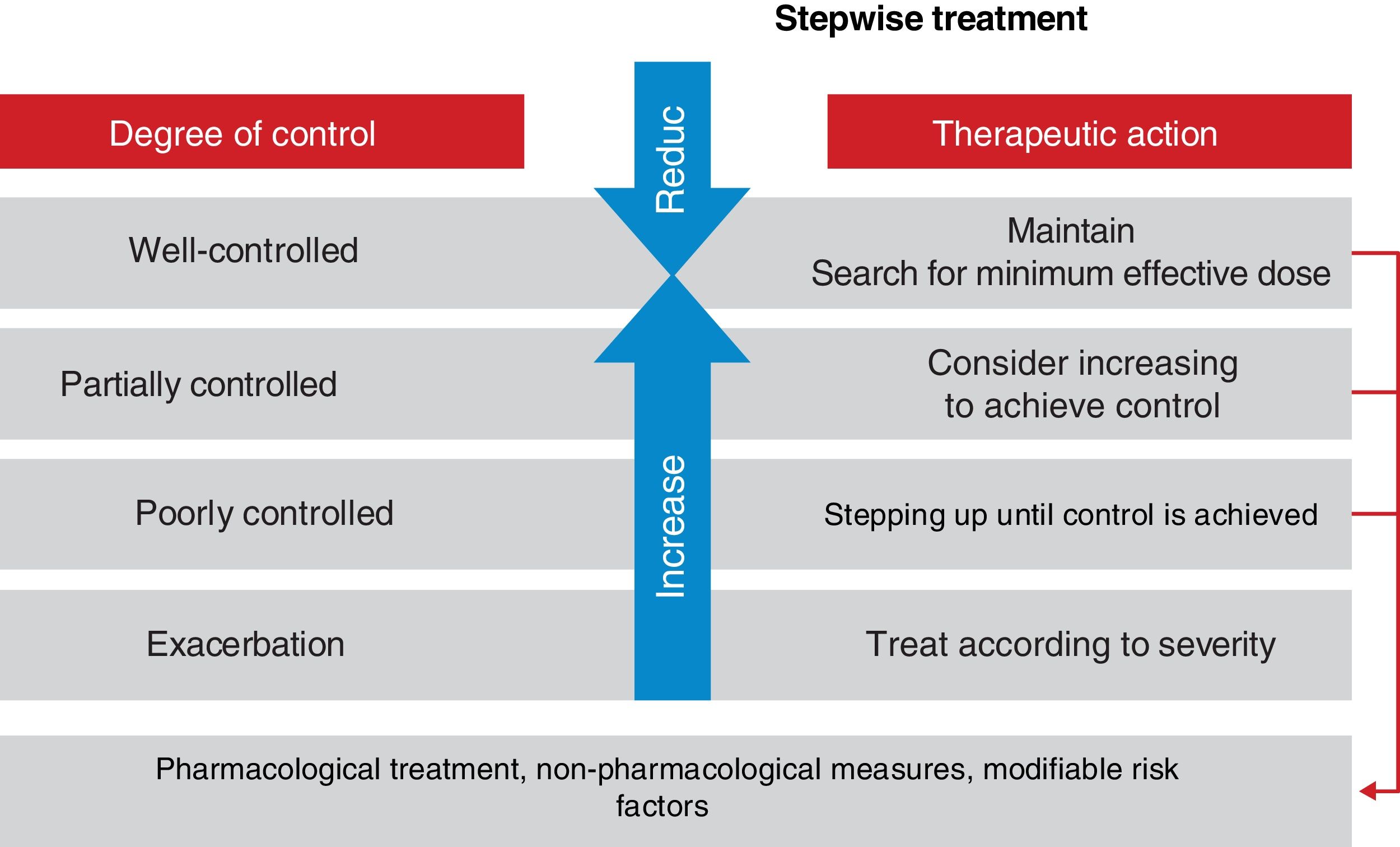
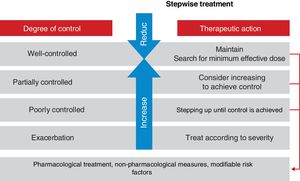
Asthma control should be evaluated at each medical visit. Once asthma treatment is started, clinical and therapeutic management of the disease should be directed toward achieving.
and maintaining control (including symptoms, exacerbations, and pulmonary function). Therefore, the degree of control will guide the decisions on maintenance treatment and dose adjustment, according to the therapeutic steps shown in the corresponding section.
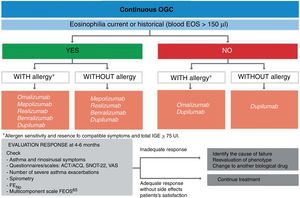
2.7RemissionWith the advent of biological therapy, the concept of “remission” in asthma has been reconsidered. It could be defined as the situation in which there is no disease activity, either spontaneously or as a result of treatment. Two types have been proposed: clinical remission, defined as the absence, for at least 12 months, of symptoms and exacerbations without the use of systemic steroids, in addition to optimization and stabilization of pulmonary function; and complete remission, when the patients also have no hyperresponse and bronchial inflammation.205
In clinical practice, it is possible to achieve remission without treatment, particularly in childhood-onset asthma. A study carried out in 119 children with allergic asthma, followed for 30 years, revealed that complete remission (defined according to strict criteria) was obtained in 22% of the cases, especially in those with better baseline pulmonary function or improvement in the transition to adulthood.206 In another study carried out in 200 adults diagnosed with asthma in the last year and with a subsequent follow-up of 5 years, 16% achieved remission, although with a less strict remission criterion (absence of asthma symptoms without medication for at least 1 year).207
However, the concept of “clinical remission” with treatment has its limitations. A study of 31 well-controlled asthma patients, treated with an ICS and followed up for 1 year, showed that the risk of exacerbations persisted in almost half of them. This risk was higher in those with blood or sputum eosinophilia.200 Another study conducted in 347 patients treated with mepolizumab, with a mean follow-up of 3.5 years, reported a progressive decline in pulmonary function, even reaching levels below baseline.208
The concept of “remission,” with or without treatment, should encompass the absence of clinical manifestations, hyperresponsiveness and bronchial inflammation for a prolonged period of time. However, confirmatory evidence is required for its validation. This should verify that patients in remission maintain stabilized pulmonary function and do not suffer exacerbations. At the time of writing this new version of GEMA, a broad consensus is underway to establish a definition of this concept.
2.8Control and classification of severity in children2.8.1Clinical severityThe classification of severity is different according to the moment at which asthma is evaluated: at the onset, at the time of diagnosis or thereafter once control of the disease has been achieved. In the first case, the level of severity depends on the frequency and intensity of symptoms (number of attacks and between-attack status: mainly exercise tolerance and nighttime symptoms), the need for a rescue bronchodilator and the values of respiratory function tests. In small children in whom lung function testing is not feasible, severity is only classified according to symptomatology.
Some children with asthma present symptoms intermittently, episodically, more or less frequently, while others suffer from more persistent symptoms. The type of moderate or severe asthma is determined by the frequency and intensity of the symptoms. In any case, the classification of severity is established once treatment is started, based on the medication necessary to keep the child well-controlled.
In this way, the patient who requires step 5 or 6 treatment will have severe asthma, the one who needs step 3 or 4, a moderate asthma, the one who requires step 1 or 2, a mild asthma.
Childhood asthma varies substantially over time, even during a single year, which makes its classification difficult. Most young children experience asthma symptoms during viral infections only; they may experience, therefore, moderate or severe asthma in the winter and remain asymptomatic in spring and summer seasons. In order to typify correctly a case of asthma in children, it is necessary to specify, in addition to severity, the triggering factors in the individual patient and the degree of control of asthma.
2.8.2ControlEl Asthma control is defined by the extent to which clinical manifestations have declined or disappeared with the treatment prescribed.209 It includes two components: current symptom control and future risk (future consequences of such control).113
The current control of symptoms is evaluated by the presence and frequency of symptoms, both at daytime and nighttime, the need of rescue medication and the presence of some limitation for daily life activities. The criteria established to define the degree of control vary from one guideline to another, but generally it is classified as good or poorly controlled asthma, although some guidelines also introduce the concept of partially controlled.113
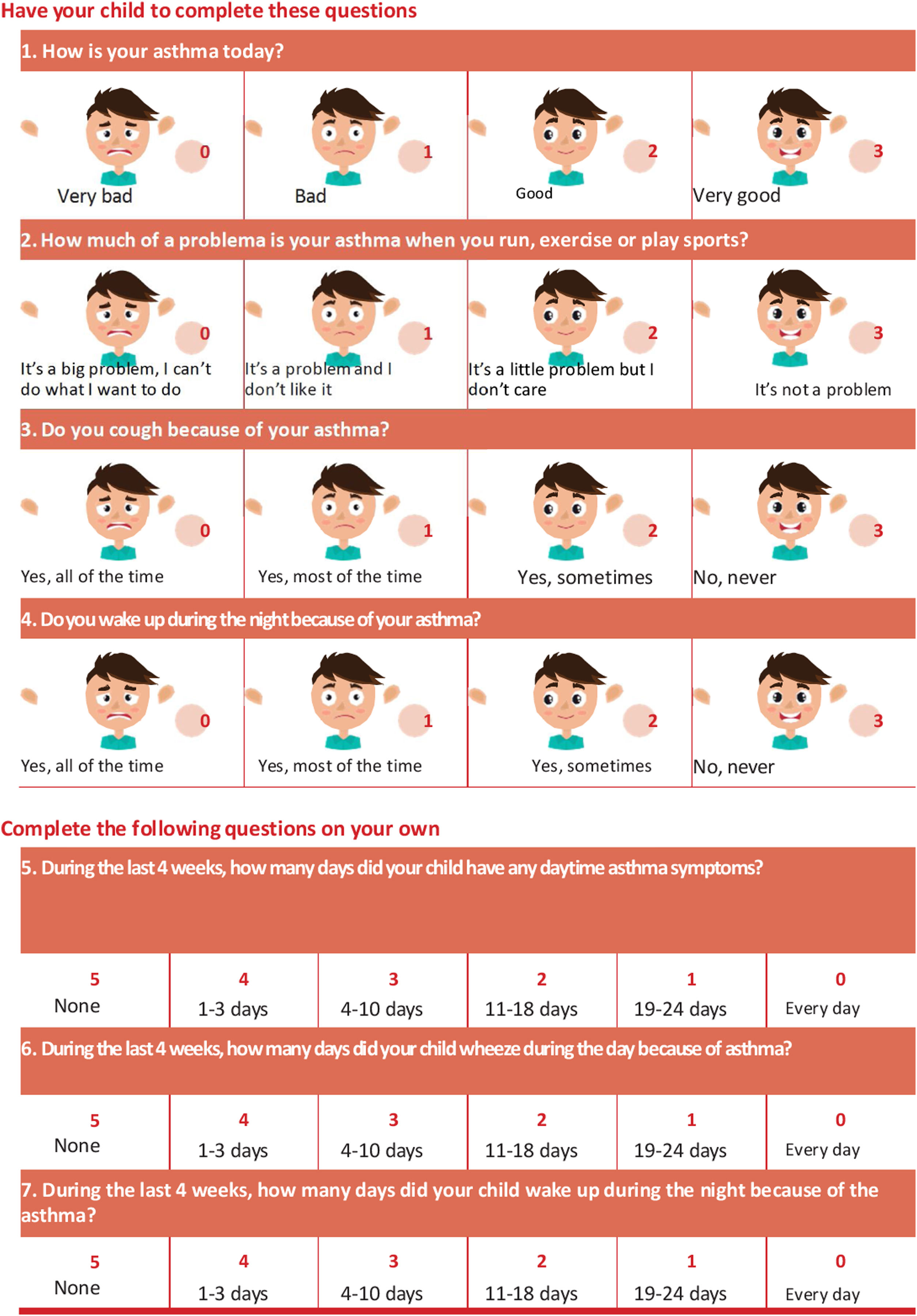
To facilitate symptom control evaluation, there are available specific Spanish validated questionnaires. One of these questionnaires is the Asthma Control Questionnaire in Children (CAN) (Control de Asma en Niños), with a version for 9-14 year-old children and another version for parents (2-8 year-old children). This instrument evaluates 9 questions about clinical manifestations within the last 4 weeks and is scored between 0 (good control) and 36 (poor control). A patient is considered to be poorly controlled when scores are equal to or higher than 8210 (table 20). Also available is the Childhood Asthma Control Test (c-ACT),211 validated in Spanish212,213 for 4-11 year-old children, which includes 7 questions (4 for the child
Asthma Control Questionnaire in Children (CAN).210
| 1.- In the last 4 weeks, how often have you coughed during the day without having a cold? | 4.- In the last 4 weeks, how often have you had wheezing at night? | 7.- When the child exercises (plays, runs, etc.) or bursts out laughing, does he/she coughs or wheezes? |
| 4. More than once a day3. Once a day2. 3 to 6 times a week1. Once or twice a week0. Never | 4. More than once a night3. Once a night2. 3 to 6 times a week1. Once or twice a week0. Never | 4. Always3. Almost always2. Sometimes1. Almost never0. Never |
| 2.- In the last 4 weeks, how often have you coughed at night without having a cold? | 5.- In the last 4 weeks, how often have you had breathing difficulty during the day? | 8.- In the last 4 weeks, how many times has he/she had to visit the emergency department because of his/her asthma? |
| 4. More than once a night3. Once a night2. 3 to 6 times a week1. Once or twice a week0. Never | 4. More than once a day3. Once a day2. 3 to 6 times a week1. Once or twice a week0. Never | 4. More than 3 times3. 3 times2. Twice1. Once0. Never |
| 3.- In the last 4 weeks, how often have had wheezing/whistling sounds inyour chest during the day? | 6.- In the last 4 weeks, how often have you had breathing difficulty during the night? | 9.- In the last 4 weeks, how many times has the child been admitted to hospital because of her/his asthma? |
| 4. More than once a day3. Once a day2. 3 to 6 times a week1. Once or twice a week0. Never | 4. More than once a night3. Once a day2. 3 to 6 times a week1. Once or twice a week0. Never | 4. More than 3 times3. 3 times2. Twice1. Once0. Never |
and 3 for the parents/caregivers). A patient is considered to be poorly controlled when the score is lower than 20 (table 21).
Pediatric Asthma Control Test (ACT) questionnaire validated in Spanish.212,213
The future risk evaluates the presence of risk factors for exacerbations (table 22), to develop a fixed airflow limitation (undertreatment with ICS, prematurity,214 environmental exposure to tobacco smoke, low FEV1, severe asthma, previous hospitalizations) and for suffering treatment-related side effects (frequent courses of oral glucocorticoids, high doses of ICS).113,215
Risk factors for asthma exacerbations in children.214,215
| – At least one exacerbation in the previous year.– Previous care in the ICU or need of intubation.– Excessive use of SABA.– Persistent and/or uncontrolled symptoms.– Lack of adherence to treatment*, inadequate inhalation technique.– Low FEV1. Positive bronchodilation test.– Exposure to allergens in case of allergy/atopy.– Exposure to tobacco smoke.– Comorbidities: obesity, allergic rhinitis, food allergy.– Important psychological or socioeconomic problems.– Other: peripheral blood or sputum eosinophilia; increase of FENO in routine control visits. |
In addition to the control of clinical symptoms and pulmonary function, measurement of FENO has been advocated as an approach to assess the control of inflammation. Although potentially useful in some patients, FENO measurement does not seem to add relevant benefits to the aforementioned follow-up and treatment strategies.215
4.1Recommendations| 2.1. Asthma should be suspected in a patient with wheezing, dyspnea (or breathing difficulty), cough and chest tightness of variable intensity and frequency. | R2 |
| 2.2. En In case of suspected asthma, seasonal variations and personal or family history of asthma or atopy are important aspects to be considered, although none of these or none of the signs or symptoms, especially when isolated, are specific of asthma. | R2 |
| 2.3. The diagnosis of asthma should be based on objective measures of functional involvement. Spirometry with a bronchodilation test is the diagnostic study of choice. | R2 |
| 2.4. The diagnosis of asthma should be considered in the presence of daily variability of peak expiratory flow (PEF) >20%, or an increased fractional exhaled nitric oxide (FENO) >40 ppb in patients who have not been treated with glucocorticoids, particularly in association with reduced FEV1. | R2 |
| 2.5. Non-specific bronchial challenge test should be considered to exclude the diagnosis of asthma. | R2 |
| 2.6. Periodic spirometry testing (at least once a year) are recommended for children with asthma requiring continuous treatment. | R2 |
| 2.7. In children, except for specialized consultation, it is not necessary to measure FENO routinely. | R2 |
| 2.8. Allergy studies are especially indicated when aeroallergens are suspected to be involved in the development of asthma or its exacerbations, or when other associated atopic diseases are present. | R2 |
| 2.9. The diagnosis of allergic asthma will be based on the concordance between the patient's clinical history and the results of diagnostic studies. | R2 |
| 2.10. The severity of asthma (in adults and children) will be established according to the minimum maintenance treatment needed to achieve control. In untreated patients, the severity of asthma should be established at the beginning of treatment, with further re-evaluation once control is attained. | R2 |
| 2.11. The severity of asthma (in adults and children) is not necessarily a constant feature and can change over time (months or years), so that periodic re-evaluation is required. | R2 |
| 2.12. Control of asthma (in adults and children) should be evaluated at each consultation, and treatment should be adjusted to achieve and maintain control. Control has two main components that should be identified: current control and future risk. | R2 |
| 2.13. In the objective assessment of the degree of current control of asthma (in adults and children), it is recommended using validated questionnaires for symptoms (preferably ACT in adults, and cACT and CAN in children). In the assessment of future risk of exacerbations, recommendations include questioning on previous events, spirometry, use of inhaled glucocorticoids and reliever/rescue medication, comorbidities and, in selected cases, inflammatory biomarkers (peripheral blood or sputum eosinophils and FENO). | R2 |
The main objective of asthma management is to achieve and maintain control of the disease as quick as possible, in addition to prevent exacerbations and chronic airflow obstruction and to reduce mortality at maximum. With a properly designed treatment plan, therapeutic targets (table 23) can be achieved in a large majority of patients in terms of daily symptom control (current control domain) and prevention of both exacerbations and excessive loss of pulmonary function (future risk domain).
Asthma treatment goals.
| In the domain of current asthma control |
| • To prevent daytime, nighttime and exercise-related symptoms. |
| • Use of short-acting β2-agonists no more often than twice a month. |
| • To maintain a normal or near-normal pulmonary function. |
| • No restrictions on daily life activities and physical exercise. |
| • To fulfil the expectations of both patients and their families. |
| In the domain of future risk |
| • To prevent exacerbations and mortality. |
| • To minimize progressive loss of pulmonary function. |
| • To avoid treatment-related adverse effects. |
| Avoid therapeutic inertia |
To attain these objectives a global and individualized long-term strategy must be followed based on an optimally adjusted pharmacological treatment along with supervision measures, environmental control and asthma education activities.223 Pharmacological treatment should be adjusted according to the patient's degree of control, considering the most effective therapeutic options, safety and cost of the different alternatives, and taking into account the patient's satisfaction with the degree of control achieved. Patients should be periodically evaluated to determine whether objectives are being achieved. Clinical inertia and causative factors of inertia on the part of the patient, the physician and the healthcare system should be avoided.
3.1.1Pharmacological treatmentTreatment of asthma should follow an overall plan, established by consensus of the physician and the patient (and eventually by the patient's family), in which the goals, the interventions to achieve them, and the criteria for schedule modification or adaptation according to changing disease circumstances must be made clear. Distinguishing between the current control domain and the future risk domain in the control of the disease is relevant, because it has been documented that these domains may respond differently to treatment224,225. For example, some patients may have a good daily control of asthma symptoms and yet experience exacerbations, and vice versa.
Treatment should be adjusted continuously, so that the patient remains always in a well-controlled status. This cyclic treatment adjustment means that asthma control should be objectively assessed (chapter 2.6), that the patient is being treated to achieve control, and that treatment is periodically checked to maintain asthma control (fig. 6). That is, if the patient is not well controlled, treatment must be stepped up as needed in order to regain control, always taking into account the role of non-pharmacological measures, treatment adherence and risk factors susceptible to be modified.
If asthma has been controlled for at least 3 months, maintenance therapy may be gradually decreased in order to determine the minimum treatment needs that are required to maintain control226. A simple scoring system (FEOS scale) that combines data of different clinical (ACT, previous exacerbations) and functional (spirometric values) variables has been developed, to determine the risk after stepping down treatment in patients with controlled asthma227.
Drugs used to treat asthma are classified as controller or maintenance medications and reliever medications, also called “rescue” medications. Controller or maintenance medications should be administered continuously during prolonged periods of time, and include inhaled glucocorticoids (ICS) or systemic glucocorticoids, leukotriene receptor antagonists (LTRA), long-acting ß2-adrenergic agonists (LABA), tiotropium and monoclonal antibodies (omalizumab, mepolizumab, reslizumab, benralizumab, and dupilumab). Chromones and sustained-release theophylline have fallen into disuse because of their lower efficacy.
Reliever medications are used on-demand for rapid treatment or prevention of bronchoconstriction, and include inhaled short-acting ß2-adrenergic agonists (SABA) (table 24) and inhaled short-acting anticholinergics (ipratropium bromide). The use on-demand of the combinations budesonide/formoterol, beclomethasone/formoterol or beclomethasone/salbutamol can also be considered reliever medications.
Characteristics of inhaled β2-adrenergic agonists.
| Amount per puff (μg) | Time of effect (minutes) | ||||
|---|---|---|---|---|---|
| Drug | Pressurized inhaler | Dry power | Onset | Maximum | Duration |
| Short-acting | |||||
| Salbutamol | 100 | 100 | 3-5 | 60-90 | 180-360 |
| Terbutalina | - | 500 | 3-5 | 60-90 | 180-360 |
| Long-acting | |||||
| Formoterol | 12 | 4.5-9-12 | 3-5 | 60-90 | 660-720 |
| Salmeterol | 25 | 50 | 20-45 | 120-240 | 660-720 |
| Vilanterol | - | 22 | 3-5 | 180-240 | 1.440 |
| Indacaterol | - | 125* | 5 | 120-240 | 1.440 |
The six treatment steps (fig. 7) aimed at achieving asthma control are the following:
Therapeutic steps for maintenance treatment in adult asthma.
ICS: Inhaled glucocorticoid; LABA: Long-acting β2- adrenergic agonist; LTRA: Leukotriene receptor antagonist; SABA: Short-acting β-adrenergic agonist.
aAfter confirmation of adequate treatment adherence and use of the inhaler (s). bLAMA: tiotropium or glycopyrronium. cWithout maintenance treatment. dOn-demand ICS+formoterol can be used when this maintenance combination is also used.
Different treatment options can currently be considered for this step. A correct clinical and functional assessment of the patient is required for an adequate selection of treatment.
The association budesonide/formoterol on-demand can be used.228 In a randomized study on adult asthma patients with approximately half of patients having intermittent asthma and in which an open-label design was used to reflect clinical practice conditions,229 the use of budesonide/formoterol on-demand was superior to salbutamol on-demand in the prevention of exacerbations. In a small study of patients with intermittent asthma and increased fractional exhaled nitric oxide (FENO) in which both budesonide/formoterol and formoterol on-demand were compared, the combination showed a higher reduction of FENO levels.230 The association salbutamol/beclomethasone dipropionate on-demand can also be used.231 However, these indications are not included in the technical specifications of these drugs. In addition, cost-benefit studies have not been carried out.
Inhaled SABA (salbutamol or terbutaline) exclusively on-demand can be used in patients with occasional and mild daytime symptoms (maximum twice a month) and without nighttime symptoms.232,233 The patient should be asymptomatic between episodes and maintain a normal pulmonary function, and not having suffered from exacerbations in the previous year as well as not having risk factors for exacerbations (table 24).232
Although SABA at the recommended doses does not increase the risk of severe exacerbation or death,234 its excessive use (3 or more inhalers a year) is associated with a higher risk of exacerbations, use of healthcare services and negative impact in the patients’ health, particularly when used as monotherapy.235 Up to a third of patients abuses of this medication, being a phenomenon of worldwide distribution.236–238 SABA abuse is an indicator of poor control, which should alert on the need to start or optimize maintenance treatment with ICS, along with the rest of the therapies used in asthma.235
The use of inhaled SABA on-demand more than twice a month for the treatment of symptoms (independently of its preventive use before exercise) or having been suffered from exacerbations in the last year or a FEV1 <80% indicates an inadequate control of asthma and requires the use of maintenance therapy.239–241
Inhaled SABAs administered 10-15minutes before exercise are the drugs of choice to prevent exercise-induced bronchoconstriction.242
An inhaled anticholinergic as a reliever medication is only recommended in those rare cases of intolerance to SABA agents.228
4.1.1.2STEP 2The treatment of choice at this step is an inhaled glucocorticoid (ICS) (beclomethasone, budesonide, ciclesonide, fluticasone or mometasone) at low doses and administered daily.243–246 In general, this is the first step for most patients with persistent asthma who have not been previously treated. The usual dose ranges between 200 and 400μg/day of budesonide or equivalent. Continuous administration of ICS is the most effective maintenance treatment for persistent asthma, both for the control of daily symptoms and to reduce the risk of exacerbations240,246,247,248
Table 25 includes the approximate equipotent doses of ICS (low, medium, high) based on results of studies with efficacy/safety designs and reported in the corresponding authorized technical specifications of these compounds (available online: https://cima.aemps.es/cima/publico/home.html). However, although these results seem to be questioned by data of another pharmacodynamic study carried out in a small sample of patients,249 the evidence is insufficient to make changes250 and further complementary studies are warranted.
Approximate potency of ICS (based on results of efficacy/safety studies).
| Low dose (μg/day) | Medium dose (μg/day) | High dose (μg/day) | |
|---|---|---|---|
| Budesonide | 200-400 | 401-800 | 801-1,600 |
| Beclomethasone dipropionate | 200-500 | 501-1,000 | 1,001-2,000 |
| Extrafine beclomethasone* | 100-200 | 201-400 | >400 |
| Ciclesonide | 80-160 | 161-320 | 321-1,280 |
| Fluticasone propionate | 100-250 | 251-500 | 501-1,000 |
| Fluticasone furoate | - | 92 | 184 |
| Mometasona furoate** | |||
| Twisthaler®† | 200 | 400 | 800 |
| Breezhaler®†† | 62.5 | 127.5 | 260 |
| Breezhaler®††,††† | - | - | 136 |
Two clinical trials showed that a strategy of using a combination of budesonide/formoterol in a single inhaler on-demand compared to continuous ICS treatment in mild persistent asthma, was not inferior in preventing exacerbations (the rate of which was similarly low); however, it was inferior in the maintenance of asthma control and in the increase of pulmonary function.251,252 In a randomized open-label study,229budesonide twice a day plus salbutamol on-demand and budesonide/formoterol on-demand were similar regarding annual exacerbation rates.
Also, a similar result with beclomethasone/salbutamol has been observed.231
Results of the aforementioned studies may provide indirect evidence of a possible indication of the combinations of low dose ICS with LABA or SABA (e.g. budesonide/formoterol, beclomethasone/formoterol or beclomethasone/salbutamol), administered exclusively on-demand, in the treatment of step 2 in patients with low treatment adherence and in which specific educational interventions have been unsuccessful. However, no studies have been specifically designed to assess this therapeutic indication.
At this level, an alternative treatment includes leukotriene receptor antagonists (LTRA) or anti-leukotrienes (montelukast and zafirlukast),253,254 although ICS are more effective for long-term treatment.253 Patients who are well controlled on ICS at low doses fail to maintain the same level of asthma control with montelukast.255
LTRA would be particularly indicated as alternative drug in patients who are unable or unwilling to receive ICS or have adverse effects with ICS, have difficulties with the inhaler technique, or suffer from concomitant allergic rhinitis.256,257
In patients who have not previously received maintenance treatment with ICS, the combination of ICS at low doses and LABA as initial treatment as compared with ICS at low doses, improves symptoms and pulmonary function but has a higher cost and it does not reduce the risk of exacerbations.258
4.1.1.3STEP 3First-line treatment at this step is a combined inhaled treatment with ICS at low doses and a LABA (salmeterol, formoterol, vilanterol or indacaterol),259–264 which can be administered using a single device (preferred option) or separate inhalers. By using this combination a more pronounced reduction of symptoms, improvement of pulmonary function, and reduction of exacerbations and use of reliever medications is obtained as compared to increasing the dose of ICS. However, an appropriate individualized risk/benefit assessment for both strategies is required.
Treatment with LABA should always be accompanied by an ICS. LABA agents must never be used as monotherapy because of a higher risk of hospitalizations and life-threatening exacerbations.265,266 ICS/LABA combinations commercialized in Spain include: fluticasone propionate with salmeterol, budesonide with formoterol, beclomethasone dipropionate with formoterol, fluticasone propionate with formoterol, fluticasone furoate with vilanterol267 and indacaterol268 with mometasone.269
Formoterol is a rapid-onset LABA. For this reason, if budesonide/formoterol or beclomethasone/formoterol combinations are chosen, they can be used as both maintenance and reliever therapy (MART strategy). This strategy leads to reduced exacerbations and a better asthma control, despite requiring a lesser amount of ICS.242,270–277 It may be assumed that other ICS combinations (fluticasone propionate) with formoterol may be effective as MART strategy, although there is no evidence of its use as maintenance and on-demand treatment and this indication is not included their technical specifications.
In any case, MART therapy always should be administered using a single inhaler device.
A further option at this step includes increasing ICS doses up to medium doses, but this approach is less effective than adding a LABA.278–280 Alternatively, ICS at low doses associated with a LTRA may be used. This option has been found to be superior to IGS monotherapy and although it is not as effective as the IGS and LABA combination, has an excellent safety profile.281–284 However, the addition of an LTRA does not appear allowing to reduce the ICS dose.285
4.1.1.4STEP 4The first-line treatment at this step is the combination an ICS at medium doses with a LABA.258,260,262,286,287
For patients who have had at least one exacerbation in the previous year, the combination of a ICS at low doses (budesonide or beclomethasone) and formoterol, using the MART strategy, is more effective in reducing exacerbations than the same dose of an ICS and LABA in a fixed schedule, or higher doses of ICS.277,278
Alternatively, the combination of an ICS at medium doses with a LTRA can be used, although the addition of LABA to the ICS is more effective in preventing exacerbations, control of daily symptoms and improving pulmonary function.280 In patients with uncontrolled asthma despite the aforementioned treatment, triple therapy with ICS at medium doses, LABA and LAMA (tiotropium or glycopyrronium) in a single inhaler288 or different inhalers289 may be considered. However, this option has not been compared with the standard strategy of increasing the doses of ICS in the ICS+LABA combination of proven efficacy for preventing severe exacerbations, so that adequate studies are needed to define the position of the triple therapy in this therapeutic step.290
4.1.1.5STEP 5The next step consists of increasing the dose of ICS up to a high dose in combination with LABA.260,262,291 ICS at medium and high doses are usually administered twice daily, although a greater therapeutic efficacy can be achieved with budesonide by increasing the dosing frequency up to 4 times a day.292
Other drugs can be added for maintenance therapy, with a subgroup of patients improving with the addition of LTRA.293,294
In patients insufficiently controlled with the combination of an ICS at high doses and LABA, who show post-bronchodilator FEV1/FVC ≤ 70%,295 the addition of tiotropium (in different inhalers) or glycopyrronium (in a single inhaler [pMDI Modulite© and Breezhaler©]), provides an improvement of pulmonary function and a reduction of exacerbations.288,289,295–299 With the same indication, in Latin America and the United States, there is another triple combination of a GCI/LABA/LAMA (fluticasone f./vilanterol/umeclidinium) (approved by the FDA), after demonstrating a significant improvement in lung function;300 however, this combination is not available in Europe for the treatment of asthma (it has been rejected by the EMA), as it has not shown a significant reduction in exacerbations.300
Macrolide antibiotics, particularly azithromycin administered 3 days/week for several months, may play a role as an add-on medication in patients with severe non-eosinophilic asthma and frequent exacerbations,301,302 as well as in eosinophilic asthma303 (see chapter 7).
4.1.1.6STEP 6For asthma patients who remain uncontrolled and with frequent exacerbations, the addition of biologic drugs should be considered after a specialized evaluation and according to the endophenotype of the patient.
In cases of uncontrolled severe allergic asthma, the anti-IgE monoclonal antibody (omalizumab) by the subcutaneous route can be added, which improves daily symptoms and decreases exacerbations,304–307 increasing the overall control of the disease (see chapter 7).
In patients with uncontrolled severe eosinophilic asthma, independently of the presence of allergy, biologic drugs targeting the interleukin-5 (IL-5) pathway can be used. Currently, anti-IL-5 monoclonal antibodies, mepolizumab and reslizumab, and the anti-IL-5 receptor a chain (IL-5Ra), benralizumab, are approved as additional treatment of eosinophilic uncontrolled severe asthma (severe refractory eosinophilic asthma)308–314 (see chapter 7).
Dupilumab is a human monoclonal antibody directed against the interleukin-4 receptor subunit a (IL-4Ra) of IL-4 that blocks the effects of IL-4 and IL-13 is approved as additional treatment in patients older than 12 years of age with uncontrolled severe asthma with increased eosinophils and/or FENO (see chapter 7).
The human monoclonal antibody directed against thymic stromal lymphopoietin (TSLP) is authorized as an add-on medication of T2 and non-T2 uncontrolled severe asthma (see additional information in chapter 7).315,316
In cases in which the administration of biologic agents has failed, the indication of endobronchial thermoplasty may be considered317 (see chapter 7).
The last therapeutic option when all other alternatives have failed is the administration of systemic glucocorticoids (always used at the lowest effective dose and for the minimum period of time possible)318,221 even though they are also associated with adverse effects, occasionally serious (see chapter 7).
3.1.3Inhalers and nebulizersInhaled therapy is the preferred administration route for the treatment of asthma as it acts directly on the lungs, delivers a greater amount of drug into the airways, elicits a rapid response and is associated with few or no systemic effects.222,319–323
The main disadvantage of this route is the difficulty of the inhalation technique of the different devices.216,324–326
Currently available inhalation devices include: the conventional pressurized inhaler (pMDI) and the Modulite® system, which can be used with or without a spacer, the breath actuated inhaler (BAI) k-haler® and Easy-breathe®, the soft mist inhaler (SMI) Respimat®, the dry powder inhalers (DPI) (Accuhaler®, Aerolizer®, Breezhaler®, Easyhaler®, Ellipta®, Forspiro®, Genuair®, Handihaler®, Nexthaler®, Spiromax®,
Turbuhaler®, Twisthaler® and Zonda®) and the nebulizers (jet, ultrasonic or vibrating mesh). Each of them has their own technical characteristics that should be considered when prescribed (table 26).324
Aerodynamic properties provided by inhalers (based in part on Giner 2016).216
| Pulmonary deposition (%) | Oropharyngeal deposition (%) | MADM | |||
|---|---|---|---|---|---|
| In vivo | In vitro | In vivo | In vitro | (μm) | |
| pMDI | |||||
| - Conventional pMDI | 7.8-34 | - | 53.9-82.2 | - | 1.4-8 |
| - Conventional pMDI with spacer | 11.2-68.3 | - | 31.2 | 40 | 2-3.2 |
| - pMDI autodisparo | 50-60 | - | 30 | - | - |
| - Modulite® | 31-34 | - | 33-58 | - | 1-2 |
| - Alvesco® | 50-52 | - | 32.9 | - | - |
| - pMDI Aerosphere* | 37.7217,218 | 58-61219 | 62 | - | 3-3.2220 |
| BAI SMI | |||||
| - k-haler® | 44.7221 | - | 23-30 | - | - |
| DPI (by alphabetical order) | |||||
| - Respimat® | 40-53 | - | 19.3-39 | - | - |
| - Accuhaler® | 7.6-18 | 15-30 | - | - | 3.5 |
| - Aerolizer® | 13-20 | 21.7-28 | 73 | - | 1.9-7.9 |
| - Breezhaler® | 36 | 39 | - | 45 | 2.8 |
| - Easyhaler® | 18.5-31 | 29 | - | - | 2.2-3.0222 |
| - Ellipta® | - | - | - | - | 2-4.8 |
| - Genuair® | 30.1 | - | 54.7 | - | - |
| - Handihaler® | 17.8 | 17.3-22 | - | 71 | 3.9 |
| - Inhalador Ingelheim® | 16 | - | 59 | - | - |
| - Nexthaler® | 56 | - | 43 | - | 1.4-1.5 |
| - Spinhaler® | 11.5 | - | 30.9 | - | - |
| - Turbuhaler® | 14.2-38 | 28 | 53-71.6 | 57.3-69.3 | 1.7-5.4 |
| - Twisthaler® | 36-37 | - | - | - | 2-2.2 |
MADM: mean aerodynamic diameter mass; BAI: breath-actuated inhaler; DPI: dry powder inhaler; pMDI: pressurized metered-dose inhaler; SMI: soft miss inhaler. The comparison of values among devices should be considered with caution because of differences in the methods and drugs used for estimating the corresponding values, as well as differences in human studies, which were performed in diverse clinical settings (healthy and ill subjects with different diseases and degrees of severity), inspiratory flows and ages.
All inhaler devices if correctly used provide en efficient deposition of the drug in the lung.320
The use of spacers is recommended for pMDI. Spacers circumvent coordination issues, improve the distribution and the amount of drug reaching the bronchial tree, reduce the deposition of drug particles in the oropharynx, decrease cough and the possibility of oral candidiasis (that may be associated with the use of ICS), decrease systemic bioavailability and, hence, the risk of systemic effects.327–330
Healthcare professionals involved in the care of patients with asthma should know the inhalation techniques of each of the devices; knowledge, however, is still insufficient.331,332
Given that the proper use of inhalers is a crucial aspect in the treatment of patients with asthma, all healthcare professionals involved, doctors, nurses and pharmacists especially those from the community due to their accessibility, should be involved in the instruction and review of the inhalation technique.333–340
The patient should be periodically trained and controlled in the use of the prescribed inhaler device, explaining its characteristics, the appropriate technique, demonstrating how it is used, then asking the patient to perform the maneuvers (with a placebo device) and correcting the possible mistakes.323,341–343
Whenever pharmacologically possible, a single type of inhaler device should be used.344,345
After the instruction in the use of the device, the patient should be given a brochure with description of the technique and receive information on how to find demonstration videos showing the correct inhalation technique.321,322,216,342,343
It is important to take advantage of control visits, performance of pulmonary function tests and admissions to the hospital to check the patient's inhalation technique.342
Hydrofluorocarbon (HFC) propellants in current pressurized cartridge inhalers (pMDIs) contribute to global warming as greenhouse gases.346,347 New less polluting HFC propellants are being investigated. Until these are available, the use of dry powder or mist devices may be preferable in new patients >6 years or with inspiratory flow >30 L/min. Changing the inhaler, for non-clinical reasons, could pose a risk of disease deterioration and/or promote low therapeutic adherence (including poor inhalation technique with the new device). Replaced inhalers and cartridges will be deposited at the convenient point of the integrated packaging management and collection system (SIGRE) of pharmacies for correct recycling.
3.2Other treatments3.2.1Smoking and environmental controlSmokers with asthma have more severe symptoms, a poorer response to ICS treatment, even in patients with mild asthma,348 and an accelerated loss of pulmonary function,349,350 so that a step-up in treatment is often required.351 The proportion of asthmatic smokers is high and similar to that in the general population. Moreover, since longitudinal studies have found a relationship between tobacco use and asthma in both adults and adolescents,352 the primary objective in environmental control is getting the patient to stop smoking. To this purpose, smokers should receive full information of the most appropriate quitting methods.353 Exposure to both environmental contaminants and passive smoking aggravates the course of asthma and constitute a risk factor for asthma development in childhood.354 Administrative regulations banning smoking in public spaces are being having a highly positive impact.355,356 Also, passive exposure to smoke of electronic cigarettes has been related with a higher risk for exacerbations and asthma symptoms,357,358 and active exposure to severe effects of respiratory health,359 so that vaping cannot be recommended as a method to quit.
Some asthma patients, particularly those with sinonasal polyposis, may experience exacerbations when administered acetylsalicylic acid or other non-steroidal anti-inflammatory drugs (NSAID). Many of these reactions are serious or even fatal,360 so that it is necessary that patients are correctly diagnosed based on evident data in the medical history (several reactions to different NSAIDs) or by means of an oral challenge test which, in severe cases, can be replaced with bronchial or nasal inhalation challenge testing.361,362 This issue is more comprehensively explained in chapter 8.5 (acetylsalicylic acid-exacerbated respiratory disease). These patients, however, among their environmental measures, should avoid the use of analgesic or anti-inflammatory treatments with drugs of the NSAID therapeutic class.
Specific recommendations should be considered in allergic asthma, once sensitizations to different allergens had been confirmed in each patient. The most effective measures are those enabling a dramatic decrease of exposure levels, such as those applicable to many patients with occupational asthma (job change) or asthma due to animal dander (removal of animals from the patient's home) or cockroach allergy (wise use of pesticides).363–368
Isolated individual interventions, such as the use of mattress covers or acaricides have not shown to be effective, not even in reducing exposure levels.369–371
However, in a recent randomized study, the use of impermeable bed covers was effective for preventing exacerbations in children and adolescents with allergic asthma triggered by dust mites.372
The use of combined specific measures has been associated with a significant reduction in the level of allergen exposure and, in consequence, of benefits in clinical efficacy.363,373,374 In a randomized trial of 937 patients with uncontrolled moderate to severe asthma and sensitization to at least one domestic allergen, in which combined measures were applied (impermeable covers, vacuum cleaners and air purifiers in the bedroom both with HEPA filters, cockroach disinsection plans), associated with a general education program, for one year, obtained a significant reduction in symptoms and unscheduled medical visits.363
Finally, the two more recent systematic reviews of the effect of combined interventions showed favorable outcomes.367,375
3.2.2Allergen immunotherapySubcutaneous immunotherapy with allergen extracts is an effective treatment in well-controlled allergic asthma with low or medium treatment levels (therapeutic steps 2 to 4), provided that a clinically relevant IgE-mediated sensitization against common aeroallergens has been demonstrated and well-characterized and standardized allergen extracts are used,376,377 avoiding complex mixtures.378,379 However, many patients with mild intermittent asthma (step1) suffer from moderate or severe allergic rhinitis concomitantly, which would justify the prescription of immunotherapy.380 Subcutaneous immunotherapy should not be prescribed to patients with uncontrolled severe asthma, because its efficacy is not well documented and a high risk of serious, even fatal, adverse reactions.379,381 For this reason, subcutaneous immunotherapy should only be prescribed by specialist physicians with experience in this type of treatment and administered in centers equipped with the basic resources for the immediate treatment of a possible adverse reaction.
The search for safer and more convenient alternatives for the patient has led to investigate the efficacy of sublingual immunotherapy. Some systematic reviews conclude that oral immunotherapy with capsules or lyophilized extracts can significantly reduce clinical manifestations and the use of rescue medication in children, adolescents and adults with allergic asthma.377,382–384
Most clinical trials showing clinical efficacy were performed with well-characterized extracts and at much higher doses than those usually prescribed for subcutaneous immunotherapy. The tolerability profile of sublingual immunotherapy is optimal and fatal reactions have not been reported.377,384
Sublingual immunotherapy with an oral lyophilized mite extract when added to regular pharmacological maintenance treatment is able to reduce the number of moderate to severe exacerbations385 and to improve the control of the disease, with a very favorable safety profile. Therefore, its use is recommendable for adult patients with moderately controlled or partially controlled asthma.380
When there are several immunotherapy alternatives available, priority should be given to the use of those that are considered registered drugs with well-established efficacy, safety, and quality data.
At the moment, no comparative studies on the cost-effectiveness of immunotherapy versus conventional pharmacotherapy are yet available, and they are not likely to be performed since their complex design makes them still unfeasible.
However, immunotherapy is not only useful in controlling disease manifestations, but it also offers additional advantages over pharmacotherapy, such as the maintenance of clinical benefits for several years after treatment discontinuation,386,387 a decrease in the risk of developing asthma in patients with allergic rhinitis,387,388 or the occurrence of new sensitizations in monosensitive patients.389 Finally, immunotherapy has been found to be cost-effective in comparison with pharmacotherapy alone in patients with the coexistence of allergic rhinoconjunctivitis and asthma.390,391
3.2.3Influenza and pneumococcal vaccinationInfluenza392,393 and pneumococcal394,395 vaccines have not been shown to be effective in preventing asthma exacerbations.
However, since it is a cost-effective approach, and due to the high risk of complications in patients with chronic diseases396,397 and a higher risk of therapeutic failure in children,398 annual influenza vaccination should be considered in patients with moderate and severe asthma, both in adults and children. Similarly, and given that asthma population have a high risk of invasive pneumococcal disease,399,400 different international401 and national402 consensus documents as well as the National Healthcare System403 recommend the administration of pneumococcal vaccine in patients with severe asthma.
3.3Education3.3.1ObjectivesEducation of asthma patients is an essential component of treatment, because reduces the risk of exacerbations, improves quality of life and decreases healthcare costs,262,404 thus becoming an indispensable part of the overall management of the disease.228,404–410 The main goal of education is to provide patients with the knowledge and skills they need to improve self-care and treatment compliance. This results in a better adherence to treatment and, in consequence, in an optimal control of the disease. In addition, education promotes patient's self-control of asthma. Self-control is the situation in which the patient monitors their symptoms and applies self-management following a plan agreed with his/her doctor. Self-control supported by a healthcare professional reduces the number of consultations and exacerbations, and improves quality of life without increasing costs.411,412
3.3.2Knowledge and skillsFrom a practical point of view,413 education should consider two major aspects: transmission of knowledge and acquisition of skills and competences (table 27). Regarding the information that the patient should receive about asthma, their needs, previous knowledge, beliefs,414 age, severity of asthma, and the degree of involvement necessary in his/her self-control and treatment should be considered.
In Information and basic skills that should be learned by a patient with asthma.
| 1. To know that asthma is a chronic disease requiring continuous treatment even if symptoms are absent. |
| 2. To know the differences between inflammation and bronchoconstriction. |
| 3. To be able to differentiate between inflammation “controller” drugs and obstruction “reliever” drugs. |
| 4. To recognize the symptoms of the disease. |
| 5. To use inhalers correctly. |
| 6. To identify triggers and avoid triggering factors as much as possible. |
| 7. To monitor symptoms and peak expiratory flow (PEF). |
| 8. To recognize the signs and symptoms of asthma worsening (loss of control). |
| 9. To act in case of asthma worsening in order to prevent an attack or exacerbation. |
These interventions should include:415 self-management of symptoms or PEF monitoring, written action plans, and regular assessments of asthma control, asthma treatment and abilities of the healthcare personnel.411
Interventions without written action plans are less effective415,416 Actions that are exclusively informative are ineffective.408,416 Regarding the skills to be developed, patients will be trained in taking the prescribed medication, particularly in the technique of their inhalation devices,321,322,324,325,417 in the recognition of exacerbations and how to act early, and in the avoidance of allergenic triggers.418,419
Minimal educational interventions reduced to the essentials (mini-action plan, avoidance behaviors and revision of inhalation technique) have shown efficacy if they are administered repeatedly at follow-up visits.420
3.3.3Action planThe education program should include establishing an action plan, which consists of a set of individualized written instructions in which asthma severity, disease control and the usually prescribed treatment are taken into account. The main objective of the action plan is the early detection of asthma worsening and the rapid adoption of measures to achieve quick remission. Depending on the patient's and the physician's preferences,421–423 the level of control on which the action plan should be based can be assessed in terms of severity and frequency of asthma symptoms, as well as through daily home recording of PEF. This plan should include two basic components:424–426 the usual treatment in situation of clinical stability of the disease and actions to be implemented in case of asthma worsening (table 28). This action plan will be reviewed at every clinical visit, either scheduled or unscheduled, as well as the time of hospital admissions or visits to the emergency department.
Action plans improve the patient's quality of life, but a systematic review did not find other beneficial or detrimental effects with the use of a written action plan.427
3.3.4Treatment adherencePatient's adherence to treatment is a critical factor for achieving and maintaining disease control. It is estimated that adherence in asthma patients is lower than 50%.428–430
Low adherence is associated with increased morbimortality as well as with a greater use of healthcare resources.431,432
Three types of patients with low adherence or non-adherence have been described: erratic (due to forgetfulness to take medication), deliberated (or intentionally non-adherence where the patient decides not to take medications) and involuntary or unwitting (due to failure in understanding the disease and/or its treatment).433,434
Treatment adherence should be evaluated at each medical visit using a reasonably validated method, such as the Test of Adherence to Inhalers (TAI) and pharmacy dispending medication or the combination of both.435–437
The education program should include the assessment of the level of adherence, promoting the appropriate corrective measures in case of low adherence and adapting them to the patient's pattern of non-adherence.
Participation of the patient in the choice of the inhaler provides greater therapeutic adherence and control of the disease. Therefore, patients should be involved in the selection of the inhaler device.330,332,344,345,438–441
Non-adherence to control medication in severe asthma can be detected by the FENO suppression test.442
3.3.5Other aspects to be consideredFor education to be effective, a confidence relationship between the healthcare team and the patients should be established, so that patients can raise their doubts, concerns and fears. The healthcare provider should use a simple and understandable language towards both the patients and their relatives, ensuring that all concepts have been understood and encourage the patients to put forward their doubts and queries. Also, common objectives with the patient should be established, always based on written and individualized plans.
An appropriate agreement between the patient's opinions and expectations and his/her physician is one of the factors related to asthma control.443
Patients and their families should be encouraged to raise doubts and queries regarding the information received or emerging as a result of the medical visits, allowing sufficient time to be solved on the next visit.228
Since education is a continuous process and not an isolated event, each visit should give the opportunity to review, strengthen and increase the patients’ knowledge and skills; hence, it is indispensable that education should be agreed on and accepted by the whole healthcare team.408
Table 29 describes the educational tasks that should be undertaken at each visit. Once properly trained, the nursing and pharmacy staff should actively participate in the organization and management of education programs.334,444–446
Educational tasks to be implemented at each visit.
| Communication | Information | Instruction | |
|---|---|---|---|
| Initial visit | Assess expectationsAgree on objectives Discuss adherence issues | Basic concepts on asthma and its treatment | Inhalation techniqueSelf-monitoring |
| Second visit | Evaluate achievements regarding expectations and objectivesDiscuss adherence issues | Reinforce information provided at the initial visit. Inform about environmental avoidance measures | Reinforce inhalation techniqueHow to avoid triggersInterpretation of recordsSelf-management plan |
| Revisions | Evaluate achievements regarding expectations and objectivesDiscuss adherence issues and environmental avoidance measures | Reinforce the whole information | Review and reinforce inhalation techniqueReview and reinforce self-monitoring and the self-management plan |
Individualized discharge programs assisted by trained nursing personnel prevent readmissions due to exacerbations.447
Educational interventions carried out in the Primary Care setting reduce unscheduled visits and the inappropriate use of drugs, such as antibiotics.448
In the interventions to potentiate self-care, patients’ sociocultural differences should be considered.414
Educational interventions cannot exclusively be developed in the clinical setting. Interventions of self-care in schools or by other patients with asthma provide a better control, a reduction of exacerbations and an improvement of quality of life. Also, they can positively influence on adolescents to quit smoking.449,450
The use of telemedicine improved adherence to treatment431 through inhaler monitoring devices451 or reminder alarms.452 It also improves symptoms and decreases the use of medical care.453 Teleconsultation improves asthma control and quality of life454 (see section 9.4).
The efficacy of the patient's self-control in asthma is very positive. For interventions on the patient's self-management to be effective, it is necessary to combine the active participation of the patient, with training and motivation of professionals integrated into a healthcare system that values the self-control in asthma patients.455
Educational workshops are a useful tool as a complement to individualized care, being more profitable when performed during the periods of time when patients present more symptoms.456
The community pharmacist, due to its accessibility and frequent use by the patient, can identify poorly controlled patients especially those who abuse SABA agents or have low adherence to anti-inflammatory maintenance treatment. The community pharmacist can offer health education improving adherence, asthma control and obtaining better clinical and economic outcomes. If necessary, he/she can refer the patient to medical consultation.340,457–460
4.2Recommendations| 3.1. SABA agents, when administered 10-15min before the exercise, are the drugs of choice to prevent exercise-induced bronchoconstriction. | R1 |
| 3.2. In step 1,budesonide/formoterol, beclomethasone/formoterol or beclomethasone/salbutamol on-demand can be used, although this strategy is not approved in technical specifications and the cost-effectiveness is unknown. | R2 |
| 3.3. First-choice treatment (step 2) is an ICS at low doses used on a daily basis. LTRA can be considered as an alternative treatment. | R1 |
| 3.4. In step 2, an alternative could be the use of ICS at low doses with LABA or SABA (e.g. budesonide/formoterol, beclomethasone/formoterol, or beclomethasone/salbutamol) on-demand in patients with low adherence to treatment in whom a specific education had previously failed. However, this strategy is not approved in the products technical specifications and the cost-effectiveness is unknown. | R2 |
| 3.5. For moderate persistent asthma, the first-line treatment is the combination of an ICS at low doses (step 3) or medium doses (step 4) with inhaled LABA. | R1 |
| 3.6. For moderate persistent asthma, ICS at low (step 3) or medium (step 4) doses associated with LTRA may be considered as an alternative option. | R1 |
| 3.7. The combination of budesonide/formoterol or beclomethasone/formoterol can be used as maintenance and on-demand treatment (reliever). | R1 |
| 3.8. In severe persistent asthma (step 5) first-line treatment is an ICS at high doses in combination with LABA. | R1 |
| 3.9. In patients with severe persistent asthma (step 5 or 6) uncontrolled with the combination of an ICS at high doses and LABA, with post-bronchodilation FEV1/FVC ≤ 70%, the addition of tiotropium or glycopyrronium has shown to improve pulmonary function and to reduce exacerbations. | R2 |
| 3.10. SABA, budesinode/formoterol or beclomethasone/formoterol combinations and, in selected cases, short-acting anticholinergics (ipratropium bromide), are the drugs that can be used as reliever medications (in all steps). | R1 |
| 3.11. Inhalation is the route of choice in the management of asthma. | R1 |
| 3.12. All healthcare professionals taking care of asthma patients should be involved in teaching the inhalation technique and control of inhaled therapy. | R1 |
| 3.13. The patient should participate in the selection of the inhaler device. | R1 |
| 3.14. It is recommendable the use of a single type of inhaler or at least similar inhalers. | R2 |
| 3.15. Patients should be trained on the inhalation technique of inhaler devices and their technique should be periodically supervised. | R1 |
| 3.16. Smoking cessation is recommended in smokers with asthma. | R1 |
| 3.17. In allergic asthma, specific combined measures of environmental control according to sensitization of the patient. | R2 |
| 3.18. In well-controlled allergic asthma with low or medium treatment levels (steps 1 to 4), allergen immunotherapy is recommended when clinically relevant IgE-mediated sensitization against common aeroallergens has been demonstrate, and well standardized extracts are used. | R1 |
| 3.19. Allergen immunotherapy should be prescribed by experienced specialized physicians. All administration of subcutaneous immunotherapy and the first use of sublingual immunotherapy should be carried out in centers with available basic resources for immediate treatment of a possible adverse reaction. | R2 |
| 3.20. When different alternatives of immunotherapy are available, the use of those based on registered medicines with well-established efficacy, safety and quality should be priorized. | R2 |
| 3.21. Patients with asthma should follow a formal education program of their disease. Informative actions alone have not been shown to be effective. | R1 |
| 3.22. Patients with asthma should be provided with a written action plan in order to detect early asthma worsening and to be able to implement actions for rapid remission. | R1 |
| 3.23. It is indispensable to determine the level of adherence to treatment in each individual patient. To this purpose, the use of validated methods such as the TAI questionnaire or electronic registry of pharmacy dispensing medicines is recommended. | R2 |
| 3.24. Self-control interventions to be effective should combine the active participation of the patient, the healthcare professional and the healthcare system. | R1 |
- •
Concept: an asthma exacerbation is defined by an episode of deterioration of the baseline clinical status of a patient that implies the need of administering specific treatment.
- •
Synonyms: in addition to crisis, it can receive other names such as agudization, exacerbation, or asthma attack.
- •
Identification: it can be clinically identified by an increase of symptoms, need of reliever medication, or worsening of pulmonary function in comparison of usual daily variation in a given patient.461
- •
Onset: depending on how fast exacerbation occurs, two types are identified: rapid-onset with progression in less than 3hours, and slow-onset (usually developing in days or weeks). The identification of the type of exacerbation is important because of differences in causative factors, pathogenesis and prognosis.462,463
Rapid-onset exacerbations develop by a mechanism of bronchoconstriction, are associated with a higher initial severity and vital risk than slow-onset exacerbations, although therapeutic response is usually more rapid and favorable. Triggering factors include inhaled allergens, drugs (NSAID or ß-blockers), food (due to food allergy, particularly milk and egg in childhood, and panallergens related to lipid transfer proteins in dried fruits, fruits and vegetables; or additives and preservatives), or emotional stress.
Slow-onset exacerbations account for more than 80% of asthma attacks attended in the emergency setting, and is mainly caused by an inflammatory mechanism, so that treatment response is slower. Slow-onset exacerbations are commonly caused by upper respiratory tract infections or a poor disease control.
- •
Severity: the intensity of exacerbations is variable with some attacks occasionally showing mild or symptoms that may be undetectable by the patient, while other episodes are very severe and life-threatening.
- •
Vital risk: a series of factors that increase the probability of suffering from life-threatening exacerbations have been reported. These factors are related to the characteristics of the current and past exacerbation episodes, adequate control of the chronic disease, and presence of a specific comorbidity (table 30).464–466
Table 30.Risk factors for life-threatening asthma exacerbation.
A. Related to the asthma exacerbation:1. Current exacerbation of rapid-onset.2. Previous episodes requiring medical consultation or hospital admissiona) Multiple visits to the emergency department in the previous year.b) Frequent hospitalizations in the previous year.c) Previous episodes of ICU admission, intubation or mechanical ventilation. B. Related to chronic asthma disease and its adequate control:1. Absence of periodic control.2. Abuse of a short-acting β2-adrenergic agonist. C. Cardiovascular comorbidity. D. Psychological, psychiatric and social conditions that difficult treatment adherence: alexithymia, denial attitudes, anxiety, depression, psychosis. ICU: intensive care unit.
Assessment of the severity of the exacerbation episode determines its treatment (fig. 8),467 and is carried out in two steps:
- •
Initial or static (pretreatment) evaluation: aimed at identifying signs and symptoms and objectively measuring the degree of airflow obstruction by determining FEV1 or peak expiratory flow (PEF) and their impact on gas exchange, in order to establish the level of severity of the exacerbation (table 31).
Table 31.Assessment of severity of asthma exacerbation.
Mild attack Moderate attack Severe attack Life-threatening attack Dyspnea Mild Moderate Intense Agonal breathing, respiratory arrest Speech Paragraphs Sentences Words Absent Respiratory rate (x’) Increased >20 >25 Bradypnea, apnea Heart rate (x’) <100 >100 >120 Bradycardia, cardiac arrest Blood pressure Normal Normal Normal Hypotension Use of accessory muscles Absent Present Very evident Paradoxical thoracoabdominal movement, or absent Wheezing Present Present Present Silence on auscultation Level of consciousness Normal Normal Normal Decreased or coma FEV1 or PEF (reference values) >70% <70% <50% Not applicable SaO2 >95% <95% <90% <90% PaO2 mm Hg Normal <80 (hypoxemia) <60 (partial respiratory failure) <60 PaCO2 Normal <40 <40 >45 (hypercapnic respiratory failure) FEV1: forced expiratory volume in one second; PEF: peak expiratory’ flow; x’: per minute; SaO2: oxyhemoglobin saturation; PaO2: arterial oxygen partial pressure; PaCO2: arterial partial pressure of carbon dioxide.
- •
Dynamic (post-treatment) evaluation: aimed to measure changes obtained in the degree of airflow obstruction as compared to initial values, and to assess the need of other diagnostic studies.
Therapeutic management of asthma exacerbation in adults.
FEV1: forced expiratory volume in one second; PEF: peak expiratory flow; SaO2: oxyhemoglobin saturation; pMDI: pressurized metered− dose inhaler; NEB: nebulized; i.v.: intravenous route; SGC: systemic glucocorticoids; ICS: inhaled glucocorticoid; NIMV: non-invasive mechanical ventilation; IMV; invasive mechanical ventilation; min: minute; Mg: magnesium; h: hour; μg: micrograms; 1st: first.
The objective of assessment is to determine factors described in table 31. The presence of signs of a life threatening asthma attack makes it necessary to consider the possibility of admission to the ICU.
Signs and symptoms that are not indicative of life-threatening asthma have a low clinical usefulness due to a poor correlation with the degree of obstruction and the large variability in their interpretation.468,469
The objective assessment of the degree of airflow obstruction by spirometry (FEV1) or using a peak expiratory flow (PEF) meter is crucial to ascertain the initial severity and evaluate treatment response. It is preferable to use the percentage value of the previous best value of the patient in the last two years, but if this datum is unknown, the percentage value in relation to the predicted value can be used. According to the values obtained, exacerbations are classified as mild, if FEV1 or PEF are equal to or greater than 70%; moderate, if FEV1 or PEF values range between 70 and 50%; and severe, if these values are lower than 50%. Life-threatening asthma attack is usually associated with values lower than 33%. The initial therapeutic response of airflow obstruction is the main prognostic factor in the assessment of the exacerbation episode.469–472
Measurement of oxygen saturation by pulse oximetry is easy to obtain in all patients and has a complementary role. Values lower than 90-92%, with or without supplemental oxygen therapy can be associated with hypercapnia and life-threatening crisis; therefore, in these cases, arterial blood gases analysis is indicated.473
Other complementary studies at the beginning of an asthma attack, such as chest X-rays or an electrocardiogram, are indicated in case of fever or suspicion of infection (pneumonia), pain or intense dyspnea that may suggest the presence of pneumothorax or pneumomediastinum, or when therapeutic response measured by objective parameters, is not appropriate and in case of a life-threatening asthma exacerbation.474–476
4.3TreatmentThe immediate objective when treating an asthma attack is to preserve the patient's life, reverting airflow obstruction and hypoxemia as soon as possible, and thereafter to set up or review the therapeutic plan to prevent further attacks. The pharmacological treatment the usually recommended doses are shown in table 32. Treatment according to severity is shown in fig. 8.
Drugs and doses commonly used for treating asthma exacerbations.
| Therapeutic groups | Drugs | Doses |
|---|---|---|
| First-choice | ||
| βz-adrenergic agonists | Salbutamol | pMDI+spacer: 200-800μg (2-8 puffs of 100μg/puff) every 10-15 min during the first hour |
| NEB intermittent: 2.5-5 mg every 20 min during the first hour | ||
| NEB continuous: 10-15 mg/hour | ||
| Anticholinergics | Ipratropium bromide | pMDI+spacer: 80-160μg (4-8 puffs of 20μg every 10-15 min |
| NEB intermittent: 0.5 mg every 20 min | ||
| Prednisone | Oral route on discharge: 50 mg every/24 hours (5-7 days) | |
| Oral route on admission: 20-40 mg every/12 hours | ||
| Hydrocortisone | i.v.: 100-200 mg every/6 hours | |
| Inhaled glucocorticoids | Fluticasone propionate | pMDI+spacer: 500μg (2 puffs of 250μg/puff) every 10-15 min |
| Budesonide | pMDI+spacer: 800μg (4 puffs of 200μg/puff) every 10-15 min | |
| NEB: 0.5 mg every 20 min during the first hour | ||
| Magnesium sulfate i.v. | i.v.: 2 g infused over 20 min (one time only) | |
| Alternative in case of previous failure | ||
| βz-adrenergic agonists i.v. | Salbutamol | i.v.: 200μg in 30 min followed by 0.1-0.2μg/kg/min |
| Magnesium sulfate inhaled | NEB: 145-384 mg in isotonic solution |
pMDl: pressurized inhaler; NEB: nebulized; i.v.; intravenous route.
In clinical practice, it is difficult to differentiate a mild exacerbation from a transient loss of asthma control, since changes observed will be close to the normal range of variation for a given patient.461
Milder attacks can be managed at home by the patient him/herself or in primary care centers, provided a correct clinical and respiratory function assessment has been carried out and treatment response can safely be achieved within the first 2hours.
Asthma patients who have been provided with written action plans, including home PEF monitoring and how to act in case of loss of control, have an excellent and readily usable tool for managing mild exacerbations.477 In order to quickly implement the adequate measures, patients should be trained in identifying the early indicators of exacerbations and be ready to act immediately according to their assigned action plan, which should include the actions to be implemented according to the response to treatment.
The treatment schedule to be followed does not depend on the setting where the patient is being cared for. The therapeutic regimen should include the administration of short-acting ß2-agonists (SABA), such as salbutamol or terbutaline, and inhaled (ICS) or oral glucocorticoids. The addition of ipratropium bromide is not necessary for mild attacks, and antibiotics should not be routinely prescribed.
Inhaled SABA are the most effective and rapidly acting bronchodilators for treating asthma exacerbations. Salbutamol at doses of 200 to 400μg (2 to 4 puffs) with spacer is used.478,479
Treatment with salbutamol at doses of 2 puffs every 3-4hours can be continued until remission of the exacerbation episode.
If a favorable outcome is observed within the first 2hours of treatment (symptom resolution, PEF over 80% predicted or personal best value) and if this clinical response is maintained for 3-4hours, no more treatments are necessary.
The lack of response requires referral of the patient to a hospital emergency department.
The use of systemic glucocorticoids accelerates resolution of exacerbations and prevents relapses.480 Except for very mild attacks, systemic glucocorticoids should always be administered as early as possible,481,482 particularly in the following cases:
- •
Pulmonary obstruction cannot be reversed with inhaled SABA.
- •
The patient is already taken oral glucocorticoids.
- •
The patient has treated him/herself a previous loss of asthma control with other therapeutic options without success.
- •
There is a history of previous exacerbations requiring oral glucocorticoids.
The daily dose of prednisone is 0.5-1mg/kg of the ideal body weight (or equivalent doses of other steroids), up to 50mg; this dose should be maintained for 5 to 7 days, and may be discontinued without down-titration in order to achieve a quick improvement and prevent early relapses.482,483
The administration of glucocorticoids by the oral, intramuscular or intravenous route provides similar biological results, but the oral route is less invasive and cheaper.482,484–486
If response to inhaled bronchodilator treatment within the first hours is satisfactory, no hospital referral is required. Patients should be instructed on the need for adequate adherence to the treatment prescribed, their maintenance treatment plan should be reviewed, and a minimal asthma education intervention should be provided.487,488
4.3.2Moderate and severe exacerbationsThe first measure consists of immediate oxygen administration, with a flow providing a saturation over 90% (95% in pregnant women or in patients with concomitant heart disease).489
In severe exacerbations with greater airflow obstruction and risk of hypercapnia, the use of oxygen with controlled FiO2 to obtain saturations around 93-95% is preferable than the use of high-flow oxygen therapy with which saturations around 100% can be achieved.489,490
In patients with severe exacerbations, the use of capnography to assess the trend to hypercapnia can be considered.491
Inhaled short-acting ß2-adrenergic agonists (SABA) are the first-line bronchodilator treatment. Both the dose and the dosing intervals should be individualized according to the choice of the administration system and the therapeutic response.
There is evidence that the use of a pressurized inhaler with spacer is the most cost-effective system;492 however, cost-effectiveness is lower in patients with very severe exacerbations.
It has been shown that the administration of SABA using a nebulizer or a pMDI inhaler with spacer has a similar clinical efficacy in terms of pulmonary function, length of stay in an emergency department, and risk of hospitalization. However, the dose when using a pMDI inhaler is lower.492–496
There is some debate as to whether nebulized treatment should be administered continuous or intermittently.497,498 A practical approach may include an initial continuous nebulization therapy to stabilize the patient followed by intermittent therapy.
There is no evidence to support the use of a route other than inhalation for the administration of bronchodilator medication.499 The intravenous route, with a very slow continuous infusion, should be used when there is no response to inhalation therapy in patients under mechanical ventilation and monitored in an ICU.
Similarly, no beneficial effects have been demonstrated when adding intravenous medication to the inhaled therapy.499
The use of parenteral epinephrine (subcutaneous or intravenous) is not indicated for treating exacerbations, except when these occur in a patient with anaphylaxis. In this case, the intramuscular administration is the route of choice because higher and more quickly plasma concentrations are obtained as compared with the subcutaneous route, as well as there is a greater safety margin.500–502
When administered in aerosol form, doses higher than 2mg, equivalent to 5mg salbutamol are required as lower doses are ineffective.503
The intravenous administration of epinephrine would only be indicated in case of cardiac arrest or in hypotensive patients who do not respond to intravenous volume replacement and multiple doses of intramuscular epinephrine.504,505
The use of ipratropium bromide during the initial phase of moderate or severe exacerbations concomitantly with a SABA is associated with a greater increase in pulmonary function (estimated by FEV1 or PEF) and a decrease in hospitalizations as compared to the use of a SABA alone.506,507
Systemic glucocorticoids accelerate the resolution of asthma attacks and prevent relapses.482,506,508 They should be prescribed early, within the first hour of treatment in the emergency room, since their effect starts 4-6hours after administration. They are especially indicated if no improvement is seen after the first dose of SABA, if the patient was already receiving them or if previous exacerbation episodes requiring these systemic glucocorticoids had occurred.
The preferred administration route of glucocorticoids is the oral route, as it is as effective as the intravenous administration,509 less invasive and cheaper.484,485 The intravenous route is limited to patients with severe dyspnea preventing swallowing and patients with vomiting or under mechanical ventilation.
The daily dose is 50mg of prednisone, as a single morning dose481 for 5-7 days, with no down-titration being necessary.510,511
Early use of high doses of ICS within the first hour of treatment reduces the need for hospital admission as in the case with systemic administration of glucocorticoids.508
The use of ICS together with systemic glucocorticoids provides even a higher reduction in the number of hospital admissions.508
Theophylline drugs should not be used in exacerbation episodes because of their lower efficacy and safety as compared with salbutamol.512
Routine administration of magnesium sulfate is not indicated, although in selected patients experiencing severe obstruction (FEV1 25-30% of predicted) or persistent hypoxemia, a single dose of 2g administered by infusion reduces the need for hospitalization.513–515
A systematic review of patients with severe exacerbations treated with intravenous magnesium sulfate showed a mild improvement of pulmonary function only.516
However, a more recent systematic review showed beneficial effects of inhaled magnesium sulfate added to SABA or SABA plus ipratropium bromide, reducing hospital admissions, in addition to a mild improvement of pulmonary function.517
Heliox, a mixture of helium and oxygen, in 80/20 70/30 proportion, has no place in the routine management of exacerbations due to the lack of consistent data regarding the efficacy of this compound. However, it may be considered in patients who do not respond to the usual treatment,518,519 particularly to nebulizing SABA.520
Regarding leukotriene antagonists, no data supporting their use either orally or intravenously are available. There is no evidence supporting the use of antibiotics, except in the presence of a clearly symptomatic respiratory infection.
4.3.3Treatment failureThe use of non-invasive mechanical ventilation may be an option in severe exacerbations resistant to treatment. It allows improvement of the respiratory rate, dyspnea, and, in particular, airflow obstruction due to a direct effect of positive pressure, or indirectly contributing to a better distribution of aerosols.521
Close monitoring is necessary so as not to delay the use of invasive mechanical ventilation in patients with an imminent life-threatening compromise.
4.4Hospitalization criteriaThe rate of hospital admission in asthma patients attended in the emergency setting is around 20%,522 although there is a large variability among different countries.523,524 It is well known that adherence to guidelines is associated with a lower risk of hospitalization.523 A systematic review522 identified the degree of pulmonary function impairment as the most important risk factor for in-patient care.
The decision to hospitalize a patient should be made within the first three hours after the start of treatment of the exacerbation episode, given that decision-making is rarely modified by longer periods of monitorization.525
However, assessment of the patient's clinical condition and pulmonary function within the first hour after admission to the emergency room already allows to predict the need for in-patient care.526,527
Criteria for admission to the hospital or to the ICU are summarized in table 33.
Criteria for hospital admission and ICU admission.
| Criteria for hospital admission | Criteria for ICU admission |
|---|---|
| Remain symptomatic after treatment | Respiratory arrest |
| O2 requirement to maitain SaO2 >92% | Decrease in the level of consciousness |
| – PEF or FEV1 <50-60% after treatment.529– PEF or FEV1=50-70% on arrival. A minimum observation period of 12 hours is advisable.– There is no functional parameter that defines when a patient should be discharged, although PEF <75% and variability higher than 25% are associated with a higher rate of re-admissions530 | Progressive functional deterioration despite treatment |
| Previous life-threatening exacerbation with history of intubation and ventilation, hospital admission or visit to the emergency department due to recent asthma | SaO2 <90% despite supplementary O2PaCO2 >45 mm Hg=alarming sign of muscle exhaustion |
| Failure of treatment with oral glucocorticoids in the outpatient setting | Hypercapnia, need of ventilatory support or pneumothorax |
| Impossibility to ensure necessary care measures at home | |
| Respiratory (pneumonia, pneumothorax, pneumomediastinum) or non-respiratory comorbidities |
ICU: intensive care unit; SaO2, arterial oxygen saturation; PEF, peak expiratory flow; FEV1, forced expiratory volume in one second; PaCO2, arterial partial pressure of carbon dioxide.
There are no functional parameters that allow a patient to be discharged with complete safety, so that the decision is usually the result of the doctor's clinical observation of the patient's condition and data of arterial oxygen saturation.531
Patients may be discharged from hospital if they are capable of following their prescribed treatment at home, are paucisymptomatic or there is a reduced need for reliever medication.530
However, it is highly recommended to have an objective pulmonary function test, such as spirometry or PEF. FEV1 or PEF values >70% and with minimal symptoms can be criteria for discharge.532 If FEV1 or PEF values are between 50% and 70%, possible risk factors should be considered (table 33).
Before discharge from the hospital, it is necessary to deliver a minimum education plan including checking of the inhalation technique and the provision of a written action plan (see chapter 3.4.3.). Also, an appointment with the patient's attending physician will be scheduled within the next five days.488
Figure 9 shows an algorithm for the patients’ hospital admission or discharge.
4.6Referral and control after dischargeThe care of patients who have suffered an asthma attack does not finish at the time of hospital discharge, and all patients should be assessed after the acute episode.
All patients should be evaluated by his/her family physician within five days after discharge,488 as well as those who had suffered from a severe exacerbation by the pneumologist or allergologist within one month.531Table 34 shows criteria for referral to the next healthcare level.
Criteria for specialized evaluation of patients within a month after an asthma exacerbation episode.531
| – Severe or life-threatening exacerbation.– Repeated exacerbations requiring care in the emergency department.473,476– Exacerbations that require in-patient care,476,533 uncontrolled severe asthma, particularly in corticosteroid-dependent asthma, allergic bronchopulmonary aspergillosis, vasculitis.– Pregnancy.534– Exacerbations triggered by NSAID, aeroallergens, food allergens or presenting with anaphylaxia.– Known associated comorbidities.– Clinical suspicion of vocal cord dysfunction, nasal polyposis, rhinosinusitis, gastroesophageal reflux, sleep apnea-hypopnea syndrome, asthma-COPD overlap syndrome |
NSAID: non-steroidal anti-inflammatory drug; COPD: chronic obstructive pulmonary disease.
| 4.1. The initial assessment of the patient with an exacerbation episode should include the analysis of the life-threatening risk, level of severity, and degree of airflow obstruction. | R2 |
| 4.2. Depending on the signs and degree of airflow obstruction, the patient with an asthma exacerbation episode should be classified into four levels of severity: mild, moderate, severe, and life-threatening. | R2 |
| 4.3. The degree of airflow obstruction will be objectively established by means of spirometry (FEV1) or peak expiratory flow (PEF)measurement. | R2 |
| 4.4. In patients with asthma exacerbation, it is recommended to consider the initial therapeutic response of airflow obstruction and signs of severity, in order to establish the approach that should be followed. | R2 |
| 4.5. Treatment with SABA is recommended in mild exacerbation episodes. | R1 |
| 4.6. In moderate or severe exacerbations, early administration of systemic glucocorticoids and oxygen at the lowest concentration ensuring SaO2 >90% is recommended. | R1 |
| 4.7. The decision of hospital admission should be made within the first three hours after starting treatment of the exacerbation episode, because the level of bronchodilation achieved cannot be increased significantly beyond this period. | R2 |
| 4.8. Patients with FEV1 or PEF >70% (predicted or best personal value) and with minimal symptoms can be discharged from the hospital. | R2 |
| 4.9. Before hospital discharge, a minimum education plan including assessment of the patient's inhalation technique should be delivered, and a written action plan should be provided. | R2 |
| 4.10. After an exacerbation, it is recommended that the patient should be evaluated by his/her family physician within five days and, if necessary, by a specialist within a month. | R2 |
The education of the child with asthma and his/her family increases the quality of life, reduces the risk of exacerbations and the cost of healthcare, the reasons for which education is one the fundamental pillars of treatment. Its objective is for the child to achieve a normal life for his/her age including physical exercise and sport activities.535
Education is essential to improve treatment adherence and to achieve control of the disease.536,537
Education should be developed in all healthcare settings in which children with asthma are attended.538
Education will be primary addressed to the family during early childhood and, from 8-9 years, should be especially addressed to the child, in order to promote personal autonomy and to achieve the maximum degree of self-care.539
Home education programs may be beneficial for children with poorly controlled asthma and are potentially profitable.540
For education to be effective, it is essential to identify the educational needs and the factors that affect the behavior of the patient and/or his/her family.541
Key aspects of education are shown in table 35.535
Key aspects of the education of a child with asthma.
| Topic area | Key points |
|---|---|
| Asthma | – Concept of asthma (chronic disease, variability)– Symptoms exacerbation/between exacerbations– Bronchoconstriction– Inflammation |
| Environmental measures | – Counselling against smoking– Triggering factors (allergens, viruses, exercise, etc.)– How to identify and avoidance measures |
| Treatment | – Bronchodilators (rescue treatment)– Anti-inflammatory drugs (maintenance treatment)– Side-effects– Exacerbations (how to recognize initial symptoms and early action)– Inmunotherapy |
| Inhalers | – Importance of inhaled medication– Inhalation technique– Maintenance of the system– Errors/forgetfulness |
| Self-control | – PEF. Best personal value– Symptoms registry– Personalized written action plan |
| Lifestyle | – School attendance– Practice of sports– Autonomy |
PEF: peak expiratory flow.
The education of children with asthma is more effective when accompanied by personalized written action plans (table 36),542,543 which address maintenance treatment (table 37)544 and management of asthma exacerbations (table 38).545 Every education plan should be reviewed periodically.
Components of a personalized action plan.
| Action plan for treating asthma exacerbation at home |
| • Recognize asthma symptoms and the onset of an exacerbation for using early short-acting bronchodilators and on-demand when symptoms appear.• Recognize warning signs and when to seek help from the doctor or go to the emergency department. |
| Plan of self-control/control by the family |
| • Rules for avoiding specific asthma triggers in children.• Daily use of preventive medication: doses, frequency and route of administration.• Changes of preventive medication according to the severity and frequency of symptoms (symptom diary) and/or measurement of peak expiratory flow (home recording of PEF).• When to go to his/her pediatrician because asthma is not controlled.• Prevention and treatment of exertional asthma. |
PEF: peak expiratory flow.
Written action plan to maintain asthma control.
| Your usual treatment (preventive):Every day I take: ____________________________________________________Before exercise I take: ________________________________________________ | ||
| WHEN TO INCREASE PREVENTIVE TREATMENT | ||
| Assess your level of asthma control: | ||
| In the last week you have had: | ||
| Asthma symptoms more than twice a day? | No | Yes |
| Activity or physical exercise limited by your asthma? | No | Yes |
| Night awakenings due to asthma? | No | Yes |
| Need of rescue medication more than twice a day? | No | Yes |
| If you measure (PEF), your PEF is lower than _______________________________ | No | Yes |
| If you have answered “Yes” to 3 or more questions, your asthma is not well controlled and to increase a step in your treatment may be necessary | ||
| HOW TO INCREASE TREATMENT | ||
| Increased treatment from _____________________________________to ______________________________________________________________and assess improvement every day. Maintain this treatment for ___________days. | ||
| In case of an exacerbation, treatment will be started based on the action plan for the management of exacerbations and will attend a medical consultation for a new assessment. | ||
Action plan for treating an asthma exacerbation at home.
| What is an ASTHMA EXACERBATION EPISODE and HOW TO ACT AT HOME? |
| An asthma exacerbation episode is a sudden or progressive worsening of symptoms:– Increased cough (continuous, nocturnal or with exercise).– Whistling sound.– Fatigue (difficult breathing).– Feeling of chest tightness.– Decrease of PEF (if you use the peak-flow meter). |
| There are symptoms that warn us that an exacerbation can be severe (warning signs):– Bluish color of the lips.– Ribs sink when breathing.– Difficulty speaking.– Numbness.Warning signs indicate that medical assistance should be immediately requested! |
| What to do at home in the presence of an exacerbation episode?– Keep calm.– Treat symptoms as early as possible.– Start medication at home.– Never wait to see if symptoms disappear spontaneously.– After starting medication, observe for 1 hour and assess response. |
| USE OF MEDICATION:Take your rapid rescue medication: salbutamol _____________ with spacer, 2-4 puffs, separated by 30-60 seconds. This dose can be repeated very 20 minutes, up to a maximum of 3 times.If symptoms do not improve in 1 hour, start taking oral glucocorticoids ___________________ (1 mg/kg/day, maximum 40 mg/day), during 3-5 days and go to the healthcare center or emergency department. |
| Take your anti-inflammatory medication___________________ times a day, all days, according to the indications given by your pediatrician. |
| ASSESS RESPONSE TO TREATMENTIf you improve in one hour and improvement is maintained for 4 hours, continue with salbutamol: 2-4 puffs every 4-6 hours (depending on symptoms) and visit your pediatrician in 24-48 hours.If you do not improve or the improvement is not maintained and you relapse again: go to an emergency departmenti |
| Si If you know how to control exacerbations, the duration of symptoms will be shorter and your quality of life will improve. |
In children, written action plans based on measurement of PEF do not provide benefits as compared with plans based on monitoring of symptoms, so that PEF-based plans are not generally recommended.542,546 However, on an individual basis, children and adolescents with severe asthma and low perception of symptoms could benefit from plans based on PEF monitoring.547,548
5.2Maintenance treatment5.2.1DrugsInhaled corticosteroids (ICS). ICS are the first-line of treatment. In children older than 3 years of age, the efficacy of daily ICS has been conclusively established, with improvement of clinical, parameters and bronchial inflammation parameters, better quality of life, and decrease in the risk of both exacerbations and hospitalizations.549,550
Infants and preschool children treated with daily ICS experience fewer recurrent asthma/wheezing episodes,551,542 with a better treatment response being obtained by those showing risk factors of developing persistent asthma (Asthma Predictive Index [API]),552,553 while viral-induced episodic wheezing shows limited response.554 A treatment trial followed by evaluation of response is recommended.555
Treatment with ICS, either continuously or intermittently, does not modify the natural history of the disease.556,557
In preschool and children, the use of controller drugs (ICS or montelukast) at regular doses or intermittently at the onset of symptoms is not recommended.558–560
Early intermittent therapy with ICS at high doses given to infants and preschool children with moderate-severe episodic wheezing and risk factors (API+) at the onset of symptoms have shown to be effective in reducing the severity and duration of exacerbations,550,561,562 but further safety studies are needed to establish a generalized recommendation of this therapy. It could be an option in highly selected cases in which education and acceptance by families are guaranteed.563,564
When administered at usual doses, ICS are safe drugs for the management of childhood asthma. There is usually a decrease in the growth rate at the beginning of treatment (1-3 years), although this is a transient effect and does not influence final growth or final height. However, the final height of children treated with ICS over prolonged periods is lower, an effect proved to be dose-dependent.565,566
It is difficult to establish the equivalent doses of ICSs mostly used in pediatric age.567 Comparable doses of ICS drugs for use in the pediatric age group are tentatively shown in table 39., taking into account that the lowest dose that maintains patient's control must be sought.
Leukotriene receptor antagonists (LTRA). In preschool children with virus-induced asthma/weezing episodes, LTRA are associated with a modest reduction of symptoms and the need of oral glucocorticoids as compared with placebo.559,568,569 Although a definite beneficial effect remains unclear, a clinical trial to assess response to LTRA may be conducted, which could be stopped if the expected response is not obtained.568 More evidence is needed to determine whether there is a responder phenotype to montelukast.570
If asthma symptom cannot be controlled with ICS at low doses, increasing ICS at medium doses is more effective than the association with montelukast.571
Association of long-acting ß2-adrenergic agonists and ICS. It has been approved for use in children over 4 years of age. The use of a LABA when administered with an ICSs is safe, but should never be used as monotherapy.572,573
One study showed a decrease in exacerbations and the need for systemic glucocorticoids in children aged 4-11 years with formoterol/budesonide administered in a single inhaler, both as maintenance treatment and as reliever (MART strategy),574 although some authors consider that the evidence in this age group is limited.575
En In children aged between 6 and 11 years with uncontrolled persistent asthma with low doses of ICS, doubling the ICS dose has a similar effect on clinical control and lung function than adding a LABA.576 However, the clinical phenotype and the heterogeneity of the individual response to ICS, LTRA and LABA should be assessed,539,577 therefore, it is necessary to closely monitor the response to treatment in children with uncontrolled asthma using ICS.
Tiotropium. It is a long-acting muscarinic antagonist. It can be used in children from 6 years of age with poorly controlled severe asthma treated with ICS at high doses plus a LABA. The dose is 5μg once a day.578 A study in children aged 1 to 5 years concluded that tolerability of tiotropium is good in preschool children and that this agent can reduce the number of exacerbations.579
Biologics. Biologics are drugs indicated in severe uncontrolled asthma that are aimed at treating the underlying inflammation by blocking different mediators. In the pediatric field, there are currently three biological agents available, all of them being monoclonal antibodies that are administered subcutaneously. Omalizumab targets IgE, mepolizumab blocks IL-5, and dupilumab targets IL-4 and IL-13. They have shown efficacy and safety and are recommended for children aged 6 and above.580,581 Their characteristics, indications, doses, and modes of administration are indicated in section 7.5 (Severe uncontrolled asthma in children).
Immunotherapy (IT). When biologically standardized extracts are used and appropriately selected in sensitized patients, immunotherapy has been shown to provide a beneficial effect by reducing symptoms, the need of reliever and maintenance medication, and decreasing bronchial hyperresponsiveness (both specific and non-specific).582
Also, IT prevents the development of new sensitizations and asthma in children with rhinitis.583,584
5.2.2Treatment according to the level of severity, control and future riskConsidering that the main objective is to achieve control with the least possible medication, the treatment should be continuously adjusted, escalating or de-escalating the therapeutic step based on the level of control, always considering non-pharmacological measures, therapeutic adherence, and modifiable risk factors (Figure 10).
The maintenance treatment will be initiated based on the initial severity level (recurrence or intensity of symptoms). Subsequently and retrospectively, the severity will be classified according to the level of treatment necessary to maintain symptom control.
- •
Step 1. Children who have occasional asthma symptoms, without nighttime symptoms, and without risk factors for exacerbation, should only use bronchodilators on-demand. In the case of infrequent asthma symptoms but with risk factor(s) for exacerbation (table 19), the corresponding treatment for step 2 should be initiated. It is important to perform a careful evaluation, ensuring that the symptoms are truly intermittent and not persistent. From the age of 12, the use of formoterol associated with ICS could be considered.
- •
Step 2. Children who require treatment with SABA two or more times per month, without symptoms between episodes and with normal lung function, should initiate treatment with low doses of ICS or consider montelukast as an alternative.
- •
Step 3-4. Children with more than 6-8 asthma episodes per year, symptoms between episodes, asthma-related awakenings once a week, and/or impaired pulmonary function should adjust their treatment to step 3 or 4. In this step, there are three options: ICS at medium doses, adding a LABA to ICS at low dose (step 3), or association of a LABA with ICS at medium doses (step 4), and in children under 4 years of age, montelukast associated with low doses of ICS.
- •
Step 5-6. In children with persistent symptoms, the need for short courses of oral corticosteroids, wheezing with minimal exertion, and impaired pulmonary function, treatment should be initiated at step 5 (ICS at high doses/LABA). Once control is achieved, the treatment should be stepped down to the lowest effective dose.585
If control is not achieved in children less than 4 years of age, it is necessary to consider adding one or more of the following drugs: LABA (off-label use), tiotropium (off-label use in children under 6 years), macrolides, or even oral glucocorticoids. In children of more than 6 years of age, options include tiotropium, monoclonal antibodies, or oral glucocorticoids (see chapter 3, “Severe Asthma”).
In children older than 12 years, it is possible to apply the so-called MART therapy, that is, ICS/formoterol, both as maintenance and rescue treatment.
5.3Evaluation and treatment of exacerbations5.3.1Evaluation of severityThe following factors should be considered: time course of the exacerbation episode, pharmacological treatment administered, presence of associated diseases, and possible risk factors (previous intubation or ICU admission, hospitalizations in the previous year, frequent need of admission to the emergency department in the previous year and/or use of oral glucocorticoids, and excessive use of SABA in the preceding weeks).
Severity assessment is mainly based on clinical criteria (respiratory rate, presence of wheezing and sternocleidomastoid retractions). Although no clinical scale is considered to be well validated586,587 the Pulmonary Score (table 40)588 has been found to be useful and applicable to all ages. The combination of symptoms and arterial oxygen saturation (SaO2) allows completing an estimation of the severity of the exacerbation episode (table 41).
Pulmonary Score for clinical assessment of asthma exacerbation in children.*
| Score | Respiratory rate | Wheezing | Use of sternocleidomastoid muscle | |
|---|---|---|---|---|
| <6 years | ≥ 6 years | |||
| 0 | <30 | <20 | No | No |
| 1 | 31-45 | 21-35 | End of expiration | Slight increase |
| 2 | 46-60 | 36-50 | Throughout expiration (stethoscope) | Increased |
| 3 | >60 | >50 | Inspiration and expiration without stethoscope** | Maximum activity |
Overall evaluation of the severity of asthma exacerbation in children by integrating the Pulmonary Score and the arterial oxygen saturation.
| Pulmonary Score | SaO2 | |
|---|---|---|
| Mild | 0-3 | >94% |
| Moderate | 4-6 | 91-94% |
| Severe | 7-9 | <91% |
SaO2: arterial oxygen saturation. In case of disagreement between clinical score and arterial oxygen saturation, the score indicating higher degree of severity will be used.
Inhaled short-acting ß2-adrenergic agonists (SABA). These agents constitute the first-line treatment due to their higher effectiveness and lower incidence of side effects.589 They should preferably be administered via a pressurized inhaler with a spacer chamber, since this way of administration is as effective as nebulizers for treating an acute asthma episode.590–593
The recommended doses and dosing intervals depend on the severity of the exacerbation episode and the response to the initial doses.594 The most commonly used drug is salbutamol, which is available as a solution for use with a nebulizer and a pressurized inhaler. The latter must be administered in sequences of 2-10 puffs of 100μg until response is obtained. In mild exacerbations, a series of 2-4 puffs may be sufficient, although up to 10 puffs may be necessary for severe exacerbations.
Nebulized SABA should be restricted to those cases in which the patient requires oxygen supply for SaO2 normalization, although a recent randomized clinical trial (RCT) showed that even in severe exacerbations, the administration of salbutamol and ipratropium bromide with spacer chamber and facial mask with oxygen by means of a nasal cannula was more effective than using a nebulizer.595
Continuous nebulization does not offer greater advantages compared to intermittent nebulization when the same total doses are administered.596,597
Ipratropium bromide. The use of frequent doses, every 20minutes, of ipratropium bromide for the first 2hours in case of severe asthma exacerbations or moderate exacerbations not responding to initial treatment with SABA, has been shown to be effective and safe.588,598 The nebulized dose is 250μg for children weighing less than 30kg y 500μg for those weighing more than 30kg. The dose for inhaled use with a spacer chamber is 40-80μg (2-4 puffs). The maximum effect, which tends to decrease gradually, is observed with the first doses, so that inhalations beyond the first 24-48hours should not be maintained.599
In infants, the use of ipratropium combined with inhaled SABA has been shown to be effective in the treatment of the most severe exacerbations.600 The effect of this association administered by an inhaler seems to be higher as compared to that administered by nebulization.595
Systemic glucocorticoids. The efficacy of systemic glucocorticoids in preschool children with mild to moderate acute episodes of wheezing induced by viral infections has been questioned; hence, its use should be restricted to more severe exacerbations (1-2mg/kg/day).569,601,602 In children over 5 years of age, these agents have shown benefit after early use,603 with the oral route being preferred over the intravenous or intramuscular routes, except for circumstances in which oral intake is not feasible.604,605 Systemic glucocorticoids should be administered in moderate-severe exacerbations, and may be considered for mild exacerbations when sufficient improvement with bronchodilators has not been achieved or the child has a history of severe attacks (in this case, early administration). Prednisolone at doses of 1-2mg/kg/day (maximum 40mg) for 3 to 5 days or until resolution of the asthma attack is the most commonly used drug.606,607
Dexamethasone is being used as an alternative. The effect of administering a single dose of dexamethasone orally (at 0.3-0.6mg/kg) is not inferior to that of administering prednisolone orally (at 1mg/kg/day) during 3 days of treatment.608–611
Inhaled glucocorticoids. There is insufficient evidence to recommend the use of ICS as an alternative612 or additional treatment to systemic glucocorticoids613,614 in the management of asthma exacerbations. Larger studies are needed, with better methodological quality and cost-effectiveness analysis,615 as well as safety studies.612
Magnesium sulfate. It can be used in severe exacerbations with unsuccessful response to the initial treatment,616,617 but its use does not prevent hospitalizations.618 The drug is administered intravenously as a single dose of 40mg/kg (up to 2g) over 20minutes.
Nebulized magnesium sulfate together with a ß2-adrenergic agonist in the treatment of an asthma exacerbation seems to have benefits in the improvement of pulmonary function.619,620
5.3.3Therapeutic regimensTreatment of an asthma exacerbation episode depends on its severity and follows the flow chart shown in Figure 11. Doses of drugs and duration of administration should be modified according to the severity of the exacerbation and the response to treatment.
When SaO2 is below 94%, oxygen therapy is required to maintain SaO2 between 94-98%.621,622 An SaO2 <92% after initial treatment with inhaled bronchodilators can be used as a marker to select the most severely ill patients who should be hospitalized for starting intensive treatment.621,622
During the first 2 hour of treatment and in children with moderate/severe exacerbation unresponsive to first-line therapy, the use of a high-flow nasal cannula seems to be superior to conventional oxygen therapy to reduce breathing difficulty.623,624 However, more studies are needed to demonstrate the general efficacy of this approach in the management of asthma and respiratory insufficiency in the emergency setting.625
Regarding non-invasive ventilation (NIV), the current available evidence does not allow to confirm or exclude its use in exacerbation episodes refractory to the usual treatment.626
Mild and moderate exacerbations can be treated in the primary healthcare setting.
In the presence of severe exacerbation or suspicion of complications, history of high-risk exacerbations or lack of response to treatment, patients should be referred to the hospital in a medicalized ambulance.
Follow-up. It is necessary to evaluate the degree of the control of symptoms in the previous weeks, the presence of risk factors, possible triggering factors, and previous treatment. Also, it is important to assess the level of therapeutic adherence and to supervise that the inhalation technique is correct. A written action plan must be reviewed or provided and a follow-up visit arranged.544
4.4Recommendations| 5.1. The education of the child with asthma and his/her family is recommended because increases the quality of life and reduces the risk of exacerbations and healthcare costs. | R1 |
| 5.2. In the education of a child with asthma, it is recommended to include written personalized management action plans, addressing maintenance treatment and how to treat exacerbations. | R1 |
| 5.3. Inhaled corticosteroids (ICS) is recommended as first-line treatment for the control of persistent asthma in children of any age. | R1 |
| 5.4.Montelukast can be tried as an alternative to ICS for maintenance therapy. | R2 |
| 5.5. Treatment with LABA can be considered in children older than 4 years of age but always combined with ICS. LABA monotherapy should never be administered. | R1 |
| 5.6. In the treatment of children with allergic asthma, immunotherapy should be considered provided that biologically strandardized extracts are used and patients are appropriately selected. | R1 |
| 5.7. In children aged 6 years or older with insufficiently controlled severe persistent asthma with high doses of ICS and LABA and/or LTRA and/or tiotropium, the use of biologics or monoclonal antibodies is recommended. | R1 |
| 5.8. Before considering that an asthma patient is poorly controlled and stepping up treatment, the diagnosis of asthma should be confirmed, treatment adherence and inhalation technique should be evaluated, and other comorbidities excluded. | R1 |
| 5.9. Early and repeated administration of SABA at high doses is the first-line of treatment of asthma exacerbations in children. | R1 |
| 5.10. It is recommended to indidualize drug doses according to severity of the exacerbation and the response to treatment. | R2 |
| 5.11. Early use of systemic glucocorticoids is recommended in moderate and severe exacerbations; in mild exacerbation, an individualized assessment of its use is recommended. | R1 |
| 5.12. In the presence of SaO2 <92% after an initial treatment with inhaled bronchodilators, admission to the hospital to start intensive therapy is recommended. | R2 |
| 5.13. A pMDI with spacer should preferably be used to administer bronchodilators, especially in mild-to-moderate attacks. | R1 |
| 5.14. The degree of control, risk factors, therapeutic adherence, and inhalation technique should be assessed, a written action plan should be provided, and the follow-up of children with exacerbations should be ensured. | R2 |
The term rhinitis defines the inflammatory process of the nasal mucosa, which is characterized by the following clinical symptoms: anterior or posterior rhinorrhea, sneezing, block of nasal passages or congestion and/or nasal pruritus/itching. These symptoms should be present for two or more consecutive days and for more than one hour on most of the days.627–629
Rhinitis is a syndrome that encompasses several phenotypes. Rhinitis has the highest prevalence of all diseases, and it has been estimated that 100% of the population (children and adults) suffer from 1 to 10 episodes of infectious rhinitis annually630 (table 42). Allergic rhinitis (AR) is the most prevalent of all chronic diseases, affecting 22-41% of the European population631 and 12.6% of children aged 0-18 years.632 The prevalence of non-allergic rhinitis (NAR) is not so well estimated, with the highest rates in children under 6 years (up to 24.9%) and around 10% in children older than 15 years of age.633
Phenotypes of rhinitis.
| Infectious | Non-infectious | ||
|---|---|---|---|
| Viral | Bacterial | Allergic/local allergic | Non-allergic |
| • Intermittent/persistent• Seasonal/perennial• Occupational• Mild/moderate/severe | • Occupational rhinitis• Drug-induced rhinitis• Gustatory rhinitis• Hormonal rhinitis• Reactive rhinopathy (nasal hyperreactivity/old vasomotor rhinitis)• Dry/atrophic/sicca rhinitis• Idiopathic rhinitis | ||
In Spain, rhinitis is the most common reason for consultation in Allergology (62% in adults and 53.8% in children).634,635 The ISAAC study reported a prevalence of rhinoconjunctivitis of 7.9% in Spanish children aged 6-7 years (with an annual increase of 0.33) and 15% among those aged 13-14 years (annual increase of 0.10).636
AR-associated costs are high. A study carried out in Spain (FERIN project) reported that the cost per patient per year was €2,326.70 (direct costs €553.80; indirect costs €1,772.90).637
6.2Diagnosis and classificationThe diagnosis of AR is mainly based on clinical manifestations, although symptoms do not enable to assess the cause, pathophysiology or the specific rhinitis phenotype; therefore, complementary diagnostic tests are necessary to establish the etiological diagnosis in cases of rhinitis of moderate to severe intensity.627
An initial approach to the classification (phenotyping) of rhinitis should establish whether the patient presents an infectious or non-infectious rhinitis, and subsequently classify rhinitis based on positivity of allergy tests and the correlation with the patient's symptoms. Two main rhinitis phenotypes are defined: AR and NAR. NAR includes a heterogeneous group of phenotypes of different pathogenetic mechanisms638 (table 42).
Family history of allergy, seasonal manifestation of symptoms, concomitant ocular and nasal symptoms and its relationship with exposure to aeroallergens are clinical data with a high predictive value for suspicion of AR639 (fig. 12).
The most efficient complementary tests for the diagnosis of rhinitis are allergic tests: skin prick testing or intradermal puncture with standardized allergic extracts and determination of specific serum IgE against allergens, preferably against recombinant allergens.638 A high percentage of patients with positive allergic tests do not have the disease or positive allergens are not clinically relevant, so that clinical correlation is indispensable to establish the diagnosis.640
The specific nasal challenge (or provocation) test with allergens is the reference test for the diagnosis AR and can be necessary in the case of a high clinical suspicion and negative results of intradermal testing or specific serum IgE.641,642
A specific AR phenotype, named local AR, has been described, which is characterized by negativity of systemic allergic tests (intradermal tests or specific serum IgE) and positive specific nasal challenge test.643
Other complementary tests that can be useful in the study of nasal function include an objective assessment of obstruction (acoustic rhinometry, active anterior rhinomanometry, measurement of peak nasal inspiratory flow),644 assessment of nasal inflammation (nasal nitric oxide [nNO], nasal cytology, biopsy),645 and assessment of olfactory function by olfactometry.646
AR is an IgE-mediated chronic inflammatory immunological disorder of the nasal mucosa that causes a myriad of symptoms, including nasal obstruction/congestion, nasal and ocular itching, sneezing bouts, and rhinorrhea after inhalation of environmental allergens.647
AR can be classified according to different criteria. On the basis of triggering allergens, AR can be classified into seasonal (outdoors such as pollens and fungal spores mainly) or perennial (indoors such as dust mites, insects, animal dangers or other fungal spores), and on the basis of temporal criterium as intermittent or persistent (symptoms present for more than 4 days a week and for more than 4 consecutive weeks). This last classification has been validated and has been shown to better reflect the actual clinical condition of patients.648
The severity of AR is evaluated on the basis of the impact on the quality of life (sleep disturbance, impairment of daily life activities, leisure and/or sport activities, impairment of school or job tasks, and the consideration of symptoms as bothersome), differentiating into mild (none affected) moderate (one to three) or severe (all affected). This classification has been validated in children and adults, with and without treatment649,650 (table 43). A visual analogue scale can also be used to assess severity of AR.652
Classification of allergic rhinits.
| 1. According to duration | |
|---|---|
| Intermittent | Persistent |
| Symptoms are present for ≤ 4 days a week or for ≤ 4 consecutive weeks | Symptoms are present for >4 days a week for >4 consecutive weeks. |
| 2. According to severity | ||
|---|---|---|
| Mild | Moderate | Severe |
| None of the following items is present:– Sleep disturbance– Impairment of daily, leisure and/or sports activities– Impairment of school and job tasks– Symptoms are bothersome | • - One,• - Two,• - or three of the aforementioned items are present | The four items are present |
In the last few years, and in a similar way to that established in asthma, it has been proposed to evaluate the control of rhinitis using validated questionnaires (such as the Rhinitis Control Assessment Test)653 or using a visual analogue scale (available as applications for mobile devices).654
6.3Rhinitis and asthmaMultiple epidemiological, physiopathological, and therapeutic studies have shown an association between rhinitis and asthma.627
Factors determining why some patients with AR will develop asthma are unclear (table 44), although it is known that both AR and NAR are risk factors for asthma.655,656
Interrelationship between rhinitis and asthma: risk factors for asthma.
| – Allergic rhinitis.– Non-allergic rhinitis.– Characteristics of aeroallergens.– Number of sensitizations.– Intensity of sensitization.– Severity and duration of rhinitis.– Number of associated allergic diseases (rhinitis, conjunctivitis, dermatitis). |
Sensitization to different types of aeroallergens and specific profiles are associated with different allergic clinical features (rhinitis with/without conjunctivitis with/without asthma) and different levels of severity.649,657
According to some studies, the association with asthma would be greater in cases of more severe and prolonged AR,658,659 higher number of sensitizations,658,660,661 higher specific IgE levels662 and in the presence of various associated allergic diseases (rhinitis, conjunctivitis, dermatitis).663,664
The prevalence of rhinitis in patients with asthma is very high and much higher than in the general population.665 In Spain, two studies showed a prevalence of rhinitis in patients diagnosed with asthma of 71% and 89.5%, respectively.666 Also, it has been shown a parallel increase in the prevalence of asthma and rhinitis.667
Suffering from rhinitis aggravates asthma,668 worsens asthma control669 and asthma symptoms,670 and increases the use of healthcare resources.671,672
Inflammatory changes in the bronchial mucosa of non-asthmatic patients with AR have been observed,673 as well as nasal eosinophilic inflammation in asthma patients without nasal symptoms.674
Treatment of AR with intranasal glucocorticoids may improve some aspects of asthma, such as pulmonary function,675 symptom score, quality of life or the use of reliever or rescue medication,676 the level of asthma control,677 and exacerbations in the pediatric population.678,679
6.4Treatment of allergic rhinitisThe treatment strategy of allergic rhinitis includes patient education, avoidance of allergens and contaminants, pharmacotherapy and allergen-specific immunotherapy. At the time of selecting the pharmacological treatment, efficacy, safety, cost-effectiveness relationship, patients’ preferences, severity of disease and the presence of comorbidities should be evaluated. Pharmacological treatment of allergic rhinitis should include clear-cut recommendations that will have to be implemented in a stepwise approach according to severity (fig. 13).
Algorithm of treatment of allergic rhinitis.627,675,676
LTRA: leukotriene receptor antagonists; GC: glucocorticoids. *In short time periods, usually less than 5 days.
Second generation H1-antihistamines (non-sedating) (bilastine, cetirizine, desloratadine, ebastine, fexofenadine, levocetirizine, loratadine, mizolastine and rupatadine) administered by the oral route, improve symptoms both in adults and children, such as rhinorrhea, szeening, nasal itching and ocular symptoms, although are less effective to relieve nasal obstruction, and should be preferred over sedating antihistamines for their favorable risk-benefit ratio.638
Topical H1-antihistamines (azelastine, emedastine, epinastine, levocabastine and olopatadine) have a rapid effect on symptoms, are more effective for nasal congestion than oral antihistamines and more effective for ocular symptoms, although are less effective for nasal congestion than intranasal glucocorticoids (INGC), and have been shown to reduce symptoms and improve quality of life versus placebo, without relevant side effects except for a bitter taste.638
INGC (budesonide, ciclesonide, fluticasone, mometasone and triamcinolone) are very effective drugs for reducing nasal and ocular symptoms, even when administered intermittently, and are superior to oral antihistamines and montelukast. Their use may be associated with some minor adverse effects, such as epistaxis or headache, but a relevant effect neither on the hypothalamic-pituitary axis nor on the growth of children has been demonstrated.638
The combination of a glucocorticoid and an intranasal antihistamine (fluticanose propionate and azelastine or mometasone furoate and olopatadine) in a single device has shown a rapid and more effective effect than the use of INGC or intranasal antihistamines in monotherapy, with the only relevant adverse effect of its bitter taste. It is recommended in more severe or uncontrolled cases or as a second-line treatment after failure of monotherapy.638,680–682
Montelukast has consistently shown to reduce symptoms and to improve quality of life as compared with placebo, although to a lower extent than INGC and similarly to oral antihistamines, with good safety data. It is neither recommended as monotherapy nor as first-line treatment.638
Decongestants, both oral and intranasal, have shown to be effective to reduce nasal congestion in the short-time, but adverse effects outweigh the benefits especially in the presence of other comorbidities, so that its generalized use is not recommended. Intranasal decongestants used for more than 5 days may cause rhinitis medicamentosa.638
Oral or parenteral glucocorticoids can improve the symptoms of RA, but should not be prescribed routinely because of their adverse effects on the hypothalamic-pituitary axis, growth and the musculoskeletal system, digestive system, control of glycemia, blood pressure and emotional status.638
Intranasal chromones (cromoglycate and nedocromil) have shown efficacy for reducing sneezing, rhinorrhea, and nasal congestion with fewer adverse effects, although these drugs are less effective than INGC.638
Intranasal anticholinergics (ipratropium bromide) reduce rhinorrhea, although are associated with some adverse effects, such as nasopharyngeal irritation, headache, and oral and nasal mucosa dryness. It is recommended to be added to INGC to improve excessive rhinorrhea.638
The anti-IgE monoclonal antibody, omalizumab, has show to reduce symptoms and the use of rescue medication as well as to improve quality of life as compared with placebo, with a low risk of local reactions at the site of injection or anaphylaxis. Its use could be considered as an add-on treatment in severe uncontrolled cases or to reduce the risk of anaphylaxis in patients treated with allergenic vaccines, although at the present time AR is not included as an indication in the technical specifications of the product.638
Immunotherapy with allergens is effective and cost-effective for the treatment of adult and pediatric AR caused by pollens and dust mites when administered both subcutaneously and orally (sublingual route). It may alter the natural course of the respiratory allergic disease, decreasing the frequency of appearance of asthma and preventing new sensitizations, and is effective for treating symptoms of both asthma and rhinitis.638
The combination of several avoidance measures of indoor allergens added to baseline pharmacological treatment is also and effective option.638
The principles of treatment of rhinitis in childhood are the same than in adulthood, but special attention should be paid to adverse events. Doses should be adequate and, in some cases, the age of the patient should be considered when prescribing certain drugs.683–685
6.5Rhinosinusitis. Nasal polyposisChronic rhinosinusitis (CRS) is defined as an inflammatory disorder of the nose and paranasal sinuses, characterized by the presence of at least two symptoms, one of which should be nasal obstruction and/or rhinorrhea, and/or facial pain/pressure, and/or hyposmia/anosmia for at least 12 weeks.686 There are two phenotypes of CRS, with nasal polyps (CRSwNP) and without nasal polyps (CRSsNP), with differences in the inflammatory profile and therapeutic response to treatment.686,687
In Europe, the prevalence of CRS is 10.7%.688
Patients with CRS have a 3.5-fold higher risk for asthma.631Acetylsalicylic acid-exacerbated respiratory disease (AERD) or NSAID- exacerbated respiratory disease associated with asthma, CRSwNP, and NSAID intolerance is more severe and has a poorer prognosis.689 In patients with asthma, the prevalence of AERD is 7-15%, which increases with a greater severity of asthma.690
The severity of CRS can be evaluated using a visual analogue scale, nasal endoscopy to assess the size of polyps, and/or using validated questionnaires, such as SNOT-22, to assess the impact on the quality of life.686,691
Imaging studies do not add value to endoscopic diagnosis692 and should be reserved for surgical planning (computerized tomography), suspicion of complications or nasal or sinus tumors (magnetic resonance).693
Medical treatment of chronic rhinosinusitis with nasal polyps (CRSwNP) is based on the continuous and prolonged use of INGC (beclomethasone, mometasone, fluticasone, budesonide) (fig. 14).694 A greater efficacy of one active principle compared to another has not been demonstrated, although high doses are more effective than low doses.695–697
Treatment algorithm of nasosinusal polyposis based on the POLINA consensus.694
NSAID: non-steroidal anti-inflammatory drugs. AERD: aspirin-exacerbated respiratory disease/NSAID. COPD: chronic obstructive pulmonary disease. VAS: visual analogue scale. CRSwNP: chronic rhinosinusitis with nasal polyps. SNOT-22: Sino-Nasal Outcome Test 22.
aIn spray, drops or irrigations. bIrrigations with isotonic saline or Ringer lactate. cSee POLINA criteria for the control of CRSwNP (Figure 13.6). dShort courses from 5 días af doses 0.5-1mg/kg/day. eOpening of affected paranasal sinuses. fPossible selection according to endotype. gEvaluate more radical/extensive surgery according to doctor-patient consensus.
Short courses of oral glucocorticoids (prednisone, methylprednisolone or deflazacort, administered for 2 to 4 weeks) associated with intranasal glucocorticoids significantly improve nasal congestion and reduce the size of polyps.698
Endoscopic nasal and sinus surgery should be indicated in patients in which medical treatment has been unsuccessful to achieve an adequate control of the disease.699,700
INGC should be used after surgery for the prevention of relapses and to improve outcome.701 The need of revision surgery depends on the previous surgical procedures and postoperative medical treatment, the probability being greater in AERD.672,702
An adequate medical/surgical control of CRS improves clinical and functional parameters of asthma.703,704
Other treatment options associated with the use of INGC that have shown some efficacy are: montelukast (particularly in allergic patients or AERD)705 and clarithromycin.706
Up to 40% of patients have poor control of the disease,707 evidencing the need to identify specific phenotypes that allow predicting therapeutic success.684 Recent studies with different monoclonal antibodies, such as omalizumab (anti-IgE),708,709mepolizumab,710 reslizumab (anti-IL5),711 and dupilumab (anti-IL4-receptor a)712–714 have shown an improvement in the size of nasal polyps, nasal symptoms including olfaction, and quality of life. Mepolizumab and dupilumab have demonstrated a mild to moderate reduction in the indication of surgery.710,714 In patients with severe CRSwNP in whom pharmacological treatment and/or nasosinusal surgery have not achieved an adequate control of the disease, the use of biologics with dupilumab,714mepolizumab715 and omalizumab709 has been recently approved in the European Union.
The use of monoclonal antibodies for the treatment of CRSwNP should be considered for patients resistant to appropriate medical treatment (mainly intranasal and oral glucocorticoids) and failure of at least one endoscopic sinonasal surgical procedure716–720 as described in table 45 based on the POLINA consensus.694
Criteria for the indication of biologics in the treatment of nasal poliposis proposed by the POLINA consensus.694
However, it is necessary to conduct studies under clinical practice conditions and patient record analysis that allow for a better establishment of its effectiveness, as well as the duration of treatment and its cost-effectiveness. In the future, biomarkers should be available to identify the clinical and inflammatory characteristics of candidates to receive them.
4.5Recommendations| 6.1. It is recommended to classify allergic rhinitis according to duration into intermittent and persistent, and according to severity into mild, moderate, and severe. | R1 |
| 6.2. The diagnosis of rhinitis is established by clinical criteria and allergy testing. | R1 |
| 6.3. Patients diagnosed with asthma should be assessed for the presence of chronic rhinitis and rhinosinusitus with nasal polyps and vice versa, to implement an integral trearment strategy. | R1 |
| 6.4. For the pharmacological treatment of allergic rhinitis, it is recommended the use of oral and/or nasal topical second-generation antihistamines, intranasal glucocorticoids, or the association of these medications in case of lack of response or moderate to severe disease. | R1 |
| 6.5. In appropriately selected patients (adults and children), immunotherapy with allergen extracts is recommended for the treatment of allergic rhinitis. | R1 |
| 6.6. In patients with chronic rhinosinusitis with nasal polyposis, continuous use of intranasal glucocorticoids is recommended. The use of short-courses of oral glucocorticoids is indicated in severe cases and exacerbations. | R1 |
| 6.7. En In patients with poor control of chronic rhinosinusitis with nasal polyposis despite maximum medical treatment, it is recommended to consider the surgical option followed by post-surgical treatment with intranasal glucocorticoids. | R1 |
Severe asthma is characterized by the need to be treated with multiple drugs at high doses (steps 5-6 of GEMA and step 5 of GINA; see chapter 2.5). Severe asthma includes both controlled and uncontrolled asthma patients.721
Severe asthma is associated with a higher consumption of economic resources as compared with moderate or mild asthma.722–724
Severe uncontrolled asthma (SUA) has received multiple and varied terms and there is no consistent agreement for its terminology.
SUA is defined as the asthma disease that remains poorly controlled despite treatment in the previous year with a combination of inhaled glucocorticoids at high doses/long-acting ß2-adrenergic agonists (ICS/LABA), and long-acting anticholinergics (LAMA) or requiring maintenance oral glucocorticoids therapy (treatment for 6 months a year, independently of the doses or cumulative doses >1g of prednisone or equivalent, independently of the duration).725 Lack of control will be identified by any of the following characteristics (table 46):
- •
Asthma Control Test (ACT) <20 or Asthma Control Questionnaire (ACQ) >1.5.
- •
≥ 2 severe exacerbations or having been received ≥ 2 courses of oral glucocorticoids (≥ 3 days each) in the previous year.
- •
≥ 1 hospitalization for a severe exacerbation episode in the previous year.
- •
Chronic airflow limitation (forced expiratory volume in one second/forced vital capacity [FEV1/FVC] ratio <0.7 or FEV1 <80% predicted) after the use of an adequate treatment (as long as the better FEV1 will be higher than 80%).
Severe uncontrolled asthma: definition and control.
| It is defined as the asthma disease that persists poorly controlled despite treatment with a combination of ICS/LABA/LAMA at high doses in the previous year, or oral glucocorticoids for at least 6 months during the same period.The lack of control is shown by:– ACT <20 or ACQ >1.5.– ≥ 2 severe exacerbations or having being received ≥ 2 courses of oral glucocorticoids (≥ 3 days each) in the previous year.– ≥ 1 hospitalization for a severe exacerbation episode in the previous year.– Chronic airflow limitation (FEV1/FVC ratio <0.7 or FEV1 <80% predicted) after the use of an adequate treatment (as long as the better FEV1 will be higher than 80%). |
It is important to exclude external factors that may contribute to poor asthma control before defining SUA (section 7.2.2).726–730
Some studies have shown a prevalence of SUA between 3% and 4% among patients with asthma.731,732
SUA can be corticosteroid-dependent or corticosteroid-resistant to a higher or lesser extent.733–735
Corticosteroid-dependent SUA is defined when continuous treatment with oral or parenteral glucocorticoids for disease control is required, the disease is insensitive to glucocorticoids and shows a FEV1 ≤ 75% that does not improve significantly (≤ 15%) after treatment with oral prednisone, 40mg/day for 2 weeks.736,737
7.2Diagnosis and evaluationWhen SUA is suspected, it is advisable to perform a systematic evaluation, preferably in specialized asthma centers or units, using a multidisciplinary approach, following a sequential stepwise diagnostic algorithm726,738–741 (fig. 15).
The use of this multidimensional approach has shown good clinical results and to be cost-effective.742–744
7.2.1Diagnostic confirmation of asthmaIt has been estimated that between 12% and 30% of patients with suspected SUA do not have asthma.726,745–747
It should be confirmed that the diagnosis of asthma has been made correctly and, in case of doubt, studies aimed to demonstrate objectively the presence of airflow obstruction, variability and/or bronchial hyperresponsiveness (see chapter 2.2) should be performed. If diagnosis cannot be confirmed, other diseases mimicking asthma should be excluded through the rational and progressive use of work-up studies summarized in table 47.
Differential diagnosis in asthma: diseases mimicking asthma and their corresponding diagnostic tests.
| Differential diagnosis | Diagnostic tests |
|---|---|
| – Organic upper airway diseases– Dynamic airway collapse– Bronchial obstruction | – Spirometry with inspiratory loop– Computerized tomography (CT) obtained in inspiration and expiration of the upper airway– Fiberoptic bronchoscopy |
| – Inducible laryngeal obstruction (ILO) | – Laryngoscopy/video-stroboscopic during exacerbation or after challenge with methacholine or after ergometry |
| – Chronic obstructive pulmonary disease (emphysema) | – Chest CT– Plethysmography and CO diffusing capacity |
| – Bronchiolitis obliterans | – Chest CT obtained in inspiration/expiration– Plethysmography/air trapping– Biopsy transbronchial/pulmonary |
| – Functional dyspneas/hyperventilation syndrome | – Cuestionario de hiperpercepción (de Nijmegen)– Psychological evaluation |
| – Left heart failure | – Chest CT– Electrocardiogram/echocardiogram |
| – Bronchiectasis– Cystic fibrosis– Allergic bronchopulmonary aspergillosis (ABPA) | – Chest CT– Sweat test/genetic study– Total and Arpergillus-specific IgE/precipitins |
| – Eosinophilic granulomatosis with polyangiitis (EGPA)– Pulmonary eosinophilias | – pANCA/biopsy of organ(s) affected– Fiberoptic bronchoscopy (with bronchoalveolar lavage) |
pANCA: perinuclear anti-neutrophil cytoplasmic antibodies.
It is necessary to identify and evaluate some factors unrelated to the disease, the presence of which can contribute to poor control of asthma. These factors can be grouped into the following categories:
- •
Factors directly related to the patient: treatment adherence and inhalation technique. Up to 50% to 80% of cases of SUA are caused by inadequate adherence or by a deficient inhalation technique.732,745,748
Therefore, adherence should always be evaluated (preferably using validated questionnaires or information on dispensing prescriptions in the the community pharmacy) and the inhalation technique (direct observation) (see chapter 3.4).
- •
Factors related with comorbidities and aggravating conditions. Different diseases or processes when present concomitantly with asthma can contribute to an insufficient control of the disease. It has been shown that 92% of patients with SUA suffer from at least one of these conditions, which in turn are more prevalent than in patients without SUA.730
Table 48 summarizes the most commonly cited comorbidities and their corresponding tests for evaluation, diagnostic confirmation, and treatment approach.738,740,741,749,750
Table 48.Common comorbidities and aggravating factors of asthma with their corresponding diagnostic tests and treatment.
Comorbidity Diagnostic tests Treatment Sinonasal disease Rhinoscopy/nasal endoscopy Intranasal glucocorticoids Sinus imaging studies (CT/MR) Nasal Iavages/antileukotrienes Endonasal surgery Gastroesophageal reflux pH-metry/esophageal manometry Hygienic-dietetic counselling Treatment test with PPI Proton pump inhibitors Upper digestive endoscopy Surgical repair Obesity BMI Weight loss Bariatric surgery Sleep apnea syndrome (SAS) Polysomnography CPAP Weight loss if necessary Psychopathology (anxiety, depression) Psychologist/psychiatrist evaluation Psychotherapy/specific treatment Fibromyalgia Rheumatological evaluation Functional dyspnea Specific questionnaires (Nijmegen questionnaire) Psychotherapy Respiratory re-education Inducible laryngeal obstruction (ILO) Laryngoscopy in exacerbation or methacholine/exercise challenge Logophoniatric rehabilitation Treatment of comorbidities: reflux Drugs: NSAID, non-selective β-blockers, ACE inhibitors Clinical history Substitution Tobacco and other inhalation toxics Questioning Cessation/quit NSAID: non-steroidal anti-inflammatory; ACE: angiotensin-converting enzyme; CT: computed tomography; MR: magnetic resonance; PPI: proton pump inhibitors; BMI: body mass index; CPAP: continuous positive airway pressure.
- •
Factors related to triggers of exacerbations. It is necessary to identify whether exposure to triggers of exacerbations are present (see table 4), particularly active and passive smoking, e-cigarettes, cannabis inhalation, allergen exposure (mites, pollens, fungi, dander, cockroaches, etc.), indoor and outdoor air contamination, occupational agents, molds and harmful chemical products, drugs such as non-cardioselective ß-blockers, non-steroidal anti-inflammatory drugs (NSAID), and angiotensin-converting enzyme (ACE) inhibitors.738,740
Moreover, lack of response due to SABA abuse (by downregulation of ß2 receptors and increase of bronchial hyperresponsiveness [BHR]) has been reported.751,752
Classification into phenotypes aims to identify specific patients who are candidates for a particular treatment739,753 (see section 7.3). Currently, specific biomarkers for each phenotype/endotype are not available.754
The minimum follow-up period, by a specialist or a specialized asthma unit, to accept the diagnosis of SUA is 6 months.728,738,741
7.3Phenotypes of severe uncontrolled asthmaSevere asthma is a heterogeneous syndrome with multiple clinical variants. Over the past two decades, there has been intense research focusing on the study, discovery, and refinement of phenotypes of SUA.755–763
Phenotype is defined as an observable characteristic of severe asthma that can be associated with an underlying mechanism, named endotype. It is important to differentiate phenotype from comorbidities, since comorbidities coexist with SUA but their treatment is different.
Establishing the asthma phenotype in patients with SUA constitutes part of the diagnostic or assessment action to be carried out in these patients, as it may entail differential treatment modalities and has prognostic implications.727,764–766
Studies based on biostatistical analyses of cases clustered according to natural history, pathobiology, clinical features (age, onset, allergy symptoms, involvement of the upper respiratory tract, body mass index [BMI], acetyl salicylic acid-exacerbated respiratory disease [AERD], pulmonary function, biomarkers (peripheral blood and sputum eosinophils, immunoglobulin E [IgE], fractional exhaled nitric oxide [FENO], induced sputum neutrophil count) and therapeutic response have identified the existence of different phenotypes.739,753,767–770 Two inflammatory patterns have been defined: T2 (present in allergic and eosinophilic asthma) and non-T2. In clinical practice, three SUA phenotypes stand out with implications in treatment decision-making:
- •
Type 2 (T2) allergic phenotype.
- •
Type 2 (T2) eosinophilic phenotype.
- •
Non-T2 phenotype (table 49).
Table 49.Severe asthma phenotypes.
Phenotypes Clinical characteristics Biomarkers Treatment Allergic (T2) Allergic symptoms+Allergen sensitization(prick test and/or specific IgE) Specific IgETh2 cytokines PeriostinSputum eosinophils and neutrophils Glucocorticoids OmalizumabAnti-IL-5/anti-IL-5R- (mepolizumab, reslizumab, benralizumab)Dupilumab Tezepelumab Eosinophilic (T2) Chronic rhinosinusitis/nasalpolyposisAERDCorticoid-depedent or refractoryto glucocorticoids Blood and sputum eosinophilsIL-5Cysteinyl-leukotrienes LTRAAnti-IL-5/anti-IL-5R- (mepolizumab, reslizumab, benralizumab)Dupilumab Tezepelumab Non-T2 Lower FEV1Greater trappingSmoking history Neutrophils orpaucigranulocytic in sputumTH17 activationIL-8 Azythromycin TezepelumabThermoplasty IgE: immunoglobulin E; AERD: acetyl salicylic acid-exacerbated respiratory disease. FEV1: forced expiratory volumen in one second; LTRA: letra leukotriene receptor antagonist; IL: interleukin.
However, both T2 phenotypes may show some degree of overlap.
7.3.1Allergic asthma (T2)Allergic asthma accounts for 40-50% of severe cases of asthma, and has an atopic underlying mechanism mediated by the activation of type-2 helper T lymphocytes (Th2), the production of interleukin (IL) 4, IL-5 and IL-13, and an isotype shift within B lymphocytes towards IgE production. Allergic bronchopulmonary aspergillosis (ABPA) is a particularly severe variety of allergic asthma that shows a pure eosinophilic or mixed (eosinophilia and neutrophilia) inflammatory pattern in sputum. Periostin (an IL-13-induced cell matrix protein), which can be measured in blood and bronchial secretions, and the fractional exhaled nitric oxide (FENO) are good biomarkers of the “increased” T2 variant.771–774 The diagnosis requires the demonstration of sensitization to an allergen and the triggering of symptoms with exposure to such allergen.
7.3.2Eosinophilic asthma (T2)It accounts for more than 25% of severe asthma cases and is characterized by the presence of eosinophils in bronchial biopsies and sputum samples despite treatment with glucocorticoids at high doses. Chronic rhinosinusitis and nasal polyps may also occur. A subset of patients develops AERD. Although eosinophilic asthma is associated with a lower prevalence of atopy, IgE and FENO may be increased. Alterations of the arachidonic acid metabolism are involved in the pathogenesis of this phenotype of asthma. A high production of IL-5 may explain the eosinophilic inflammation in the absence of the traditional allergy-mediated T2 mechanism.775–778
7.3.3Non-T2 asthmaThis phenotype of asthma occurs without eosinophilia, neither in the peripheral blood, nor in sputum. It frequently shows a paucigranulocytic profile, neutrophilia, scarce local eosinophilia, low FENO levels, and a poor response to glucocorticoids. It can be accompanied by chronic airflow limitation with important air trapping and, frequently, history of smoking is present.779,780 It should be taken into account that inflammatory biomarkers of type T2 phenotype (peripheral blood eosinophils, sputum eosinophils, and FENO) are frequently suppressed by oral glucocorticoids. In our opinion, analysis of peripheral blood eosinophils and FENO should be repeated up to three times (e.g. when asthma worsens, before the administration of oral glucocorticoids), before assuming that asthma does not belong to the T2 phenotype.
In the GINA 2019, the possibility of type 2 refractory inflammation is considered, in the presence of any of the following findings in a patient taking ICS at high doses or daily oral glucocorticoids:740
- -
Peripheral blood eosinophils ≥ 150/μl, and/or FENO ≥ 20 ppb, and/or
- -
Sputum eosinophils ≥ 2%, and/or
- -
Asthma is clinically induced by allergens.
Asthma education. Asthma education activities are not different from that usually recommended for the remaining asthma population (see chapter 3.5). However, approaches such as maximizing avoidance measures and smoking cessation should be implemented, with special emphasis to confirm objectively that adherence to treatment and the inhalation technique are both correct. At present, there are different devices for remotely adherence monitoring.781,782
Background pharmacological treatment. According to the inclusion criteria defining SUA, in patients on maintenance therapy with a combination of ICS/LABA at high doses it is advisable to add, at least, a third controller drug, usually tiotropium783,784 (see chapter 3.2).
Treatment of comorbidities. If either an associated comorbid condition or an aggravating factor has been identified, the appropriate therapeutic measures should be initiated (table 48).738,740,749,750
7.4.2Phenotype-directed treatmentPatients with SUA according to the pathophysiological underlying mechanism (T2 or non-T2 asthma) and the presence or absence of different inflammatory markers are classified into the aforementioned phenotypes (see section 7.3).
Inflammation markers of T2 phenotype may be suppressed by treatment with oral glucocorticoids (OCS); therefore, they should be preferably measured before starting treatment with OCS or at the lowest possible dose, and at least on three occasions (e.g. during an exacerbation), prior to assume that a patient presents a non-T2 phenotype. In corticosteroid-dependent patients, it is important to check their historical values.
A phenotype-directed treatment algorithm is proposed in the present guideline (fig. 16 and table 50); the different monoclonal antibodies available for treating SUA are shown together with their main characteristics.
Treatment of SUA according to phenotype.
SUA: severe uncontrolled asthma; ICS: inhaled glucocorticoids; LABA: long-acting β2-adrenergic agonists; LAMA: long-acting cholinergic agonists; CRSwNP: chronic rhinosinusitis with nasal polyposis; HES: hypereosinophilic syndrome; EGPA: eosinophilic granulomatosis with polyangiitis; PMN: polymorphonuclear neutrophils; ACT: Asthma Control Test; ACQ: Asthma Control Questionnaire; SNOT-22: Sinonasal Outcome Test; VAS: visual analogue scale; FENO: fractional exhaled nitric oxide; EOS: eosinophils; aSensitivity to allergens and presence of compatible clinical features, and total IgE ≥ 75 IU; bOmalizumab if IgE ≥ 75 U/l and EOS <150μl; cDupilumab if EOS ≥300μl and/or FENO ≥50 ppb and between 150-300 EOS and FENO ≥25 ppb. Not recommended if EOS ≥1,500μl. dTezepelumab if FENO ≥25 ppb; eMepolizumab if current EOS ≥150μl and ≥300μl in the previous 12 months. fBenralizumab if current EOS ≥150μl and nasal polyposis or ≥3 severe exacerbations in the previous year or FCV <60%; gReslizumab if EOS ≥400μl.
Notes
Definitions
SUA: asthma requiring treatment with 5-6 therapeutic steps according to GEMA and presents ≥1 of the following criteria:
- –
ACT <20 or ACQ ≥1.5.
- –
≥2 courses of oral corticoids (OCS) during ≥3 days
- –
in the previous year.
- –
≥1 hospital admission due to asthma exacerbation in the previous year.
- –
FEV1 ≤ 80% predicted.
Type 2 refractory inflammation: ≥1 of the following criteria in a patient using inhaled glucocorticoids (ICS) at high doses or daily OCS:
- –
≥150 eosinophils per microliter in blood
- –
FENO ≥25 ppb/ul (American Thoracic Society Committee).
- –
≥2% eosinophils in sputum.
- –
Asthma is clinically induced by allergens.
Patients requiring maintenance treatment with oral glucocorticoids can also have an underlying type 2 inflammation. However, OCS often suppress type 2 inflammation biomarkers (blood and sputum eosinophils and FENO). Therefore, when possible, these tests should be performed before starting a short course or maintenance treatment with OCS, or when the patient receives the lowest possible dose of OCS.
Thresholds of peripheral blood eosinophilia: At least one analytical result of more than 300 Eos/μl in the last year. Low values of eosinophils may appear in patients recently treated or on chronic treatment with systemic glucorticoids. In this case, it can be useful to review the patient's historical values.
Thresholds of FENO. The cutoff value is established at 25 ppb. However, it should be considered that results of FENO measurement can altered by the recent use of systemic glucocorticoids and total dose of inhaled glucocorticoids, age. and current smoking (lower values in smokers). In the presence of high FENO levels, it is necessary to confirm that self-administration inhaled medication is correct (treatment adherence and inhalation technique).
Response to a biological drug. It is defined by:
- –
ACT score equal or higher than 20 or a significant change as compared with baseline score (≥3 points).
- –
Absence of hospital admissions or visits to the emergency room.
- –
More than 50% reduction of exacerbations.
- –
Suppression of the use of oral glucocorticoids or significant decrease of doses (≥50%).
Choice among monoclonals
The order in which biologics appear in the scheme when they coincide for the same indication only takes into account the time since each drug has been commercialized.
In the choice of biologics should be considered: blood eosinophil count, pulmonary function, use of maintenance treatment with oral glucocorticoids, presence of comorbidities: nasal polyposis/AERD, chronic urticaria, atopic dermatitis and asthma-associated diseases (eosinophilic granulomatosis with polyangiitis, eosinophilic pneumonia, allergic bronchopulmonary aspergillosis, eosinophilic esophagitis).
- –
Benralizumab (higher efficacy ≥300 eosinophils/μl): patients with poor pulmonary function, polyposis, maintenance with oral glucocorticoids and difficult access to asthma unit living far away [long distances]).
- –
Reslizumab (higher efficacy ≥400 eosinophils/μl): improves pulmonary function. Not effective for reducing OCS doses. Intravenous administration.
- –
Mepolizumab (indication from 150 eosinophils/μl but higher efficacy ≥500 eosinophils/μl): indicated in patients with ≥150 eosinophils/μl if there are historical values of ≥300 eosinophils/μl. It has been shown that allows reduction of withdrawal of OCS.
- –
Dupilumab (higher efficacy ≥300 eosinophils/μl and/or FENO ≥50 ppb): improves pulmonary function, nasal polyposis and severe dermatitis. It has been shown that allows reduction of withdrawal or OCS and increases eosinophil values. Administration every 15 days.
To choose between drugs with potential efficacy in a given patient, criteria of posology, patient's preferences, and costs should be also considered.
Thermoplasty is indicated in patients neither with emphysema/bronchiectasis/atelectasis nor with important comorbidities, without treatment with anticoagulants or immunosuppressants, and who do no present recurrent infections. FEV1 should be greater than 40% and any contraindication for fiberoptic bronchoscopy with sedation should be absent.
Biologics approved for the treatment of SUA and their characteristics.
| Biologic(SUA) | Approval:TPR Spain | Mechanism of action | Evidences | Adverse events (“frequent” according to technical specification) | Administration |
|---|---|---|---|---|---|
| Omalizumab | >6 years with severe allergic asthma and sensitization to perennial allergens with IgE between 30-1500 UI/ml and FEV1 <80% | Binds circulating IgE preventing binding to high and low affinity receptor (FcɛR1) for IgE | 34% reduction of exacerbations but no improvement of symptoms, HRQoL and pulmonary function in RCT.Efficacy in nasal polyposis | Injection site reactions, headache, upper abdominal pain | 75-600 mg s.c. route every2-4 weeks according to weight and IgE. Possible administration at home |
| Mepolizumab | ≥ 6 years with refractory eosi nophilic asthma with Eos ≥ 500 or <500 with 2 severe exacerbations or 1 hospitalization in the previous year | Blocks IL-5 from binding to the IL-5 receptor | 53% reduction of severe exacerbations and improvement of HRQoL, control of symptoms and pulmonary function in RCT. Reduces doses of maintenance OCS. Efficacy in nasal polyposis | Injection site reactions, headache, pharyngitis, pyrexia, upper abdominal pain, eczema, back pain, Hypersensitivity reactions | 6-11 years: 40 mg every4 weeks≥ 12 years: 100 mg every4 weeksPossible administration at home |
| Reslizumab | >18 years with severe eosinophilic asthma on treatment with ICS at high doses plus another controller with Eos ≥ 500 or between 400-500 and 2 severe exacerbations or 1 hospitalization in the previous year | Binds to the same domain that IL-5 receptor blocking binding of IL-5 to its receptor | 54% reduction of exacerbations in patients with ≥ 400 Eos and ≥ 1 exacerbation in the past year | Increase of blood CPK | 3 mg/kg i.v. route every 4 weeksDay hospital |
| Benralizumab | >18 years with severe eosinophilic asthma on treatment with ICS at high doses plus LABA with Eos ≥ 500 or <500 with 2 severe exacerbations or 1 hospitalization in the previous year | Binds Fα of IL-5 receptorinhibiting its activation.Induces direct elimination (by Ac-mediated cytotoxicity) of eosinophils and basophils through natural killer (NK) cells | 57% reduction of exacerbations in patients with ≥ 300 Eos and ≥ 3 exacerbations in the past year; and improvements of pulmonary function and reduction of OCS doses | Injection site reactions, pharyngitis, headache, hypersensitivity reactions | 30 mg s.c. every 8 weeks (with the first 3 doses at one month interval)Possible administration at home |
| Dupilumab | ≥ 6 years with severeasthma with T2 markers (Eos≥ 300 o FENO ≥ 25 ppb) or corticosteroid-dependent | Blocks subunit α of IL-4 receptor (anti-IL-4 and IL-13 effect) | 50% reduction of severe exacerbations and improvement of HRQoL, control of symptoms and pulmonary function in RCT. Reduces maintenance doses of OCSEfficacy in nasal polyposis | Injection site reactions, transient blood eosinophilia (4-13%) | Initial dose 400 mg followed by:200 mg s.c. every 2 weeks (severe eosinophilic asthma/T2)300 mg in corticoid-dependent or with associated atopic dermatitis. Possible administration at home.Dosage in patients between 6 and 11 yeats of age is described in section 7.5 |
| Tezepelumab | Indicated as additional maintenance treatment in adults and adolescents ≥ 12 years of age with severe asthma not adequately controlled despite ICS at high doses combined with another drug for maintenance treatment* | Human monoclonal antibodu (IgG2λ) directed against TSLP, bronchial epithelial-cell derived cytokine member of the alarmin family | Significant reduction of exacerbations (66-71%) and bronchial hyperresponsiveness, improvement of pulmonary function, control of the disease, and HRQL. It is also effective in case of blood eosinophils <150 cells/μl and FENO<25 ppb. | Injection site reactions, pharyngitis, arthralgias, skin eruption | 210 mg s.c. every 4 weeksPossible administration at home |
TPR: therapeutic positioning report; s.c.: subcutaneous; i.v.: intravenous; HRQoL: health-related quality of life; RCT: randomized controlled trial; Eos: eosinophils. FEV1: forced expiratory volumen in one second; ICS: inhaled corticosteroids; LABA: long-acting ß2-adrenergic agonist; IgE: immunoglobulin E; OCS: oral glucocorticoids; CPK: creatine phosphokinase; Ac: antibody.
Considering the level of peripheral blood or sputum eosinophils and the presence of relevant allergic clinical manifestations with confirmed sensitization to perennial aleroallergens, one of the available monoclonal antibodies will be selected (fig. 16).741
4.5.1.1Anti-IgE treatment: omalizumabMonoclonal antibody blocking IgE, with more than 15 years in clinical practice that has shown its efficacy in randomized controlled trials (RCT) reducing severe exacerbations, intensity of symptoms, use of inhaled ICS, and improvement of quality of life.785–789
Omalizumab is indicated in allergic SUA with sensitization to perennial allergens in patients aged ≥ 6 years with serum total IgE values between 30-1500 IU. The dose varies according IgE levels and body weight. The administration route is subcutaneous (s.c.) every 2 or 4 weeks.
Subsequent studies carried out in daily practice conditions have shown a decrease of exacerbations, improvement of quality of life, and reduction of OCS,790 independently of the baseline value of biomarkers791 or the eosinophil count.790
In some cases, after a prolonged period of treatment (5 years), withdrawal of omalizumab is possible. Treatment discontinuation should be performed gradually, on an individual basis, in agreement with the patient and with close monitorization of the control of asthma.792–794
Good results with the use of omalizumab in allergic bronchopulmonary aspergillosis have been reported,795,796 but up to the present time RCTs have not been carried.
4.5.1.2Anti-IL-5/IL-5Ra treatment4.5.1.2.1MepolizumabMonoclonal antibody that bocks circulating IL-5. In RCTs, the use of mepolizumab has shown to reduce exacerbations in patients with ≥ 300 eosinophils/μl in peripheral blood during the previous year, or with ≥ 150/μl at the time of treatment but with high historical values.797,798 A post hoc analysis showed a greater reduction of exacerbations (70%) in the group of patients with >500 eosinophils/μl.799 Also, this drug has shown to be effective in reducing the doses of OCS in patients on maintenance treatment with systemic glucocorticoids.800,801 It is indicated in patients with eosinophilic asthma of ≥ 6 years of age, at doses of 100mg s.c. every 4 weeks for patients aged 12 years and older, and 40mg s.c. dose every 4 weeks to patients aged 6 to 11 years.
Studies carried out in routine daily practice802 and those extended a long time have confirmed the efficacy of mepolizumab shown in clinical trials, with a favorable safety profile and stable and long-lasting effect.803,804
The efficacy of this drug has been demonstrated in patients with partial response to Mepolizumab has been also approved for the treatment of hypereosinophilic syndrome (HES). In adolescents and adult patients with severe HES, the use of this drug achieved a reduction in the percentage of patients with exacerbations (worsening of symptoms or increase in eosinophil count requiring escalation to other treatments) and a decrease in annual exacerbations (the open-label extension study confirmed the results of the randomized trial).805,806
4.5.1.2.2ReslizumabIt is a monoclonal antibody against IL-5 that has shown a significant reduction of exacerbations and improvement of current control-related variables in severe asthma with ≥ 400 eosinophils/μl.807–809 The efficacy is independent of allergic sensitization.810 A post hoc study showed a reduction in OCS burden in corticosteroid-dependent asthma.811 It is indicated in patients with eosinophilic asthma >18 years of age, at doses of 3mg/kg i.v. every 4 weeks.
Some studies in small series of patients in which treatment with other monoclonal antibodies (omalizumab and mepolizumab) have been unsuccessful, showed improvement after the use of reslizumab.812,813 Studies at 2 years demonstrate a favorable safety profile.814
4.5.1.2.3BenralizumabIt is a monoclonal antibody binding subunit a of the IL-5 receptor preventing its activation and inducing direct elimination (by antibody-dependent cell-mediated cytotoxicity) of eosinophils and basophils through NK cells; so that, it is known as anti-eosinophilic effect. In pivotal RCTs carried our in eosinophilic SUA, benralizumab has shown to reduce severe exacerbations, improve pulmonary function (FEV1), and decrease asthma symptoms,815,816 particularly in patients with peripheral blood eosinophils ≥ 300μl or ≥ 150μl on maintenance treatment with OCS. It is indicated in patients with eosinophilic asthma aged ≥ 18 years, at doses of 30mg s.c. every 4 weeks for the first 3 doses, and every 8 weeks thereafter.
Also, it has been shown a significant reduction of the doses of OCS and even withdrawal of OCS.817,818
In phase III trials, a number of baseline clinical factors were associated with a greater response, including the use of OCS, history of nasal polyposis, reduced pulmonary function based on FVC <65% and frequent exacerbations.763,819–822
Follow-up studies at 2 and 5 years have confirmed the efficacy and safety results.823,824
Subsequent studies conducted in routine clinical practice have shown a decrease of exacerbations, improvement of quality of life, and a reduction of OCS.825
4.5.1.3Anti-IL4/IL-13 treatment4.5.1.3.1DupilumabIt is a fully human monoclonal antibody binding receptor a of IL-4, blocking both IL-4 and IL-13. Initial RCTs with this drug have shown a reduction of exacerbations, improvements in quality of life, control of symptoms, and pulmonary function (FEV1) in patients with moderate to severe uncontrolled asthma over 12 years of age. These improvements were also observed in patients with peripheral blood eosinophils between 150 and 300/μl with FENO ≥ 25 ppb, although there was a higher improvement in patients with eosinophil count ≥ 300/μl and/or FENO ≥ 50 ppb.826–828 Further studies have extended the indication to patients ≥ 6 years of age with SUA and high eosinophil counts and/or FENO.829
Reduction and withdrawal of OCS has also been demonstrated in corticosteroid-dependent patients,830 and a better response in patients with higher values of eosinophils and/or FENO.828
The effect on reduction of exacerbations, pulmonary function, and reduction of OCS is maintained over time, according to an extension study of almost 3 years.831
Extension studies have confirmed a favorable safety profile.831 In relation to adverse effects, it should be noted that a patients treated with dupilumab showed eosinophilia, with counts higher than 3,000 cells/μl in a percentage of cases, although without clinical repercussion. In these studies, transient hypereosinophilia did not modify the response to treatment.828–831
4.5.1.4Treatment with anti-thymic stromal lymphopoietin (TSLP)4.5.1.4.1TezepelumabIt is a human monoclonal antibody that binds thymic stromal lymphopoietin (TSLP), a bronchial epithelium-derived cytokine of the group of alarmins. At doses of 210mg by the subcutaneous route every 4 weeks significantly reduce exacerbations (66-71%) and bronchial hyperresponsiveness,832 improves pulmonary function, control of the disease, and quality of life, independently of baseline levels of T2 inflammation biomarkers (FENO, peripheral eosinophilia, IgE) without achieving a significant reduction of OCS doses.833,834
Extension studies have confirmed the effectiveness shown in clinical trials and a favorable safety profile.835
7.4.2.2Treatment of non-T2 asthmaIn patients in whom there is no evidence of the presence of T2 inflammation biomarkers, other therapeutic options should be selected.
4.5.1.4.2TezepelumabAlthough it provides a reduction in the rate of severe exacerbations, more pronounced as more higher blood eosinophils and FENO values at baseline, it is also effective when the blood eosinophil count is <150 cells/μl and FENO <25 ppb. Therefore, it is the only biological drug that currently demonstrates efficacy in non-T2 asthma.833
4.5.1.4.3AzithromycinBecause of their immunomodulatory effect, macrolides have been used in asthma with inconsistent results.775,836,837 In the AMAZES study,838,839 it was found that azithromycin administered at doses of 500mg orally, 3 times a week during 48 weeks, reduced exacerbations and improved quality of life, independently of the inflammatory phenotype.
An individualized indication is recommended in SUA patients with triple therapy with non-T2 phenotype especially if they suffer from frequent exacerbation episodes.740,750
4.5.1.5Bronchial thermoplastyThis bronchoscopic procedure reduces the bronchial smooth muscle layer by heating the tissue through the deliver of radiofrequency energy.840,841
Results of studies of bronchial thermoplasty in patients with moderate and severe asthma showed a significant improvement of the quality of life, control of symptoms, and reduction of exacerbations.842–846 Efficacy regarding reduction of exacerbations is still present after 5 and 10 years of the procedure.840,847,848
This is a therapeutic option to be considered in patient with SUA with phenotypes unsuitable for the use of monoclonal antibodies or in which monoclonal antibodies have been unsuccessful, provided that there are no contraindications to the technique and it is applied in experienced centers.
4.5.1.6Systemic glucocorticoidsIn some patients with SUA suffering from an exacerbation episode, treatment with OCS is necessary. Patients requiring OCS courses may present adverse effects, the risk of which increase with the use of ≥ 4 courses of OCS in a year or >30 days a year.849,850
The use of OCS at the minimum necessary dose and for the shortest time possible should be reserved as one of the last alternative for patients in whom control is not achieved with other therapeutic options.851 In these circumstances, preventive or treatment measures for possible adverse effects will be considered.
Some studies with not very robust designs, carried out in small samples of patients showed that intramuscular triamcinolone depot (glucocorticoid with the addition of a fluorine group), in patients with corticosteroid-dependent asthma, compared to the usual OCSs, provided a significant reduction of exacerbations, an increase in pulmonary function, and fewer side effects.852,853 However, they are not free of adverse effects and the pharmacokinetic profile is unknown.
4.5.1.6.1Recommendations for taperingl of oral glucocorticoidsSome studies have shown that the use of OCS is not uncommon.854,855
A significant dose-response relationship between chronic use of OCS and the risk of complications has been observed.856
Every patient undergoing treatment with OCS should undergo proper monitoring and management of possible side effects. A protocol to this purpose is shown in table 51.
Protocol of monitoring side effects of systemic glucocorticoids.
| Osteoporosis | Annual height measurement.Evaluation of the risk of fractures.Bone densitometry before treatment with SGC and at one year:• If low BMD, repeat at one year.• If normal BMD, repeat at 2-3 years. |
| Adrenal insufficiency | If the use of SGC exceeds 2 consecutive weeks continuously or 3 cumulative weeks in the previous 6 months:• Evaluation of symptoms.• Baseline cortisol assay (8-9 a.m). |
| Ophthalmological | Annual ophthalmological examination.Early referral for ophthalmological examination (in the presence of cataracts and/or risk factors for glaucoma). |
| Cardiovascular | Calculate the risk according to the Framingham index.Lipid profile after one month of starting treatment with SGC, repeat every 6-12 months. |
| Hyperglycemia | Glycemia every 3-6 months on the first year, and then annually. |
| Infections/pneumonias | Alert the patients to seek medical care in the presence of fever and/or symptoms of infection.Microbiological surveillance. Serial sputum cultures. |
BMD: bone mineral density; SGC: systemic glucocorticoids.
In a recent Dephi expert consensus study,858 the authors made a series of recommendations regarding the use of OCS:
- •
Minimize OCS use as much as possible.
- •
An annual cumulative dose of 0.5 to 1g would be indicative of poor asthma control.
- •
Primary care physicians prescribing at least 3 courses of OCSs per year to a patient should consider specialist referral.
- •
In all patients who have received a cumulative dose of OCS exceeding 2g in the past year, a fasting morning cortisol test (8 a.m.) should be performed when attempting to reduce the OCS dose below 5mg/day.
- •
When no other alternative is available, a dose of ≤ 5mg/day of prednisone (or equivalent) would be considered acceptable.
Regarding tapering of OCS doses in patients starting treatment with biologics, the following should be considered:
- •
The initial tapering of high OCS doses (e.g. 20mg/day) can proceed at a faster pace (e.g. 10mg/week or 30-50% reductions every 2-4 weeks). Reduce 2.5 to 5mg every 0.5 to 2 weeks until the physiological dose is achieved (e.g. 5-10mg/day) and then proceed to a lower pace (1-2.5mg every 1-2 weeks).
- •
When a reduction in the OCS dose by 5mg/week fails, a slower and lower dose reduction of 1mg/week should be attempted.
- •
If mild symptoms occur, maintain the current dosage; they are likely to resolve as endogenous-axis recovery occurs.
- •
If intolerable symptoms occur, return to the previous (efficacious) dose and then later consider reattempting tapering at a slower pace.
Although there is no unanimous agreement, a scheme for reducing the dose of OCS in patients starting treatment with biologics has recently been proposed.859
Figure 17 shows the algorithm proposed for the treatment of corticosteroid-dependent severe asthma in adults725 and fig. 18 the algorithm for down-titration doses of OCS.
Treatment of corticosteroid-dependent severe asthma in adults (based in part on the 2022 SEPAR consensus860 (OCS: oral glucocorticoids; ACT: Asthma Control Test; ACQ: Asthma Control Questionnaire; SNOT: Sinonasal Outcome Test Questionnaire; VAS: visual analogue scale).
Algorithm for the evaluation of adrenal function during down-titration of OCS dosage.
(Modified from Menzies-Gow et al., 2022).859
*It can be directly substituted by hydrocortisone 20mg/day, preferable at breakfast.
**Do not take OCS on the previous night nor in the morning before blood sampling.
Other new molecules such as antagonists of IL-33 and its receptor are under study. Of these, astegolimab, an anti-ST2 antibody, has shown efficacy in reducing exacerbations in eosinophil-high (> 300 cells/μl) and non-T2 inflammation (< 300 cells/μl) in a similar proportion.861
Interleukin-33 blockade with itepekimab led to a lower incidence of events indicating a loss of asthma control than placebo and improved lung function in patients with moderate-to-severe asthma.862
7.5Severe uncontrolled asthma in children7.5.1Epidemiology. DefinitionSevere asthma in childhood is more common from school age863,864 with a prevalence of 2-5%.865,866 It is associated with a high morbidity,867 costs,868 and future risk of chronic obstructive pulmonary disease (COPD).869,870
The clinical presentation and response to treatment vary from infants to adolescents.871,872
In children with severe recurrent exacerbations, and in younger than 5 years of age, with or without symptoms between episodes, a diagnosis of SUA may be considered when despite a correct treatment with ICS at high doses, the following events are present:
- 1)
≥ 1 admission to an intensive care unit,
- 2)
≥ 2 hospital admissions requiring intravenous therapy or
- 3)
≥ 2 courses of OCS in the previous year.873
The definition for children older than 5 years of age coincides with that for adults.726
7.5.2EvaluationA cost-effective multidimensional, Multidisciplinary, and stepwise evaluation is necessary necesaria874,875 (fig. 19).
Up to 50% of patients present potentially avoidable factors and/or associated comorbidities responsible for difficult control of their asthma.726,876
4.5.1.7Diagnostic confirmationUp to 12-30% of patients with SUA may be diagnosed with other diseases mimicking symptoms of asthma.726
A detailed medical history, physical examination, and pre- and post-bronchodilation spirometry are necessary. Many children with SUA have normal pulmonary function,877 requiring a bronchoprovocation test. In addition, other complementary tests oriented by the clinical suspicion or atypical presentation will be necessary. Also, in children with SUA under 5 years of age and in non-atopic children, the possibility of other diagnoses is high (table 52).
Diseases mimicking severe asthma in children.
| – Bronchiolitis, bronchiolitis obliterans.– Persistent bacterial bronchitis.– Recurrent aspiration, gastroesophageal reflux, swallowing disorders.– Prematurity and related diseases (bronchopulmonary dysplasia).– Cystic fibrosis.– Endobronchial foreign body.– Congenital or acquired immunodeficiencies.– Primary ciliary dyskinesia. | – Obstruction/compression central airway.– Congenital abnormalities, including vascular rings– Tracheobronchomalacia.– Carcinoid tumor or other.– Mediastinal mass/lymphoid nodule.– Congenital heart disease.– Interstitial lung disease.– Connective tissue diseases.– Vocal cord dysfunction. |
To this purpose, the presence of comorbidities (table 48) and/or avoidable associated factors that affect asthma control should be investigated.876 The following should be carefully evaluated: lack of adherence to treatment,878 inadequate inhalation technique,879 exposure to allergens,880 tobacco smoke, and other inhaled toxic substances881 as well as the presence of psychosocial factors.
4.5.1.9Resistance to glucocorticoidsAssessing the response to steroids after the administration of a course of OCS or a dose of triamcinolone, can help to make therapeutic decisions, such as adding tiotropium or monoclonal antibodies instead of increasing treatment with OCS.882
4.5.1.10Severe asthma phenotypes in childrenAssessment of phenotypes is necessary for an adequate personalized treatment. The allergic phenotype is the most common, being frequent the presence of polysensitization, the association with other atopic comorbidities (allergic rhinitis, atopic dermatitis, food allergy) and a high T2 inflammatory profile (elevated IgE, peripheral blood eosinophilia, and increase of FENO.863,867
Non-allergic eosinophilic severe asthma is less common, and neutrophilic severe asthma is rare.
7.5.3TreatmentChildren with SUA, despite adequate management of associated factors and comorbidities, are candidates for increasing the therapeutic step.
Inhaled glucocorticoids. A few children benefit from doses of fluticasone propionate or equivalent higher than 500μg/day, which in turn are related with adverse effects.879
Oral glucocorticoids. No data are available on the efficacy of OCS in the maintenance treatment of children with asthma treated with ICS at high doses plus LABA and/or montelukast. After the introduction of tiotropium and monoclonal antibodies, they have been relegated to a second step due to their adverse effects. If necessary, they should be used at the lowest dose for the shortest period of time and monitoring their adverse effects.
Triamcinolone. Triamcinolone could be useful in children with SUA, particularly in non-adherent patients to OCS or to determine the sensitivity or response to steroids.883 However, the use of triamcinolone should be very limited because of side effects and unknown pharmacokinetics.
Tiotropium. Associated with ICS/LABA in children aged 6 years or more is an option for trying to achieve asthma control884,885 prior to the use of monoclonal antibodies.
Omalizumab. It is an anti-IgE monoclonal antibody that has shown efficacy for treating children aged 6 years or more with persistent moderate or severe allergic asthma insufficiently controlled with high doses of ICS and LABA.886,887 It reduces exacerbations, symptoms, doses of ICS, the use of rescue medication, and improves quality of life.789,888
It is administered subcutaneously every 2-4 weeks, with the dose adjusted to total IgE values and body weight. In several studies carried out in routine clinical practice in children with severe allergic asthma, after the fifth month of treatment with omalizumab, improvement in asthma control, reduction in the rates of exacerbations and admissions, and in ICS doses were observed.889
Mepolizumab. It is an anti-IL5 monoclonal antibody, effective in severe eosinophilic asthma.890,891 Currently there is indication for its use after 6 years of age, with safety and efficacy data that support the use of mepolizumab in children with severe eosinophilic asthma, with a similar than adolescents and adults.829,891 The recommended dose is 40mg between 6-11 years and 100mg from 12 years, administered subcutaneously, once every 4 weeks.
Dupilumab. It is a monoclonal antibody that blocks the shared receptor of IL-4 and IL-13, reduces exacerbations, improves pulmonary function, and decreases the need for oral glucocorticoids in severe eosinophilic asthma in adults and adolescents. Its efficacy has also been proven in children from 6 to 11 years of age, with a dose of 100mg every 2 weeks of 300mg every 4 weeks in patients weighing less than 30kg, 200mg every 2 weeks or 300mg every 4 weeks in those weighing between 30 and 60 kf, and 200mg every 2 weeks in those with a body weight greater than 60 kg.892
Macrolides. They have an immunomodulator and antibacterial effect. In highly selected cases, azithromycin may be beneficial for improving some clinical symptoms and pulmonary function in children (< 6 years of age) with persistent uncontrolled asthma,893 as well as the quality of life, without differences between the eosinophilic and non-esoinophilic phenotypes.894
In infants and preschool children the level of evidence of the studies is even lower, although emerging studies are trying to define therapeutic position alternatives.
When symptoms remain uncontrolled despite ICS at high doses combined with montelukast, either LABA (off-label indication),895tiotropium,896 macrolides or even OCS may be added, although the best therapeutic option has not yet been established. The need to stepped-up treatment should be reevaluated at each visit, trying to maintain it during the shortest possible period of time.
4.6Recommendations| 7.1. It is suggested to define severe uncontrolled asthma (SUA) as asthma disease that remains poorly controlled despite having been treated with a combination of ICS/LABA at high doses in the previous year, or oral glucocorticoids for at least 6 months during the same period. | R2 |
| 7.2. The lack of control will be objectively determined by any of the following characteristics: ACT <20 or ACQ >1.5; ≥ 2 severe exacerbation or having being treated with ≥ 2 courses of oral glucocorticoids (≥ 3 days each) in the previous year; ≥ 1 hospital admission due to severe asthma in the previous year; chronic airflow limitation (FEV1/FVC ratio <0.7 or FEV1 <80% predicted) after use of adequate treatment (as long as the best FEV1 is higher than 80%). | R2 |
| 7.3. It is recommended that diagnostic evaluation of SUA should be preferably undertaken in centers or specialized asthma units, and using a stepwise decision algorithm. | R2 |
| 7.4. It is suggested to perform a protocolized diagnostic evaluation of SUA (in adults and children) based on three key actions: 1) to confirm the diagnosis of asthma objectively; 2) to identify those factors that are external to the asthmatic disease (treatment adherence, patient's inhalation technique, comorbidities o aggravating factors, triggers of exacerbations); and 3) to establish the phenotype of severe asthma. | R2 |
| 7.5. In the absence of diagnostic confirmation, the presence of other possible disease mimicking asthma should be excluded. | R2 |
| 7.6. It is recommended to establish asthma phenotype in patients with SUA as part of the diagnostic assessment. This identification can involve a differential treatment approach and have prognostic implications. | R2 |
| 7.7. In daily clinical practice, it is suggested the use of three severe asthma phenotypes for treatment decision-making, which are the following: allergic asthma (T2), eosinophilic asthma (T2), and non-T2 asthma. | R2 |
| 7.8. The general treatment of SUA includes: the prescription of drugs recommended in steps 5 and 6 (ICS/LABA combination at high doses and a third controller drug preferably tiotropium), adherence to an asthma education program, treatment of comorbidities/aggravating factors, and prevention/treatment of side effects of glucocorticoids. | R2 |
| 7.9. Given that inflammation markers of phenotype T2 may be suppressed by treatment with OCS, it is recommended assessing these markers before starting treatment of OCS, or with the lowest possible dose, and at least on three occasions (e.g. during an exacerbation) prior to assuming that the patient presents a non-T2 asthma. | R2 |
| 7.10. In the treatment of T2 SUA, on the basis of the level eosinophils in the peripheral blood and sputum, and the presence of relevant allergic clinical manifestations with confirmed sensitization to perennial aeroallergens, one or other of the available monoclonal antibodies will be chosen: omalizumab, mepolizumab, reslizumab, or benralizumab. | R1 |
| 7.11. In case of non-T2 asthma, treatment with azithromycin or bronchial thermoplasty or systemic glucocorticoids is recommended. | R2 |
| 7.12. In In children 6 years of age or older, depending on the inflammatory phenotype, one or the other of the monoclonal antibodies, omalizumab, mepolizumab or dupilumab, will be chosen. | R1 |
Asthma and chronic obstructive pulmonary disease (COPD) are two different chronic respiratory diseases,897 although it is common to find the characteristics of both diseases in a single patient.898
Asthma and smoking,899,900 low pulmonary function in childhood,901 exposure to irritants902 or environmental contamination903 can contribute to the development of associated COPD in adulthood.
The GesEPOC-GEMA consensus defines asthma-COPD overlap syndrome (ACOS) as the presence of persistent chronic airflow limitation (CAL) (crucial for diagnostic confirmation), in a current smoker or ex-smoker patient (main risk factor), who presents characteristics of asthma (clinical, biological or functional).904
Different definitions of ACOS have been proposed,905–912 the most recent of which are based on two types of patients:
- •
Patients with asthma who smoke and develop chronic airway obstruction.
- •
Patients with COPD and eosinophilia.904,911,913,914
The prevalence of ACOS varies according to the source considered and criteria used for definition,915–917 with estimates between 1.6% and 4.5% in the general population, and between 15% and 25% in patients with obstructive respiratory disease.907,918–932
Patients with ACOS have more symptoms, poorer quality of life, higher risk of exacerbations, more accelerated loss of pulmonary function, higher incidence of comorbidities and greater consumption of healthcare resources905,906,927,933–937 as compared to patients with asthma or COPD, but a better survival when treated with inhaled glucocorticoids (ICS).907,919,938,939
The mortality of chronic respiratory disease is higher in patients with ACOS or COPD than in those without chronic airway obstruction.940–942
8.1.2Diagnostic confirmationThe following sequential diagnostic evaluation is proposed (fig. 20):913,943
- •
To confirm that the patient meets criteria for COPD (> 35 years, smoker >10 pack-years, post-bronchodilation forced expiratory volume in one second/forced vital capacity [FEV1/FVC] <70% [assessing the lower limit of normal, particularly at extreme ages]).909,944
- •
If the patient also meets criteria for asthma,909,945 ACOS is confirmed.
Diagnostic confirmation of asthma and COPD overlap syndrome (ACOS).
*Maintain after treatment with ICS/LABA (6 months). In some cases added after a course of oral steroids (15 days). ACOS: asthma-COPD overlap syndrome; ICS: inhaled glucocorticoids; LABA: long-acting ß2- adrenergic agonist; BDT: bronchodilation test; LNN: lower limit of normal.
If the patient does not meet complete criteria for asthma, the presence of a very positive bronchodilation test (FEV1 post-bronchodilation ≥ 15% and 400ml) or blood eosinophilia (≥ 300 eosinophils/μl), confirms the diagnosis of ACOS.
8.1.3TreatmentAlthough the initial treatment does not differ between patients with pure asthma and those with overlap syndrome, in patients with COPD, a diagnosis of ACOS predicts the response to ICS.946,947 There are proposals for the treatment of ACOS according to its treatable features948,949 that should be agreed upon.
Therapeutic recommendations in patients with ACOS
- •
If the diagnostic evaluation only confirms asthma, it will be treated according to GEMA guidelines,943 avoiding monotherapy with long-acting ß2-adrenergic agonist (LABA).
- •
If the diagnostic evaluation only confirms COPD, it will be treated according to GesEPOC guidelines944 avoiding monotherapy with ICS.
- •
If the evaluation confirms ACOS: start with a combination of ICS at low or moderated doses according to symptoms,950 associated with a LABA.951–955
- •
In case of persistence of exacerbations or relevant symptoms, it is recommended adding a long-acting muscarinic antagonist (LAMA).956,957
- •
Treatment of comorbidities.
- •
Treatment with biologics: the role of omalizumab958–963 or anti-leukin-5 (anti-IL-5) (benralizumab964,965 or mepolizumab963,966,967) in ACOS still remains unclear.968
- •
Other treatments (when necessary): smoking cessation, respiratory rehabilitation, oxygen therapy.
- •
Patients should be referred to specialized consultation in case of lack of response or partial response to the prescribed treatment.
- •
Periodic follow-up assessments should be established.
Asthma is the most common respiratory disease in pregnancy and affects between 2% and 13% of all pregnant women.969 Up to 18% of pregnant women with asthma experienced worsening of asthma during gestation, increasing to 50% in case of severe asthma.969–971 This may be due to hormonal and mechanical changes, fear by pregnant women to use medications and the degree of previous control of the disease.972
8.2.1Effects of asthma on pregnancyAlthough the risk is low, pregnant women with asthma may present maternal and fetal complications. Poor asthma control is associated with prematurity, loss of pregnancy, low birthweight, and increased perinatal mortality, whereas in the mother there is an increased risk of pre-eclampsia, placenta previa, and gestational diabetes.973,974 Also, asthma exacerbations during pregnancy are associated with a higher risk of complications during the gestational period, adverse perinatal events, and respiratory conditions during early infancy in their children.975 Prevention of asthma exacerbations is essential for reducing the risk of complications.976
Poor adherence to treatment977 and upper respiratory tract infections are the most common trigger factors for exacerbations.969 Women with other comorbidities, such as rhinitis, obesity, sudden increase of body weight during the first trimester of gestation, and smoking habit have a poorer control of asthma during pregnancy.978,979
8.2.2Treatment of asthma in pregnancyVirtually all drugs used in the treatment of asthma cross the placental barrier; however, the advantage of treating asthma during pregnancy outweighs the potential shortcomings of the use of medication.969,972,978,979
The appropriate use of ICS, LABA, montelukast, and theophylline is not associated with an increase of fetal abnormalities.980
ICS prevent asthma exacerbations during pregnancy.981
Budesonide and other ICS are safe drugs.982,983 A study carried out in 2014 in neonates born from mothers treated with inhaled budesonide during pregnancy did not show a higher rate of teratogenesis (3.8%) as compared to the general population (3.5%).984
Although safety studies of ß2-agonists during pregnancy are not totally conclusive, and a recent study revealed a slightly higher risk for the incidence of cleft palate and gastroschisis985 but advice against the use of these compounds has not been established.986
Oral glucocorticoids (OCS) cause teratogenic effects, and their use should be restricted to asthma exacerbations and severe asthma.987 A study carried out in 250 pregnant women with asthma treated with omalizumab did not reveal a high risk of congenital malformations.988 However, starting the administration of omalizumab during pregnancy is not recommended because of the risk of anaphylaxis.988,989
The same algorithms for the treatment of exacerbations in non-pregnant women with asthma should be followed, but ensuring an adequate oxygenation (SaO2 >95%) and monitoring of the fetus.969,972
Control of asthma and prevention of exacerbations can be improved during pregnancy using measurement of FENO, questionnaires such as the Pregnancy Asthma Control Test (p-CAT) or the Asthma Control Questionnaire (ACQ) or telehealth.990–993
8.3Occupational asthmaOccupational asthma (OA) is asthma induced by work exposure and caused by agents exclusively found in the workplace (table 53). It is the most common occupational respiratory disease and the risk attributable to workplace exposure is 10% to 25%; it has been estimated that this etiology is present in one out 6 adults with asthma.996,997
Causative agents of occupational asthma.994,995
| Class | Agent | Jobs/activities at risk of exposure |
|---|---|---|
| High molecular weight | ||
| Animals | Mites, rats, crustaceans, mammal dander, etc. | Laboratory workers, farmers, veterinarians, seafood processors |
| Cereals and flours | Cereal powders, wheat, barley, oats, com | Bakery, baker's shop, pastry-making, beer industry |
| Enzymes | Amylase, alcalase | Pharmaceutical companies, baker's shops |
| Latex | Latex | Healthcare personnel |
| Low molecular weight | ||
| Diisocyanates | Toluene diisocyanate (TDI), methylene diisocyanate (MDI) and hexamethylene diisocyanate (HDI) | Polyurethane foams, varnish, plastics, insulators, gun spray painting |
| Acid anhydrides | Phthalic acid, trimellitic acid, maleic anhydride, trimellitic anhydride | Resins and plastics, chemical and adhesive industries |
| Metals | Nickel, platinum, cobalt, chrome, stainless steel salts | Platinum refinery, polishers, grinding, tanners |
| Sanitary ware | ||
| Glutaraldehyde and chlorhexidine | Carpentry, electronic welding | |
| Red cedar and tropical wood | ||
| Biocides | Penicilin, spiramycin, tetracycline | Pharmaceutical industry |
| Nickel, platinum, cobalt, chrome, stainless steel salts | Platinum refinery, polishers, grinding, tanners | |
| Antibiotics | Glutaraldehyde and chlorhexidine | Sanitary ware |
| Irritants | ||
| Bleach/hydrogen chloride | Chlorine, ammonia, CIH | Cleaning |
| Smokers | Smokes | Firefighters |
| Gases | NO2, SO2, ozone | Metallurgy, agriculture |
| Other | Resin, acetic acid, caustic soda | Sanitary ware, chemical industry |
- •
Immunological OA: induced by sensitization to specific agents which are present in the workplace, through a mechanism associated with a specific immunological response.994 High molecular weight (HMW) agents (proteins or glycopeptides >10 kDa) causing production of specific IgE and the typical allergic response are the most common. Low molecular weight (LMW) agents are chemical products causing asthma through an unclear mechanisms suggesting sensitization. OA induced by HMW agents is associated with rhinitis and conjunctivitis and characterized by an earlier reaction, whereas OA induced by low molecular weight agents presents higher bronchial hyperreactivity and more severe clinical manifestations.998,999
- •
Non-immunological OA: induced by irritants in the absence of sensitization.1000 The reactive airways dysfunction syndrome (RADS)1001 is the most representative form of this type of asthma. The term irritant-induced asthma is currently used, which includes cases of asthma occurring after one or more exposures to high concentration levels of irritants.1002
- •
Exposure level: the higher the level, the greater the risk of developing asthma caused by both HMW or LMW agents.1003,1004
- •
Atopy: particularly in those exposed to HMW agents.1005
- •
Rhinitis: often accompanying or preceding asthma produced by HMW.995,1006
- •
Tobacco: an association may exist with the development of asthma caused by HMW and LMW agents, which act through an IgE-mediated mechanism.1007
The diagnosis of asthma and its relationship with the patient's workplace should be confirmed.1000 Diagnostic tests are shown in table 54 and the diagnostic algorithm is presented in fig. 21. Methacholine challenge test has a high negative predictive value for the diagnosis of OA due to its high sensitivity (87-95%), in particular, if the patient has been recently exposed, but the specificity is low (36-40%).1012,1013
Diagnostic tests in occupational asthma.
| Diagnostic tests | Diagnostic value |
|---|---|
| Clinical and work history | Essential but low positive predictive diagnostic value1008. |
| Immunological tests | – IgE sensitization → intradermal tests/prick testIdentify the Allergen.– Positivity only indicates that sensitization exists995. |
| PEF monitoring: working vs. non-working period | – Sensitivity: 81-87%.– Specificity: 74-89%.1009 |
| Non-specific bronchial hyperresponsiveness: working vs. non-working period | – Associated to PEF monitoring.– Added value but with no increase neither in sensitivity or specificity.1010 |
| Induced sputum | – Eosinophilic pattern in most cases (> 3%).– Improves the sensitivity of specific bronchoprovocation test.1000 |
| Fractional exhaled nitric oxide (FENO) | - Additional information to the specific bronchoprovocation test if induced sputum is not available.1000 |
| Specific bronchial provocation test | – Inhalation of the suspicious agent at increasing doses.– Serial FEV1 monitorization.– Is the most reliable and the reference test to confirm OA.1011 |
Diagnostic algorithm of occupational asthma.
OA: occupational asthma; RADS: reactive airway dysfunction syndrome; SBPT: specific bronchial provocation test; PEF: peak expiratory flow. *Measurements performed after 15 days of a working period and 15 days of sick leave; sputum: analysis of the change in the number of eosinophils.
Bronchial provocation using the specific agent is the most accepted test for the diagnostic confirmation of OA.1014
8.3.4TreatmentThe patient with OA caused by a sensitizing agent should be removed from the source of exposure.1010,1015 Workers with irritant-induced asthma may continue to work provided they are transferred to lower exposure areas together with the implementation of industrial hygienic measures to reduce exposure.
In approximately 70% of patients, asthma symptoms and bronchial hyperresponsiveness (BHR) persist for several years after having been removed from the site of exposure.994
8.4Physical exercise-induced asthmaExercised-induced asthma is defined as a narrowing of the lower airways that is triggered by strenuous physical exercise.1016
Exercise-induced bronchoconstriction is more frequent among patients diagnosed with asthma, but may be also be present in non-asthmatic subjects.1017,1018
Exercise-induced asthma is more common in patients with poorly controlled asthma.1019,1020
Exercise-induced asthma is caused by the increased osmolarity at the airway surface due to cooling and dehydration induced by hyperventilation.1021
It is associated with the release of mediators, such as prostaglandins, leukotrienes and histamine. Exercise-induced asthma may be the expression of a genetic predisposition and the environmental interaction of pollutants and the resulting oxidative stress,1022 among other factors.
The prevalence is higher in athletes, children and adolescents, females, urban environments, and among Afro-Americans and Asiatics.1023,1024
Symptoms (cough and dyspnea with wheezing) usually occur during or after finishing the exercise, with a 2-3 hour-refractory period after their onset.1025
Self-reported symptoms are unreliable for the diagnosis. The diagnostic test is the finding of a fall of FEV1 over 10% measured 30minutes after cessation of exercise and compared with the previous FEV1 value.1026
Differential diagnosis with laryngeal and glottic disorders should be made as well as with other conditions associated with exercise-induced dyspnea, such as COPD, restrictive pulmonary diseases, obesity, anatomical defects, paralysis of diaphragm, or pulmonary fibrosis.1027
It is necessary to evaluate the degree of control of asthma and to consider the possibility of increasing a therapeutic step.
Occasional use of short-acting ß2-agonists (SABA) approximately 10minutes before exercise1017 is the treatment of choice. However, when used regularly, these agents present a gradual loss of effectiveness.1028,1029
ICS should be added when a continuous treatment with SABA is needed, since this combination reduces both the frequency and intensity of exacerbations.1030
LTRA are a therapeutic option as they have a similar efficacy to LABA for preventing exercise-induced bronchial obstruction, but are not effective when the obstructions has been already established.1031
Increasingly intense warm-up exercise before starting any sports activity may decrease the intensity of bronchoconstriction.1032,1033
Reduction of dietary sodium intake and supplementation with ascorbic acid or fish oil may diminish the severity of exacerbations.1034
8.5Aspirin-exacerbated respiratory disease (AERD)AERD or respiratory disease exacerbated by non-steroidal anti-inflammatory drugs (NSAID) refers to acute development of nasal and/or bronchial respiratory symptoms of any intensity between 30minutes and 3hours after the administration of acetylsalicylic acid (ASA) or other cyclooxygenase-1 (COX-1) inhibiting NSAIDs1013 It can be associated with cutaneous symptoms and hypotension, although this occurs rarely. The prevalence of AERD in the general population is of 0.3-2.5% but increases to 9% in subjects with asthma and is higher than 20% in patients with severe asthma.1035 In patients with concomitant asthma, chronic rhinosinusitis (CRS), and nasal polyposis (NP), the prevalence reaches 40%.1036 Avoidance of NSAID does not resolve asthma or NP.
There is a mechanism of non-IgE-mediated hypersensitivity with dysregulation of the arachidonic acid pathway by 5-LT-C4-synthase followed by overproduction of cysteinyl-leukotrienes (LT-C4, LT-D4, LT-E4) and a reduction of PG-E2.1037 There is inflammation of the mucosa with activated eosinophils and mast cells (in which the enzyme is overexpressed), basophils, and abundant platelets Blockage of COX-1 by NSAID contributes to formation and release of T lymphocytes, and to the release of preformed mediators (PGD2, histamine, and tryptase).1038 Mucous secretion, vascular permeability and bronchoconstriction are rapidly increased. IL-C2 cells of innate immune response are also involved producing type T2 cytokines.1039
8.5.1DiagnosisAERD should be suspected in any subject with asthma, with or without CRS and NP, and confirmed through a detailed clinical history showing a relationship between ingestion of a NSAID and the appearance of respiratory symptoms.1040 At the present time, sufficiently validated in vitro diagnostic tests are lacking. The use of E4 leukotriene concentration in urine (uLTE4) together with clinical findings, slightly improves the diagnostic prediction.1041 The diagnosis is confirmed by means of controlled exposure challenge with a NSAID, preferably ASA. The administration route may be oral, bronchial (inhaled), or nasal. These latter two routes are safer, although negative results do not exclude diagnosis; in this case, the result must be confirmed by using the oral route, which is the definitive test to confirm or exclude the diagnosis of AERD.1042–1044 The diagnostic algorithm of AERD with asthma symptoms is shown in fig. 22.
Diagnostic algorithm of aspirin-exacerbated respiratory disease (AERD) with asthma symptoms.954
L-ASL: lysine-acetylsalicylate.
The medical-surgical treatment of underlying diseases should be considered.1045 Improvement in patients with AERD and moderate or severe asthma after adding LTRAs to the standard treatment1046 or after endoscopic sinus surgery has been reported.1047 In addition, the administration of biological drugs can be useful in the treatment of patients with AERD. Omalizumab significant reduces the use of rescue medication in patients with severe allergic asthma and AERD1048 and leukotrienes (LT) in urine.1049 Also, some patients treated with omalizumab may finally tolerate NSAID, although this possibility should always be confirmed by means of controlled exposure tests.1050 Biological drugs targeting eosinophilic inflammation (mepolizumab,1051 reslizumab,1051 and benralizumab,1052 as well as dupilumab)1053 in patients with asthma and T2-high endotype may be potentially beneficial in patients with AERD.
COX-1 inhibitors should be avoided1054 (table 55). Selective COX-2 inhibitors (celecoxib, etoricoxib, perecoxib)1056 or partially selective COX-2 inhibitors (nabumetone, meloxicam)1057 are recommended, but in all cases after assessment of tolerability by oral controlled exposure testing. Doses of paracetamol higher than 500mg should not be recommended without assessment of tolerance.1040
Classification of some NSAIDs based on their capacity of inhibition of cyclooxygenase isoforms.1055
| Potent COX-1 and COX-2 inhibitors | Acetylsalicylic acid, diclofenac, ibuprofen, metamizol |
| Weak COX-1 and COX-2 inhibitors | Paracetamol |
| COX-2 inhibitors | |
| – Partially selective (dose-dependent COX-1 inhibition)– Highly selective | Meloxicam, nabumetoneCelecoxib, etoricoxib, parecoxib |
In selected cases (patients with uncontrolled severe asthma, recurrent nasal polyposis with several endoscopic sinus surgeries despite receiving appropriate maintenance treatment), ASA desensitization could be considered.1058 It has been shown that ASA desensitization can improve nasal symptoms, asthma control, and quality of life in patients with AERD.1059,1060 Moreover, these effects are maintained over time despite requiring lower doses of ASA,1061 although the procedure is not free from associated adverse effects.1062 The maintenance dose should not be withdrawn, as the therapeutic effect is lost and adverse reactions reappear when taking NSAID.1063 However, the cost-benefit of chronic treatment with high doses of NSAID should be evaluated. While this treatment is maintained, the patient can also tolerate other NSAIDs different from ASA.1064
Both challenge and desensitization tests are not routine techniques and should be performed by qualified personnel and with the adequate equipment for the control reactions.1045
8.6Inducible laryngeal obstructionThe ERS/ELS/ACCP Working Group has defined inducible laryngeal obstruction (ILO), formerly known as vocal cord dysfunction, as a condition that causes sudden respiratory difficulties secondary to an obstruction of the airway at the level of the glottic or supraglottic larynx. These attacks are characterized by the presence of dyspnea, stridor of laryngeal origin, and other symptoms such as cough, pharyngeal globe or dysphonia.1065
The term inducible refers to the mechanism by which the obstruction crisis is triggered, which can include physical exercise or the presence of external (odors, chemicals) or internal (gastroesophageal reflux) irritants.
Its presentation may suggest an asthma exacerbation episode, as well as other laryngeal diseases such as paralysis or dystonia. Its association with asthma is possible, which makes the diagnosis difficult. ILO is observed in about 25% of individuals with asthma, with a trend towards a higher frequency in cases of severe asthma.1066
As triggers for ILO episodes, mechanical factors (talking, shouting, and swallowing) and the smell of vinegar are more frequent than exposure to pollens and humidity, which are more characteristic of asthma exacerbation.1067
Clinical suspicion is essential for the diagnosis of ILO. There are questionnaires that can help to distinguish between asthma and ILO.1068 Flattening of the inspiratory portion of the flow-volume loop is of little value in the diagnosis of ILO,1069 but it may be suggestive. The diagnosis is confirmed by laryngeal videoendoscopy showing paradoxical adduction of the larynx during inspiration, or less frequently, during expiration. A challenge test with exercise or inhalation of mannitol or methacholine is usually required.1070
The use of dynamic computerized tomography (CT) to demonstrate paradoxical laryngeal closure during attacks has been recently proposed.1066
In the acute phase of ILO, respiratory techniques for controlling inspiratory flow may be useful. Mild sedatives (ketamine, benzodiazepines) have shown to be of help, as well as inhaling heliox (a mixture of helium and oxygen) or non-invasive ventilation.1071
Long-term treatment aims to reduce the intensity and frequency of attacks. The first step includes logophoniatric rehabilitation focused on breathing techniques and relaxation of the laryngeal muscles.
In refractory cases or in patients who are not candidates for logophoniatric rehabilitation, infiltration of thyroarytenoid muscles with botulinum toxin may be used.1072
In selected cases of supraglottic ILO, transoral laser surgical techniques have been used successfully.1073
There is no solid evidence for the indication of tracheostomy in these patients; however, some single case reports have been published.1074
8.7Asthma and the coronavirus disease 2019 (COVID-19)COVID-19 is caused by the betacoronavirus SARS-CoV-2. This airborne infection has high transmissibility and within a few weeks from the outbreak (Wuhan [Hubei, central China]) in December 19, it became a serious pandemic.1075
The disease has a broad clinical spectrum from mild forms with a few symptoms (or asymptomatic) to influenza-like symptoms (fever, cough, myalgia, asthenia) and severe forms with bilateral pulmonary infiltrates and severe acute respiratory failure (5-20%), and in some cases, causing death (2.3-3.8%).1076–1081 The disease is less common in children,1082 in which there is a different transmission pattern and only a minority of index cases (< 20%) is identified in the pediatric age.1083 Clinical manifestations are usually milder, although infants may be more vulnerable.1084–1086
The available evidence indicates that suffering from (non-severe) asthma or allergy is not associated with a greater probability of developing, becoming more severe or dying from COVID-19.1081,1087–1091 However, in severe asthma the information available is ambivalent, some series confirmed a higher morbimortality,1092,1093 or with exacerbations in the previous year,1094 while other did not.1095,1096
It is recommended not to perform pulmonary function and induced sputum tests in infected patients and in the entire population during the waves of the pandemic (peaks). However, outside of them, they can be carried out following certain biosafety standards.1097–1099
In the treatment of patients with asthma infected by SARS-CoV-2, nebulizers will not be used for the aerosolization of drugs (but devices coupled to spacers or inhalation chambers), nor non-invasive single-limb ventilator equipment and without bacterial filter placed before the outlet port.1100–1102
There is no evidence of the deleterious effect of maintenance treatments for asthma, particularly ICS, on the prognosis of COVID-19. Therefore, patients should continue to take previously prescribed medications for their asthma. Systemic glucocorticoids should even be administered in case of exacerbations.
On the other hand, some in vitro studies have shown that ICSs reduce the replication of SARS-CoV-2 in respiratory epithelial cells.1103 In addition, the data from in vivo studies seem to suggest that treatment with ICS may offer some protective capacity against infection.1104,1105
Although the information available is limited, there could be some pharmacological interaction between some of the drugs used to treat the infection and those for the treatment of asthma (table 56).1104,1105 Very close clinical monitoring is recommended when these drugs are administered together. It is possible that in some cases it would be necessary to consider up or down dose adjustments (table 56).1106
Possible pharmacological interactions between drugs used for the treatment of COVID-19 and those used for asthma (based on the proposals from the Neumo SEFH Group 2020).1106
Patients with severe asthma on treatment with biological drugs do not present a greater severity of SARS-CoV-2 infection.1095,1096
However, in case of infection, it is advisable to postpone the administration of the biological agent until its resolution.
There is no evidence against vaccination for COVID-19 in patients with asthma. It would be contraindicated in patients with previous anaphylaxis reactions to the vaccine or any of its components.1107
8.8Allergic bronchopulmonary aspergillosis (ABPA)8.8.1Concept and definitionABPA is a respiratory disease due to a hypersensitivity reaction caused by bronchial colonization by Aspergillus fumigatus, which mainly affects patients with asthma and/or cystic fibrosis (CF).1108
Repeated inhalation of Aspergillus fumigatus spores generates a type I (IgE-mediated), type III (IgG-mediated immune complex), and type IV (cell-mediated) hypersensitivity response in susceptible hosts without tissue invasion.1109,1110
The Prevalence of ABPA ranges between 0.7-3.5% in asthma and 9% in CF.1111,1112
The most frequent symptoms are cough, respiratory distress, expectoration of mucus plugs, wheezing and more rarely chest pain or hemoptysis.1109,1113,1114
8.8.2Diagnostic confirmationThe diagnosis of ABPA is based on the combination of clinical, radiological, and laboratory data. The ISHAM criteria of 2013, updated in 2016, are currently the most widely used1114,1115 (table 57).
Diagnostic criteria ISHAM 2013, updated 2016.
| Predisposing conditions |
| – Asthma.– Cystic fibrosis.– COPD.– Post-tuberculous fibrocavitary disease. |
| Obligatory criteria (both should be present) |
| – A. fumigatus-specific IgE >0.35 kUA/l (or positive cutaneous reaction if specific IgE is not available).– Elevated serum total IgE >1000 IU/ml (if patients fulfill all “other criteria”, an IgE value <1000 IU/ml can be accepted). |
| Other criteria (at least two of three) |
| – A. fumigatus-specific serum IgG antibodies >27 mgA/l.– Total eosinophilia >500 cells/μl in steroid naïve patients.– Radiograhic lung opacities consistent with ABPA. |
More recently, Asano et al.1116 have proposed new diagnostic criteria for aspergillosis/allergic bronchopulmonary mycosis, with greater sensitivity and specificity compared to those of Patterson and Rosenberg and those of the ISHAM, even in atypical cases without asthma or without positive cultures for Aspergillus.
Total IgE serum level is a useful test both in the diagnosis and in the follow-up of ABPA. The most widely used cut-off point for diagnosis is >1,000 IU/ml1117,1118 and it decreases with treatment.1119
Most patients exhibit Aspergillus sensitization demonstrated by skin testing. Specific IgE >0.35 Ku/l has a sensitivity of 100% and a specificity of 66.2%.1114
Specific IgG against A. fumigatus is present in 69-90% of patients with ABPA. A cut-off point of 27mg/l has high sensitivity and specificity.1118
In relation to peripheral eosinophilia, a blood eosinophil count >500 cells/l has been considered to be a relevant criterion for diagnosis.1120
The most common finding on chest radiographs are infiltrates, whereas bronchiectasis, mucoid impactation, centrilobular nodules, and tree-in-bud opacities among others are frequent findings on chest CT.1121 The presence of high-attenuation mucus (HAM) visually denser than the paraspinal skeletal muscle is a pathognomonic finding of ABPA.
8.8.3ClassificationOn the basis of high-resolution CT findings of the lung, the disease is classified into:
- •
Serological ABPA.
- •
ABPA with bronchiectasis.
- •
ABPA with high-attenuation mucus plugs.
- •
ABPA with chronic fibrosis1122 (table 58).
Table 58.Classification of ABPA based on radiological findings.
– ABPA-S (serological ABPA): fulfills the diagnostic criteria of ABPA with an absence of radiological manifestations.– ABPA-B (ABPA with bronchiectasis).– ABPA-HAM (ABPA-High attenuation mucus): ABPA with high-attenuation mucus plugs in high-resolution CT– ABPA-CPF (ABPA-Chronic pleuropulmonary fibrosis): fulfills the diagnostic criteria of ABPA with at least two radiological features suggestive of fibrosis (including fibocavitary lesions, pulmonary fibrosis, pleural thickening) without the presence of mucoid impaction.
Depending on the response to glucocorticoids, it is classified into 5 stages:
- •
Acute.
- •
Remission.
- •
Exacerbation.
- •
Glucocorticoid-dependent asthma.
- •
Fibrotic lung disease.1115,1123
The aims of treatment of ABPA is to control symptoms, prevent exacerbations, and limit loss of pulmonary function and structural damage especially bronchiectasis.
A reduction of IgE levels of 25-50% correlates with clinical and radiological improvement.1119
Systemic glucocorticoids are the primary therapy for ABPA. The steroids help to relieve the symptoms and decrease airflow obstruction, decrease serum IgE and reduce peripheral blood eosinophils.1108
Prednisolone is a commonly used drug for treatment, 0.5 to 1mg/kg/day for 2 weeks, followed by 0.5mg/kg every other day for 8 weeks. A subsequent taper (by 5mg every 2 weeks) over the 3 to 5 months1124 or 0.75mg/kg/day for 6 weeks followed by 0.5mg/kg/day for 6 weeks, and subsequent taper by 5mg every 6 weeks and suppression in 8-10 months.1113,1125,1126
It is advisable to add antifungal treatment with itraconazole (200mg twice daily for 26 weeks) or voriconazole to decrease the fungal load and subsequent suppression of inflammatory response.1127,1128
There are no randomized double-blind placebo-controlled trials to establish the efficacy of biologics in the treatment of ABPA. However, the involvement of type I (IgE-mediated) hypersensitivity response and Th2 pathway suggest that they could have a prominent role.
Treatment with omalizumab has been associated with an improvement of asthma symptoms, reduction of FENO levels, exacerbations, serum IgE, and requirements of the doses of glucocorticoids.1129
Single cases reports of the combination of omalizumab and mepolizumab have been published.1130
The evidence with mepolizumab1131 and benralizumab1132,1133 is limited.
Some cases have been published with sequential treatments due to a lack of response to the initial biological treatment. After failure of omalizumab, there have been cases of response to mepolizumab1134 or benralizumab;1135 or initial failure of mepolizumab with posterior response to benralizumab.1136
8.9Eosinophilic granulomatosis with polyangiitis (EGPA)8.9.1Concept and definitionEGPA (formerly known as Churg-Strauss syndrome) is a rare form of vasculitis and according to the Chapel Hill Consensus Conference (CHCC 2012) included in the group of vasculitis associated with antineutrophil cytoplasmic antibodies (ANCA).
It is characterized by eosinophil-rich necrotizing granulomatous inflammation and necrotizing vasculitis of small and medium-sized blood vessels associated with eosinophilia and asthma.1137
EGPA has been recently considered an emergent clinical variant of ANCA-associated vasculitis, in which a Th2 autoimmune response is involved in its pathogenesis.1138
In general, ANCA are positive in 30-47% of patients with EGPA and are usually p-ANCA (MPO-ANCA). Rarely, c-ANCA are seen, which difficults the differential diagnosis with granulomatosis with polyangiitis (formerly known as Wegener's disease).1139
The incidence of EGPA is lower than 4 cases per million person-years and the prevalence is lower than 31 cases per million person-years.1140
It is estimated that the age at onset ranges between 40-59 years and at the time of diagnosis, history of asthma of more than5 years is present in more than 90% of patients.1141 Some studies have shown a higher prevalence in women than in men.1142
Clinical manifestations of EGPA are heterogeneous and the clinical course is characterized by three phases: prodomal (asthma, rhinosinusitis, and nasal polyposis), eosinophilic (peripheral eosinophilia and organ involvement), and vasculitis (clinical manifestations of vasculitis of small-sized vessels). These three phases are not necessarily always present, they may even overlap, or be absent in some patients.1143,1144
Asthma in EGPA is usually severe in more than 65% of patients, glucocorticoid-dependent, and precedes to the systemic disease.1140
The presence of severe refractory and/or late-onset asthma associated with eosinophilia >10% or pulmonary infiltrates requires ruling out EGPA.
8.9.2Diagnostic confirmationAlthough biopsy (lung, nerve or skin) is the reference procedure for the diagnosis of EGPA, most patients are diagnosed according to clinical criteria.1145
The diagnosis of EGPA is based on the combination of clinical, radiological, and laboratory criteria. The presence of at least four out of the six criteria proposed in the classification of the American College of Rheumatology (ACR) in 19901146 (table 59) renders an 85% sensitivity and 99.7% specificity. This classification does not include the determination of ANCA, which can lead to overlaps with other vasculitis conditions.
Diagnostic criteria of EGPA according to the American College of Rheumatology 1990.1146
| The presence of at least four out of the six criteria: |
| 1. Asthma.2. Eosinophilia (> 10% total leukocyte count)3. Neuropathy (mono- or polyneuropathy)4. Migratory infiltrates in lungs.5. Paranasal sinus abnormalities.6. Extravascular eosinophilic infiltration in biopsy. Histological features of vasculitis. |
Recently, the ACR/European Alliance of Associations for Rheumatology (EULAR) validated criteria to help differentiating EGPA for other small- or medium-vessel vasculitis once other mimicking diseases have been excluded (table 60). A score of >6 points allows classifying vasculitis as EGPA with a sensitivity of 85% (95% CI: 77 to 91) and a specificity of 99% (95% CI: 98 to 100).
Classification criteria of EGPA according to the American College of Rheumatology/European Alliance of Associations for Rheumatology 2022.1147
| A cumulative score ≥ 6 points is necessary to classify EGPA as a small- or medium-vessel vasculitis* | |
| Clinical criteria | |
| • Obstructive airway disease | +3 |
| • Nasal polyps | +3 |
| • Mononeuritis multiplex | +1 |
| Laboratory and biopsy criteria | |
| • Blood eosinophil count >1×109/liter | +5 |
| • Biopsy with extravascular eosinophilic predominant inflammation | +2 |
| • Cytoplasmic antineutrophil cytoplasmic antibody(c-ANCA) or anti–proteinase 3 (anti-PR3) positivity | -3 |
| • Hematuria | -1 |
In an appropriate clinical context, the high diagnostic specificity of a positive ELISA test for MPO-ANCA or PR3-ANCA can obviate the need for biopsy.
Patients with positive ANCA (usually MPO-ANCA) typically have more renal and peripheral nerve involvement, whereas cardiomyopathy and possibly pulmonary infiltrates are more common in ANCA-negative cases.
Multiple prospective and retrospective studies have confirmed cardiac involvement as one of the most important risk factor for specific mortality in patients with EGPA (standardized mortality rate 3.06).1144,1146
The 2021 ACR and the Vasculitis Foundation guideline defined EGPA as active disease in the presence of new, persistent clinical signs and/or symptoms and not related to prior damage; severe disease in the presence of vasculitis with life- or organ-threatening manifestations (e.g. alveolar hemorrhage, glomerulonephritis, central nervous system vasculitis, mononeuritis multiplex, cardiac involvement, mesenteric ischemia, limb/digit ischemia); and non-severe disease in the presence of vasculitis without life- or organ-threatening manifestations (e.g., rhinosinusitis, asthma, mild systemic symptoms, uncomplicated cutaneous disease, mild inflammatory arthritis).1148
The update of the EULAR recommendations for the management of ANCA-associated vasculitis has recently been published, which classifies the disease for treatment purposes according to the presence of vital organ involvement or not (instead of severity).1149
8.9.3TreatmentWhen deciding on treatment, it is important to consider the severity and whether EGPA is active or in remission.
The treatment is divided into two parts: 1) induction phase to achieve remission, and 2) maintenance phase.
Recently, the 2021 ACR/VF guideline has established new recommendations, the majority of which are conditional, for the treatment of EGPA (fig. 23), in particular:
- a.
Induction of remission for active and severe EGPA treatment with doses of oral glucocorticoids (1mg/kg/day) or intravenous pulse (e.g. methylprednisolone i.v. 500mg/day for 3-5 days followed by oral glucocorticoids with progressive reduction up to 10mg or less), generally associated with cyclophosphamide (2mg/kg/day orally or 15mg/kg i.v. every 2 weeks for 3 doses, followed by 15mg/kg every 3 weeks for 3 doses).1148–1151
Experiences with rituximab have been published for refractory forms of EGPA,1152,1153 but controlled studies are lacking. It has been used in EGPA with renal involvement, but its efficacy is less established compared to other ANCA-associated vasculitis (especially if ANCA-negative), with cases of recurrent nasal and sinus disease and refractory asthma despite treatment with rituximab. The 2021 ACR/VF guideline consider rituximab in the following situations: ANCA-positive patients, glomerulonephritis, failure of cyclophosphamide treatment, or to preserve fertility.1148
- b.
For patients with active non-severe EGPA, induction of remission treatment includes the association of glucocorticoids (prednisone 0.5-1mg/kg/day with progressive reductuion)1145 with mepolizumab, and the association of azathioprine, mycophenolate or methotrexate as a second choice.
- c.
In patients with severe EGPA who achieve remission with cyclophosphamide (generally 3-6 months), the maintenance treatment recommended includes oral glucocorticoids at doses of 10mg or less, associated with azathioprine (2mg/kg/day), methotrexate (7.5mg/week oral or subcutaneous with 2.5mg increases every week up to a maximum dose of 20-25mg/week accompanied by folic acid 1mg/day), or mycophenolate (experience in case series only) for 12-18 months or at long-term in case of frequent relapses.999,1011,1043,1103,1105
- d.
In patients with non-severe EGPA with relapse on treatment with azathioprine, methotrexate or mycophenolate, the use of mepolizumab is recommended.
Treatment of EGPA.
OCS: oral glucocorticoids; RTX: rituximab; AZA: azathioprine; MTX: methotrexate; MMF: mycophenolate mofetil. *Severe: alveolar hemorrhage, glomerulonephritis (GNF), central nervous system vasculitis, mononeuritis multiplex, cardiac involvement, mesenteric ischemia, limb/digit ischemia. **Non-severe: rhinosinusitis, asthma, mild systemic symptoms, uncomplicated cutaneous disease, mild inflammatory arthritis. †RTX in cases of ANCA+, GNF, failue of cyclophosphamide, and to preseve fertility.
‡In selected cases consider OCS only. Modified from the 2021 ACR/VF guideline.1148
Regarding mepolizumab both the FDA and the EMA have approved its use at doses of 300mg subcutaneously every 4 weeks as add-on treatment in patients with EGPA aged 6 years and older with relapsing-remitting or refractory disease.1154 In a phase III 52-week randomized placebo-controlled trial in patients with relapsing or refractory EGPA, mepolizumab 300mg subcutaneously added to standard treatment increased the percentage of patients who achieved remission, improved their quality of life, and decreased the doses of oral glucocorticoids as compared with placebo.1155
Severe uncontrolled asthma may persiste even after conventional treatment of EGPA, being a reason for the indication of mepolizumab.
8.10Idiopathic hypereosinophilic syndrome8.10.1Concept and definitionIdiopathic hypereosinophilic syndrome (HES) is a rare disease characterized by bone marrow overproduction of eosinophils resulting in persistent high blood eosinophil levels (≥ 1500 cells/μl) with involvement of tissues and organs, without a known underlying cause.1156,1157
The diagnosis of idiopathic HES requires excluding other secondary causes of hypereosinophilia (parasitosis, drugs, entities such as EGPA, ABPA, eosinophilic pneumonia, etc.) including myeloid/lymphoid neoplasms with eosinophilia associated with FIP1L1-PDGFRA or PDGFRA, PDGFRB, FGFR1, PCM-JAK2 mutations.1158,1159
The incidence of all types of HES is estimated between 0.04-0.17 per 100,000 person-years and the prevalence is 0.15-6.3.1159,1160
In patients with chronic or persistent tissue infiltration, the release of effector molecules by activated eosinophils can result in tissue damage and organ dysfunction.1161
The clinical presentation is variable, the most frequent symptoms are cutaneous (intractable pruritus, urticaria, angioedema), pulmonary (asthma, sinusitis, pulmonary infiltrates), gastrointestinal, cardiovascular (endomyocardial fibrosis, thromboembolism), and neurological.1157,1162–1164
8.10.2Diagnostic confirmationThe diagnosis of idiopathic HES is based on the involvement and/or dysfunction of a systemic organ, directly related to an eosinophil count ≥ 1,500/μl in two or more determinations separated by 4 weeks and/or tissue eosinophilia without a secondary cause.1161
8.10.3TreatmentThe goal of treatment is to achieve sustained reduction in blood and tissue eosinophil counts to reverse and prevent damage and improve symptoms.1158
Treatment of idiopathic HES includes systemic glucocorticoids and cytotoxic and/or immunosuppressive drugs. However, they can fail to achieve complete remission of the disease and have important side effects.1165,1166
Patients receiving standard treatment for HES frequently have periods of poor disease control, with worsening symptoms and increased eosinophil counts, sometimes with organ involvement.1159
Previous studies with mepolizumab (administered at a dose of 750mg i.v. every 4 or more weeks) demonstrated a reduction in the dose of glucocorticoids and eosinophil count, with good tolerance.1167–1171
A phase III randomized, double-blind, placebo-controlled study for 32 weeks demonstrated that mepolizumab (300mg subcutaneously every 4 weeks), added to existing HES therapy, reduced disease flares in patients with uncontrolled FP1L1-PDGFRA negative disease (defined as ≥ 2 flares within the past 12 months and a blood eosinophil count ≥ 1000 cells/μL) compared with placebo. The proportion of patients who experienced one or more flares during the study was 50% lower in the mepolizumab group than in the placebo group (15 of 54 [28%] vs. 30 of 54 [56%]; p=0.002); and the relapse rate was 66% lower with mepolizumab compared with placebo (0.50 vs 1.46 flares per year, respectively; p<0.001). A 92% reduction in eosinophil count was also observed.1172
An open-label extension study in patients with FIP1L1-PDGFRA negative HES who had been previously treated with placebo or mepolizumab in the phase III trial and received mepolizumab 300mg subcutaneously every 4 weeks for 20 weeks, confirmed the reduction of flares, need of OCS, and blood eosinophil count also in the long-term.1173
4.7Recommendations| 8.1. The diagnosis of ACOS will be establish in patients with persistent chronic airflow limitation, current smokers or ex-smokers, with documented diagnosis of asthma, or in whom there is a very positive bronchodilation test or eosinophilia. | R2 |
| 8.2. All patients with ACOS will be initially treated with a combination of ICS and LABA. | R2 |
| 8.3. In patients with ACOS treated with a combination of ICS and LABA who remain symptomatic or with exacerbations, a LAMA will be added. | R2 |
| 8.4. E Drugs usually administered, LABA plus ICS, are recommended for the maintenance treatment of asthma in pregnant women. | R1 |
| 8.5. In the treatment of exacerbations in pregnant women the same algorithms than in non-pregnant women should be followed, ensuring adequate oxygenation (SaO2 >95%) and monitoring of the fetus. | R1 |
| 8.6. In order to reduce the risk of maternal and fetal complications, pregnant women with asthma should be adequately controlled for preventing severe exacerbations. | R1 |
| 8.7. In adult-onset asthma or if there is a deterioration of previous asthma, it is recommended to exclude occupational asthma. | R2 |
| 8.8. The diagnosis of occupational asthma should be confirmed by objective tests, and in cases of allergic etiopathogenesis, by immunological tests. | R2 |
| 8.9. The specific challenge test is the reference diagnostic test for immunological occupational asthma. | R2 |
| 8.10. In the treatment of immunological occupational asthma, removal of exposure to the causative agent is recommended. | R2 |
| 8.11. In exercise-induced asthma, warm-up exercises before starting the sport activity are recommended. | R1 |
| 8.12. In exercise-induced asthma, SABA used occasionally are the most effective short-term treatment option. | R1 |
| 8.13. In exercise-induced asthma, ICS reduce the frequency and intensity of symptoms, so that its use is advisable in patients usually treated with SABA. | R1 |
| 8.14. In exercise-induced asthma, LTRA is a less effective therapeutic option than ICS for preventing bronchoconstriction and are not useful to reverse an already established obstruction. | R1 |
| 8.15. It is recommended to evaluate the degree of control to determine the need for increasing a therapeutic step in known asthma patients with exercise-induced asthma. | R1 |
| 8.16. In patients with asthma and chronic rhinosinusitis with nasal polyps, it is advisable to exclude aspirin-exacerbated respiratory disease (AERD), particularly in cases of severe asthma. | R1 |
| 8.17. Patients with AERD should avoid receiving treatment any NSAIDs that are COX-1 inhibitors. | R1 |
| 8.18. In the analgesic or anti-inflammatory treatment of patients with AERD, an alternative medication of choice (opiates, systemic corticosteroids) should be used. After demonstrating their tolerability, paracetamol at doses lower than 500mg and selective COX-2 inhibitors (celecoxib, etoricoxib, parecoxib) can be used. | R2 |
| 8.19. In patients with moderate or severe asthma and AERD, adding LTRA should be considered. | R2 |
| 8.20. Desensitization with acetylsalicylic acid may be useful in selected cases. | R2 |
| 8.21. Biological drugs can be used in patients with severe uncontrolled asthma and AERD, especially in the presence of concomitant nasal polyposis. | R2 |
| 8.22. The diagnosis of inducible laryngeal obstruction (ILO), formerly known as vocal cord dysfunction, should be established after clinical suspicion and confirmation by laryngeal videoendoscopy. | R1 |
| 8.23. Treatment of the acute phase of ILO should include respiratory logophoniatric reeducation (laryngeal muscle relaxation) techniques. | R2 |
| 8.24. In the treatment of the acute phase of ILO, sedatives may be useful, whereas type A botulinum toxin or surgery is reserved for refractory cases. | R2 |
| 8.25. It is recommended to rule out ABPA in all patients with severe uncontrolled asthma. | R1 |
| 8.26. For the diagnosis of ABPA in patients with asthma, it is recommended to use a combination of clinical, laboratory, and radiological data following the ISHAM 2016 criteria. | R1 |
| 8.27. In acute ABPA it is recommended to start treatment with glucocorticoids generally associated with antifungals. | R1 |
| 8.28. In case of recurrent exacerbations of ABPA or glucocorticoid dependence, a therapeutic trial with biological drugs is recommended. | R2 |
| 8.29. It is recommended that EGPA should be ruled out in all patients with long-lasting severe uncontrolled eosinophilic asthma. | R2 |
| 8.30. For the diagnosis of EGPA in patients with asthma, it is recommended to use a combination of clinical, laboratory (eosinophil count and ANCA determination in blood) and radiological data. | R1 |
| 8.31. In severe and active EGAP, it is recommended to start treatment with systemic glucocorticoids in pulses or at high oral doses associated with cyclophosphamide or rituximab. | R2 |
| 8.32. In non-severe and active EGAP, it is recommended to start treatment with glucocorticoids associated with mepolizumab as the first choice. | R2 |
| 8.33. It is recommended to rule out secondary causes of hypereosinophilia and myeloproliferative hematological disease before diagnosing idiopathic HES. | R1 |
| 8.34. The diagnosis of idiopathic HES is established (by arbitrary expert criteria) in the presence of involvement and/or dysfunction of a systemic organ, together with an eosinophil count ≥ 1,500/μl in 2 or more determinations separated by 4 weeks, and/or tissue eosinophilia of unknown cause. | R2 |
| 8.35. The first-line treatment of idiopathic HES is glucocorticoids to which immunosuppressants or other glucocorticoid-sparing drugs can be added, but in some patients their effect may be insufficient to reduce eosinophilia. | R1 |
| 8.36. It is recommended to add mepolizumab to glucocorticoid treatment in idiopathic HES with the aim of reducing disease flares, the need for OCS, and the eosinophil count. | R1 |
Healthcare professionals should provide asthma patients with continuous care in order to ensure adequate prevention, diagnosis, control, treatment and follow-up,1174 so that coherence of coordinated healthcare over time (continuity of care)1175 is perceived by the users.
It is a priority to identify the current status of healthcare for patients with asthma1176–1181 to provide solutions in the three types of continuity of care: information (availability of data of previous episodes at different levels of care), relationship (between patients and providers), and management (coordination of actions).1182
The multidisciplinary approach, the coordination between the levels of care, the patient's involvement, and appropriate management of social and healthcare resources are the essential elements to establish an integrated care network that provides quality care to patients with asthma.1183–1185 The involvement of nursing, as demonstrated by the Finish program, is essential to achieve good asthma control.1186 Also, the collaborative practice between physicians and community pharmacists has a positive impact on the patients’ health, improving the knowledge they have of their disease, their quality of life, adherence to treatment, and control of the disease.1187,1188
Actions to be implemented for improving continuity of care in asthma are shown in table 61.
Actions aimed to improve continuity of care in asthma.
| Healthcare professionals | Patients | Administration |
|---|---|---|
| GEMA implementation1180,1189 | Education1190,1191 | National Strategic Plan in Asthma (nonexistent) |
| Coordination between healthcare levels1192,1193 | Adherence to treatment1194,1195 | Integrated healthcare processes1196 |
| Referral criteria established by consensus1193 | Action plans1189,1191 | Universal electronic medical history1197 |
| Asthma units1198 | Self-control1199–1201 | National registry of patients with severe asthma1202,1203 |
| Importance of Nursing1186 and Community Pharmacy1188 in healthcare programs | Strategic plans adapted to local characteristics1183 | |
| Use of computerized tools for asthma control1204,1205 | Provide necessary resources |
Referral to specialized care has shown to be effective for adequate management of patients with asthma in selected cases.1206–1209
Clinical practice guidelines should describe the criteria by which a patient with asthma should be referred to an asthma specialist, but an effective referral system requires good coordination between healthcare providers at the different levels of care.1209
In Spain, the consensus document on referral criteria for asthma,1193,1210 developed by professionals of Primary Care Medicine, Pneumology and Allergology, establishes the circuit to be followed by the primary care physician in the event of suspected asthma, in the evaluation of the control and follow-up of asthma patients, as well the referral of patients with asthma from primary care to specialized care in the following circumstances:
- •
To confirm the diagnosis of asthma when this is not possible with the resources available in the primary care setting.
- •
To study comorbidities when this cannot be completed in the primary care setting.
- •
Patients with severe asthma and uncontrolled asthma.
- •
Special circumstances (allergy study, occupational asthma, aspirin-exacerbated respiratory disease [AERD], exercise-induced asthma, and asthma in pregnancy).
- •
To study other diseases for the differential diagnosis with asthma.
For an adequate bidirectional communication between both levels of care and to improve continuity of care, the document proposes specific electronic referral templates and a minimum data set that should be included in specialized care reports of asthma patients.1193
9.2Asthma unitProspective data from a UK registry showed that management of patients with difficult asthma in asthma centers specialized in severe asthma resulted in improved related quality of life and less use of healthcare resources.1210 Some authors indicate that 1-day visit with extensive assessment in a specialized asthma center is beneficial and sufficient for a large group of patients with uncontrolled asthma, reducing the need of high-cost special treatments.1211
En 2015, the asthma area of the Spanish Society of Pneumology and Thoracic Surgery (SEPAR) addressed the task of establishing the necessary requirements for the provision of official accreditation standards of the different levels of care for asthma units already existing in hospitals of the Spanish National Healthcare System. Accreditation levels included basic units, specialized units, or specialized units of high complexity, with or without the distinctive of excellence, according to the fulfillment of a series of criteria.1212 Also, recently the Spanish Society of Allergology and Clinical Immunology (SEAIC) have established criteria for accreditation of Severe Asthma Units (SAU) in the Allergology Services.1213
These units coordinate the strategies aimed at improving the follow-up of patients with asthma, particularly those with severe asthma, interacting with other levels of care and with all other specialists involved in care of asthma, as well as the use of complex diagnostic and therapeutic techniques that require rigorous knowledge and application. This strategy results in a personalized clinical approach that makes it possible to recognize individual needs and carry out special pharmacological or behavioral interventions (education, follow-up of adherence to treatment).1214
Given the complexity of asthma, different specialties (Otorhinolaryngology, Gastroenterology, Endocrinology, Psychology, Pharmacy, etc.) are involved to a greater or lesser extent in the care of asthma patients. It is indispensable to have available a specialized nurse who can perform all education tasks, including training and review of the inhalation technique, treatment adherence, self-management, written action plan, and knowledge of the disease.1215
The distribution of tasks that should be assumed by the Asthma Unit is shown in fig. 24.
The development of an Asthma Unit in a healthcare areas is associated with important clinical benefits for the patient (increases considerably the percentage of patients with well-controlled asthma and reduces exacerbations substantially), with a highly favorable cost-effectiveness balance. In this respect, implementation of an Asthma Units is a beneficial option both from the perspective of efficiency for the healthcare system, and from the perspective of the patient, improving health outcomes and quality of care.1198,1216
9.3Implementation of GEMAFor a clinical practice guideline (CPG) to be applied and adopted by healthcare professionals, three indispensable sequential key steps should be addressed: diffusion, implementation, and evaluation. The diffusion of a CPG (be means of medical and scientific publications, mailing, workshops, symposia and computer-based tools via Internet) will not be effective if is not accompanied by a proper implementation.1217–1219
However, CPGs for asthma do not seem to meet this requirement. A study that aimed to evaluate the quality of these CPGs using the AGREE II instrument, found that none of them reached a score higher than 60% (minimum recommended level) in the evaluation of their corresponding implementation plans (domain 5 of AGREE checklist: applicability or implementation).1220
The correct application and implementation of a CPG, Graham proposes a series of structured and stepwise planning in order to transfer knowledge into action (knowledge-to-action).1221 The diffusion and implementation of GEMA is based in part on such principles and includes the following 8 actions:
- 1.
Specific healthcare area. For the implementation, a specific healthcare territorial area will be defined in order to assign a selected zone to a reference hospital and the various primary care teams assigned to the hospital.
- 2.
Analysis of needs and local deficiencies. An audit will be performed in order to detect weak points and deficiencies in disease management within that territory.
- 3.
Executive committee. A multidisciplinary group of experts in asthma pertaining to the implementation area will be set up. The committee will comprise expert physicians in asthma (pneumologists, allergologists, primary care physicians, and pediatricians) as well as professional representatives from the local nursing and pharmacy settings of the area.
- 4.
Development of a functional document based on GEMA.5.3 The Executive Committee will adapt evidences and recommendations of GEMA5.3 to the local healthcare reality according to the resources assigned to the area, the type of professionals, and their training level.
- 5.
Material resources. A minimal amount of material resources should be available in the area in order to ensure the application of the guideline. Specific resources will include: spirometries (of good quality throughout the area) in all centers; electronic medical history (EMH) shared among healthcare levels; standardized asthma symptom questionnaires (ACT, ACQ); placebo-containing inhalation devices to be used in education programs to instruct patients in the inhalation technique; an accredited specialized Asthma Unit in the hospital fitted with a complete technical equipment (bronchoprovocation tests, FENO, allergy skin tests, computerized tomography [CT]).
- 6.
Training plan. An educational intervention on asthma will be performed among both medical and nursing professionals working in the area.
- 7.
Professional motivation plan. Administrative authorities will be engaged in promoting adherence of professionals involved in the “Implementation Plan” by setting up appropriate motivational interventions.
- 8.
Evaluation and follow-up plan. To determine the impact of the “Implementation Plan” a set of health-related results (health outcomes) will be used in order to determine whether the proposed objectives have been achieved, and to establish appropriate adjustments if objectives were not meet. The eight indicators of healthcare quality for asthma proposed by a multidisciplinary expert group that participated in GEMA are shown in table 62.
Table 62.Healthcare quality indicators for asthma proposed by the multidisciplinary expert group (Asmaforum II).
Indicator groups Indicator Calculationo I. Diagnosis 1. C Diagnostic confirmation by means of spirometry with bronchodilation test. The diagnostic confirmation of patients with asthma is established by spirometry and bronchodilation test as an objective measurement of functional involvement. Number of patents with asthma undergoing spirometry × 100/number of patients diagnosed with asthma. 2. Sensitization study in allergic asthma. Patients with suspicion of allergic asthma should undergo a study of possible sensitization to different allergens. Number of patients diagnosed with suggestive history of allergic asthma with sensitization study performed at different allergens × 100/number of patients diagnosed with asthma. II. Non-pharmacological treatment 3. Smoking cessation. Smoking cessation is recommended in smokers with asthma. Number of smoking patients with asthma and registered recommendation to quit smoking × 100/smoking patients with asthma. 4. Education plan for patients with asthma. Patients with asthma should follow a basic education program (including knowledge of the disease and its treatment, written action, plan and inhalation technique) as part of their management. Number of patients with asthma with an asthma education program × 100/number of patients with asthma. III. Pharmacological treatment 5. Treatment of choice in persistent asthma. The treatment of choice in persistent asthma includes the use of inhaled glucocorticoids (ICS) on a daily basis. In some cases, an alternative treatment with leukotriene receptor antagonists may be justified. Number of patients on control treatment due to persistent asthma receiving ICS × 100/number of patients on control treatment due to persistent asthma. 6. Treatment of asthma in the pregnant woman. In the maintenance treatment of asthma in pregnancy, it is recommended to maintain the usually administered medication (β2-adrenergic agonists and inhaled glucocorticoids). Number of women with asthma who maintain their usual treatment (β2-adrenergic agonists and inhaled glucocorticoids) during pregnancy×100/number of pregnant women with asthma on maintenance therapy. IV. Follow-up 7. Periodic follow-up of patients. Need to establish periodic follow-up of patients based on scheduled medical visits, even in the absence of exacerbations. Number of scheduled follow-up visits (unexpected visits excluded) per patient per year × 100/number of patients with asthma on follow-up by year. 8. Periodic registry of exacerbations. Specific assessment of exacerbations is periodically evaluated. Number of patients with asthma in whom exacerbations have been evaluated and documented × 100/number of patients with asthma.
Advances in knowledge and information technology make it possible to provide medical care for chronic conditions such as asthma. The terminology used to define healthcare based on the new technologies is continually evolving. It has been proposed to use the term telehealthcare as a general term, encompassing all the different forms of telemedicine-related healthcare. This term includes.1222
- •
Tele-monitoring that involves storing and transference of patient's data.
- •
Tele-consultation is the use of technology allowing remote consultation between a patient and a clinician.
- •
Telemedicine that involves consultation among healthcare professionals.
The technology used is based on 3 main strategies:1223
- •
Support for patients’ self-management through the use of automatic medication-taking reminders (tele-reminder) to improve adherence, educational games to improve knowledge or modify the attitude towards the disease, and tele-monitoring of clinical variables (PEF, use of medication, etc.).
- •
Remote consultation with a healthcare professional.
- •
Computerized systems to aid decision-making for both physicians and patients.
The combined use of these strategies, which includes tele-case management or tele-consultation, improves the control of the disease and the quality of life of patients with asthma.1223,1224
4.8Recommendations| 9.1. To achieve quality in continuing care of asthma, coordination of different healthcare levels, involvement of the patient and nursing professionals, as well as the rational use of resources are recommended. | R1 |
| 9.2. It is suggested to promote the development of Asthma Units because they provide a better control of the disease, decreasing exacerbations, and improving health-related quality of life of patients, with a favorable cost-effectiveness balance. | R2 |
| 9.3. It is recommended to include a diffusion and implementation plan of this guideline to achieve the objectives of improving the level of training of healthcare professionals. | R2 |
| 9.4. The GEMA implementation plan proposes: implementation of actions in a local specific healthcare area; identification of local opinion leaders and engage them in this endeavor; adaptation of GEMA to the healthcare reality of the area; arrangement of an education plan for the professionals involved; and adjustment of actions according to whether objectives assessed by health outcomes have been attained. | R2 |
| 9.5. The use of telemedicine or medical tele-assistance based on strategies of “tele-cases” or tele-consultation is proposed, given that it improves control of disease and quality of life of patients with asthma. | R2 |
All the coauthor's contributed to the search for evidence, participated in the debates for decision-making and in the critical review of the final document.
FundingThe GEMA5.3 project was funded by pharmaceutical companies ALK, AstraZeneca, Chiesi, GlaxoSmithKline, Menarini and Sanofi. The viewpoints of these funding bodies did not influence the content of the guide.
Conflicts of interestThe authors of this guide declare that in the past two years, they have received honoraria for their participation in meetings, congresses, or research projects organized by the following pharmaceutical companies: ALK, AstraZeneca, Bial, Boehringer-Ingelheim, Chiesi, Esteve, GlaxoSmithKline, Leti, Menarini, MSD, Mundipharma, Novartis, Orion, Pfizer, Sanofi, Teva, and Zambón.
AcknowledgmentsThe authors would like to thank Marta Pulido, MD, for the English translation of the guideline and Luzan5 for their contribution in its diffusion.List of abbreviations:ABPA
Allergic bronchopulmonary aspergillosis
ABPA-BABPA with bronchiectasis
ABPA-CPFABPA-Chronic pleuropulmonary fibrosis
ABPA-SSerological ABPA
ABPA-HAMABPA-High attenuation mucus
AcAntibody
ACEAngiotensin-converting enzyme inhibitors
ACOSAsthma-COPD overlap syndrome
ACQAsthma Control Questionnaire
ACRAmerican College of Rheumatology
ACTAsthma Control Test
AEAdverse effects
AEMPSSpanish Agency of Medicines and Sanitary Products
AEPapSpanish Association of Primary Care Pediatrics
AERDAspirin (acetylsalicylic acid)-exacerbated respiratory disease
AIRQAsthma Impairment and Risk Questionnaire
ALATLatin American Thoracic Association
ANCAAntineutrophil cytoplasmic antibodies
APIAsthma Predictive Index
ARAllergic rhinitis
ASAAcetylsalicylic acid
ATSAmerican Thoracic Society
AZAAzathioprine
BAIBreath-actuated inhaler
BdBronchodilation
BDTBronchodilation test
BHRBronchial hyperresponsiveness
BMDBone mineral density
BMIBody mass index
c-ACTChildhood Asthma Control Test
CANAsthma Control Questionnaire in Children
CALChronic airflow limitation
CFCystic fibrosis
COCarbon monoxide
COPDChronic obstructive pulmonary disease
COVID-19Coronavirus disease 2019
COX-1Cyclooxigenase-1
COX-2Cyclooxigenase-2
CPAPContinuous positive airway pressure
CPGClinical practice guideline
CPKCreatine phosphokinase
GRAPPrimary Care Respiratory Society
CRSChronic rhinosinusitis
CRSsNPChronic rhinosinusitis without nasal polyps
CRSwNPChronic rhinosinusitis with nasal polyps
CTComputed tomography
DACDifficult-to-control asthma
DPIDry powder inhaler
EGPAEosinophilic granulomatosis with eosinophilia
EMAEuropean Medicines Agency
EMHElectronic medical history
ENTEar, nose and throat
EosEosinophils
ERSEuropean Respiratory Society
ERS/ATSEuropean Respiratory Society/American Thoracic Society
FDAFood and Drug Administration
FEF25-75Forced expiratory flow between 25% and 75% of forced vital capacity (FVC)
FENAERNational Federation of Associations of Patents with Allergic and Respiratory Diseases
FENOFractional exhaled nitric oxide
FEV1Forced expiratory volume in one second
FEOSWeighted FEV1. Exacerbations, oral corticosteroids and asthma symptoms to determine the response to a biological drug
FIOForced impulse oscillometry
FVCForced vital capacity
GCGlucocorticoids
GEMASpanish guideline for asthma management
GM-CSFGranulocyte-macrophage colony-stimulating factor
GNFGlomerulonephritis
GRAPPrimary Care Respiratory Society
HESHypereosinophilic syndrome
HFCHydrofluorocarbon
HMWHigh molecular weight
HRHazard ratio
HRQoLHealth-related quality of life
ICSInhaled corticosteroids
ICUIntensive care unit
IEImmunosuppressive effect
ICSInhaled glucocorticoids
IgEImmunoglobulin E
ILInterleukin
ILOInducible laryngeal obstruction
INGCIntranasal glucocorticoids
INPECSInstitute for Clinical and Healthcare Excellence
IOSImpulse oscillometry
ISAACInternational Study of Asthma and Allergens in Childhood
ITImmunotherapy
i.v.Intravenous
kgkilogram
LABALong-acting ß2-adrenergic agonists
LAMALong-acting muscarinic antagonist
L-ASLLysine-acetylsalicylate
LLNLower limit of normality
LMWLow molecular weight
LPTLipid transfer protein
LTRALeukotriene receptor antagonists
MAMeta-analysis
MADMMean aerodynamic diameter mass
MARTMaintenance and reliever therapy
MgMagnesium
MMFMycophenolate mofetil
MRMagnetic resonance
MTXMethotrexate
NARNon-allergic rhinitis
NEBNebulized
NIVNon-invasive ventilation
NKNatural killer
nNONasal nitric oxide
NO2Nitric oxide
NPNasal polyposis
NSAIDNon-steroidal anti-inflammatory drug
OAOccupational asthma
OCSOral glucocorticoids
OROdds ratio
pACTPregnancy Asthma Control Test
pANCAPerinuclear anti-neutrophil cytoplasmic antibodies
PaCO2arterial partial pressure of carbon dioxide
PaO2arterial oxygen partial pressure
PEFPeak expiratory flow
PDProvocation dose
pMDIPressurized metered-dose inhaler
PMNPolymorphonuclear neutrophils
ppbParts per billion
PPIProton pump inhibitor
RADSReactive airways dysfunction syndrome
RCTRandomized controlled trial
RRRisk ratio
RTXRituximab
RSVRespiratory syncytial virus
SABAShort-acting (2-adrenergic agonists
SaO2Arterial oxygen saturation
SARS-Cov-2Severe acute respiratory syndrome coronavirus 2
SBPTSpecific bronchial provocation test
s.c.Subcutaneous
SEAICSpanish Society of Allergology and Clinical Immunology
SEDISASpanish Society of Healthcare Directors
SEFACSpanish Society of Clinical Family and Community Pharmacy
SEFCSpanish Society of Clinical Pharmacology
SEFHSpanish Society of Hospital Pharmacy
SEICAPSpanish Society of Clinical Immunology, Allergology and Pediatric Asthma
SEMERGENSpanish Society of Primary Care Physicians
SEMESSpanish Society of Emergency Medicine and Emergency Care
SEMFYCSpanish Society of Family and Community Medicine
SEMGSpanish Society of General and Family Physicians
SENPSpanish Society of Pediatric Pneumology
SEORL-CCCSpanish Society of Otorhinolaryngology and Head and Neck Surgery
SO2Sulfur dioxide
SEPARSpanish Society of Pneumology and Thoracic Surgery
SEPEAPSpanish Society of Outpatient and Primary Care Pediatrics
SGCSystemic glucocorticoids
SMISoft mist inhaler
SNOT-22Sino-Nasal Outcome Test 22
SPPPortuguese Society of Pneumology
SRSystematic review
SUASevere uncontrolled asthma
TAITest of Adherence to Inhalers
Th2Type 2 helper T cells
TLT lymphocytes
TNFTumor necrosis factor
TPRTherapeutic positioning report
TSLPThymic stromal lymphopoietin
uLTE4E4 leukotriene concentration in urine
USAAUncontrolled severe allergic asthma
VASVisual analogue scale