Background. Staging systems have considerable impact on hepatocellular carcinoma (HCC) treatment approaches and outcomes. There is an unmet need to improve their stratification ability. We have evaluated four commonly used staging systems and assessed whether angiogenic biomarker vascular endothelial growth factor (VEGF) could improve their prognostic stratification.
Material and methods. Four staging systems; Okuda, Cancer of the Liver Italian Program (CLIP), Barcelona Clinic Liver Cancer (BCLC), and Child-Pugh were evaluated in 78 HCC patients; their stratification abilities were detected by Kaplan-Meier curves and log-rank test; their accuracies of predicting survival were compared with the concordance index. Serum VEGF levels were measured using ELISA method. Recursive partitioning was used to determine the optimal VEGF cutoff. The prognostic significance of VEGF cutoff and other parameters were analyzed using univariate and multivariate models.
Results. None of the staging systems demonstrated better discriminatory ability in predicting survival. The four staging systems did not reveal significant differences in probability of survival across their intermediate-advanced stages. Optimal cutoff identified for VEGF was 445 pg/mL. In advanced HCC, VEGF level (p = 0.004) and in early HCC, bilirubin level (p = 0.009) were identified as the independent prognostic factors. Survival comparison with high and low VEGF levels was significant for advanced HCC, while insignificant for early disease.
Conclusion. Staging systems with conventional parameters did not provide good prognostic stratification for survival in advanced HCC population. Serum VEGF level was an independent predictor of survival in advanced HCC, and provided more survival homogeneity within the advanced stages of conventional staging systems.
Hepatocellular carcinoma (HCC) is the fifth most common type of cancer affecting approximately one million people every year and the third most frequent cause of annual cancer-related deaths worldwide.1.–3 In many parts of the world, the incidence and mortality from HCC continues to rise as patients with cirrhosis are expected to have a longer survival due to improved medical manage-ment.4.–8 The similarity between mortality and incidence rates is indicative of poor survival of this disease.9 Because of these, in recent years, many prognostic factors of HCC have been evaluated, particularly, angiogenesis-related markers have been a subject of interest.
Angiogenesis plays a crucial role in growth and progression of HCC, which is one of the most vascularized solid tumors.10 Vascular endothelial growth factor (VEGF) is a critical mediator regulating angiogenesis in HCC.10.–12 There have been many studies suggesting a relationship between VEGF and prognosis of HCC.13.–20
In the management of HCC disease, the role of a staging system is to estimate prognosis, to define the suitable patient population to be recruited in clinical trials and to provide a common terminology to compare outcomes of these trials. Importantly, by guiding the treatment decisions, they have considerable impact on the outcomes.21 HCC is a heterogeneous disease with distinct risk factors, underlying liver function, clinical presentation, natural history, and response to therapeutic modalities, which leads to very different clinical outcomes. Widely accepted HCC staging systems are Barcelona Clinic Liver Cancer (BCLC), Cancer of the Liver Italian Program (CLIP) and Okuda systems.22.–24 Tumor-node-metastasis (TNM) staging system which is commonly used in other malignant diseases, is based on the anatomic tumor extension and does not include many important parameters affecting HCC prognosis such as underlying liver function status. Child-Pugh classification is a widely used clinical measure of hepatic functional reserve, but lacks parameters representing the tumor itself. Ideal staging systems for HCC should necessarily involve many prognostic parameters that accurately stratify patients with regard to survival outcome. Among the many staging systems introduced for HCC, there is no worldwide consensus on which one is the best in predicting prognosis yet, and it is unclear whether integration of additional prognostic variables can improve their stratification ability.21,25.–27
The aim of this study is to compare the accuracies of four commonly used staging systems at predicting survival and to investigate whether angiogenic factor VEGF can be a prognostic measure, and hence, improve prognostic stratification of these staging systems in a series of HCC patients treated with different therapeutic modalities.
Material and MethodsPatients and data collectionFrom January 2008 to January 2009, a total of 89 eligible HCC patients attending to our Hepato-logy Cirrhosis Clinics were identified at Marmara University Faculty of Medicine Hospital in Istanbul, Turkey. HCC was diagnosed by radiologic criteria or by histological confirmation as described by the American Association for the Study of Liver Diseases.28
Patients with incomplete information required for the analysis, who were lost during the follow up and patients with a history of other concurrent malignancies were excluded from the study. We obtained approval of the Central Research Ethics Committee for the study protocol and informed consent form of the patients prior to participation.
The primary outcome for the analysis was to evaluate the correlation of biomarker VEGF with overall survival and its role in the prognostic stratification of HCC disease. Required data were collected to stage patients according to Child-Pugh, Okuda, BCLC, CLIP systems and to perform statistical analysis, and the data included demographics; clinical, etiological, laboratory, and therapeutic variables; and tumor data determined by available imaging. Patients were also classified as having early or advanced disease; advanced HCC was defined as disease that surgical or locoregional therapies (radiofrequency ablation, alcohol ablation, chemo-embolization) were not suitable, or that have recurred after therapy.29
Serum samples were obtained from 78 HCC patients, 20 cirrhotic patients with no evidence of HCC, and 20 healthy adults as controls. Venous blood samples were drawn into a serum separator tube and centrifuged at 5,000 rpm for 10 min, then, samples were stored at -20 °C until analysis. VEGF serum level was quantitatively measured by Chemi-Kine sandwich ELISA kit (Chemicon International, Billerica, MA, USA) by following the company’s instructions.
Statistical analysisOverall survival was calculated by using the Kaplan-Meier method. Survival comparison among the stages of each prognostic system was performed by using the log-rank test. We also compared median survival of patients with high and low VEGF levels in each stage of four prognostic systems, and in early and advanced HCC patient groups using log-rank test.
Recursive partitioning method was used to search for the optimal VEGF cutoff value. Recursive partitioning is known as a tree analysis method, which creates a decision tree based on the likelihood ratio test to examine all possible binary splits of HCC patients and selects the VEGF value that maximally discriminates between those who survive and those who do not survive.
To identify independent prognostic factors, we fitted variables that demonstrated a p value < 0.1 in the univariate analysis to the multivariable Cox regression model.
The discriminatory abilities of four staging systems in terms of predicting survival were evaluated by concordance (c-) statistics, and compared to each other. Concordance index (c-index) is measured by calculating the area under the receiver operating characteristic (ROC) curve, and varies between 0.5 and 1.0 with a higher value for a system indicating a better predictive ability for survival.
We used independent-samples t test, one-way Anova test, and Pearson correlation test to correlate VEGF levels with various parameters and prognostic scoring systems.
Continuous data were expressed as median and range. Comparisons between groups were performed by the χ2 test (or Fisher exact test where appropriate) for categorical variables, and the Mann-Whitney U-test for continuous variables.
Statistical analysis was performed using SPSS 15.0 for Windows statistical software. P values < 0.05 were considered statistically significant.
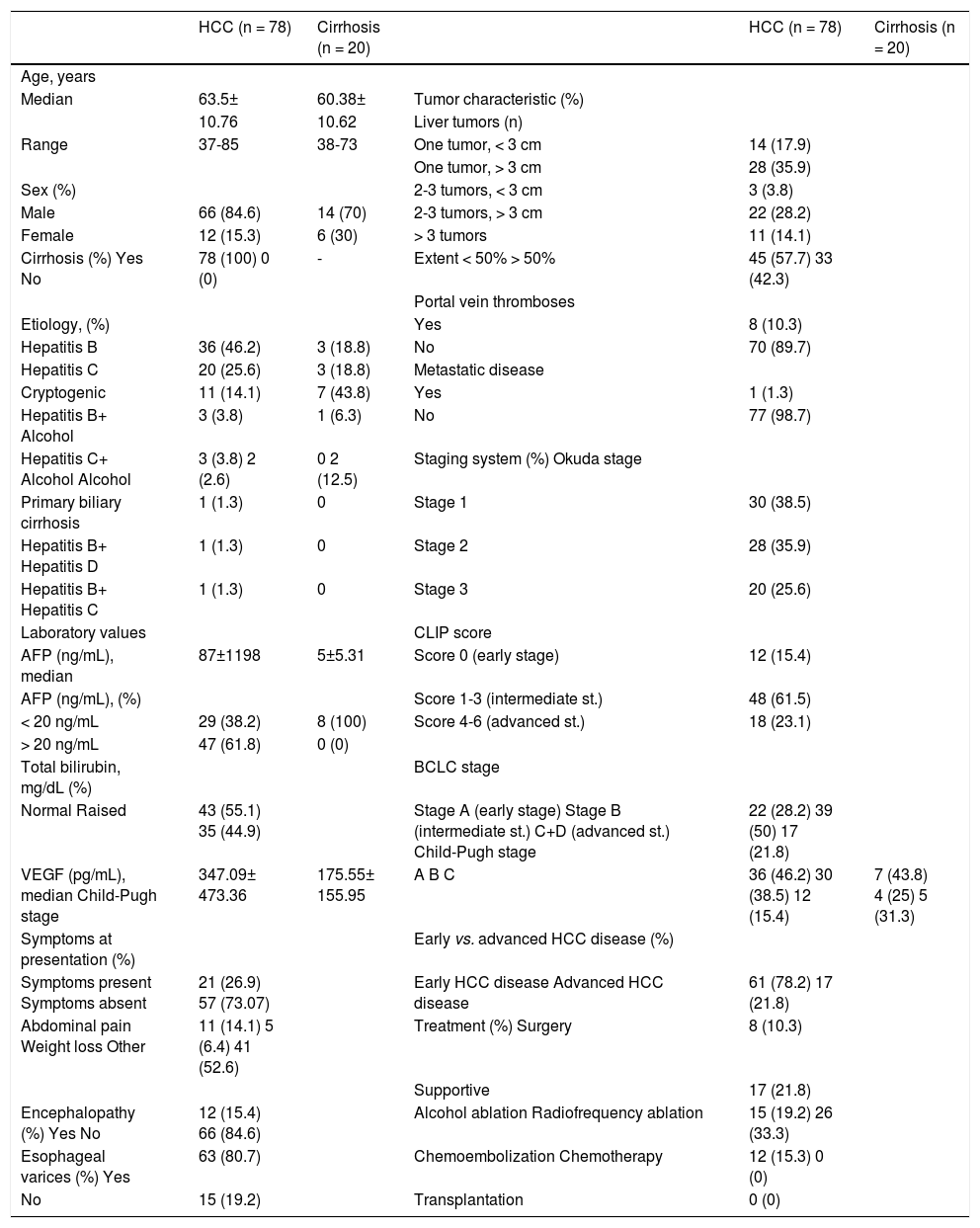
ResultsPatient characteristicsAmong the 89 HCC patients identified as eligible, 78 patients were enrolled in the study; 11 patients were excluded due to incomplete data or no follow-up. The diagnosis of HCC required liver biopsy in 4/ 78 (5%) cases. The majority of the HCC patients were male (84.6%) and the median age was 63.5 (3785) years. Median duration of follow-up was 4 ± 7 (1–60 mo) months. All HCC patients had underlying cirrhosis and 46.2% had preserved hepatic functional reserve (Child-Pugh A). The study population included 17 (21.8%) patients with an advanced tumor. Of the 78 patients, 49 (62.8%) did not survive by the time of data analysis. Table 1 summarizes patient characteristics.
Demographic and clinical characteristics of patients.
| HCC (n = 78) | Cirrhosis (n = 20) | HCC (n = 78) | Cirrhosis (n = 20) | ||
|---|---|---|---|---|---|
| Age, years | |||||
| Median | 63.5± | 60.38± | Tumor characteristic (%) | ||
| 10.76 | 10.62 | Liver tumors (n) | |||
| Range | 37-85 | 38-73 | One tumor, < 3 cm | 14 (17.9) | |
| One tumor, > 3 cm | 28 (35.9) | ||||
| Sex (%) | 2-3 tumors, < 3 cm | 3 (3.8) | |||
| Male | 66 (84.6) | 14 (70) | 2-3 tumors, > 3 cm | 22 (28.2) | |
| Female | 12 (15.3) | 6 (30) | > 3 tumors | 11 (14.1) | |
| Cirrhosis (%) Yes No | 78 (100) 0 (0) | - | Extent < 50% > 50% | 45 (57.7) 33 (42.3) | |
| Portal vein thromboses | |||||
| Etiology, (%) | Yes | 8 (10.3) | |||
| Hepatitis B | 36 (46.2) | 3 (18.8) | No | 70 (89.7) | |
| Hepatitis C | 20 (25.6) | 3 (18.8) | Metastatic disease | ||
| Cryptogenic | 11 (14.1) | 7 (43.8) | Yes | 1 (1.3) | |
| Hepatitis B+ Alcohol | 3 (3.8) | 1 (6.3) | No | 77 (98.7) | |
| Hepatitis C+ Alcohol Alcohol | 3 (3.8) 2 (2.6) | 0 2 (12.5) | Staging system (%) Okuda stage | ||
| Primary biliary cirrhosis | 1 (1.3) | 0 | Stage 1 | 30 (38.5) | |
| Hepatitis B+ Hepatitis D | 1 (1.3) | 0 | Stage 2 | 28 (35.9) | |
| Hepatitis B+ Hepatitis C | 1 (1.3) | 0 | Stage 3 | 20 (25.6) | |
| Laboratory values | CLIP score | ||||
| AFP (ng/mL), median | 87±1198 | 5±5.31 | Score 0 (early stage) | 12 (15.4) | |
| AFP (ng/mL), (%) | Score 1-3 (intermediate st.) | 48 (61.5) | |||
| < 20 ng/mL | 29 (38.2) | 8 (100) | Score 4-6 (advanced st.) | 18 (23.1) | |
| > 20 ng/mL | 47 (61.8) | 0 (0) | |||
| Total bilirubin, mg/dL (%) | BCLC stage | ||||
| Normal Raised | 43 (55.1) 35 (44.9) | Stage A (early stage) Stage B (intermediate st.) C+D (advanced st.) Child-Pugh stage | 22 (28.2) 39 (50) 17 (21.8) | ||
| VEGF (pg/mL), median Child-Pugh stage | 347.09± 473.36 | 175.55± 155.95 | A B C | 36 (46.2) 30 (38.5) 12 (15.4) | 7 (43.8) 4 (25) 5 (31.3) |
| Symptoms at presentation (%) | Early vs. advanced HCC disease (%) | ||||
| Symptoms present Symptoms absent | 21 (26.9) 57 (73.07) | Early HCC disease Advanced HCC disease | 61 (78.2) 17 (21.8) | ||
| Abdominal pain Weight loss Other | 11 (14.1) 5 (6.4) 41 (52.6) | Treatment (%) Surgery | 8 (10.3) | ||
| Supportive | 17 (21.8) | ||||
| Encephalopathy (%) Yes No | 12 (15.4) 66 (84.6) | Alcohol ablation Radiofrequency ablation | 15 (19.2) 26 (33.3) | ||
| Esophageal varices (%) Yes | 63 (80.7) | Chemoembolization Chemotherapy | 12 (15.3) 0 (0) | ||
| No | 15 (19.2) | Transplantation | 0 (0) | ||
AFP: α-fetoprotein. BCLC: Barcelona Clinic Liver Cancer. CLIP: Cancer of the Liver Italian Program. HCC: hepatocellular carcinoma. VEGF: vascular endothelial growth factor.
Four prognostic systems were analyzed separately for their ability to stratify patients into stages with survival differences by Kaplan-Meier curves. Child-Pugh and Okuda systems stratified patients more effectively into different prognostic risk groups than the BCLC and CLIP systems by log-rank comparison (p = 0.006 for Child-Pugh, p = 0.04 for Okuda, p = 0.064 for BCLC, and p = 0.112 for CLIP).
Pairwise log-rank comparison between stages within each prognostic system revealed significant survival differences between Child-Pugh stages with the exception of stages B and C (Child-Pugh A vs. B p = 0.001; A vs. C p = 0.051; B vs. C p = 0.571), and between Okuda stages with the exception of stages II and III (Okuda I vs. II p = 0.042; I vs. III p = 0.001; II vs. III p = 0.103). Within the stages of CLIP system, early CLIP stage tended to have a longer survival than the intermediate and the advanced stages, while no survival difference was demonstrated among intermediate-advanced stage comparison (CLIP early vs. intermediate p = 0.054; early vs. advanced p = 0.074; intermediate vs. advanced p = 0.537). When BCLC system stages were compared, stage A had a better prognosis than stage C-D, while no significant survival difference were found among A and B stages, among B and C-D stages (BCLC A vs. B p = 0.217; A vs. C-D p = 0.018; B vs. C-D p = 0.174) (Figure 1).
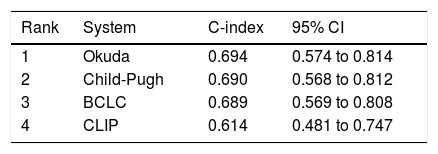
Comparison of staging systemsBy c-statistic analysis, the discriminatory abilities at predicting survival were ranked as Okuda (0.694; 95% CI, 0.574 to 0.814), Child-Pugh (0.690, 95% CI, 0,568 to 0,812), BCLC (0.689; 95% CI, 0.569 to 0.808), and CLIP (0.614; 95% CI, 0.481 to 0.747) with no statistically significant difference among each other (p > 0.05 for paired comparisons) (Table 2).
Ranking of staging systems in patients with HCC by using C-index.
| Rank | System | C-index | 95% CI |
|---|---|---|---|
| 1 | Okuda | 0.694 | 0.574 to 0.814 |
| 2 | Child-Pugh | 0.690 | 0.568 to 0.812 |
| 3 | BCLC | 0.689 | 0.569 to 0.808 |
| 4 | CLIP | 0.614 | 0.481 to 0.747 |
BCLC: Barcelona Clinic Liver Cancer. C-index: concordance index. CI: confidence interval. CLIP: Cancer of the Liver Italian Program. HCC: hepatocellular carcinoma.
At the end of follow up, 49/78 (62.8%) patients had died. The estimated median overall survival duration of 78 HCC patients was 8.0 months (95% CI, 6.2–9.8), of 61 early HCC patients was 9.0 months (95% CI, 5.96–12.04), and of 17 advanced HCC patients was 4.0 months (95% CI, 1.46–6.53). Variables that significantly or likely influenced survival in our univariate model analysis were Child-Pugh stage (p = 0.006), bilirubin level (p = 0.000), Okuda stage (p = 0.004), presence of esophageal varices (p = 0.051), BCLC stage (p = 0.064), tumor extension (p = 0.058), and VEGF cutoff 445 pg/mL (p = 0.08).
Optimal VEGF cutoff as an independent prognostic factorWhen recursive partitioning was applied, optimal cutoff identified for VEGF in terms of predicting survival was 445 pg/mL.
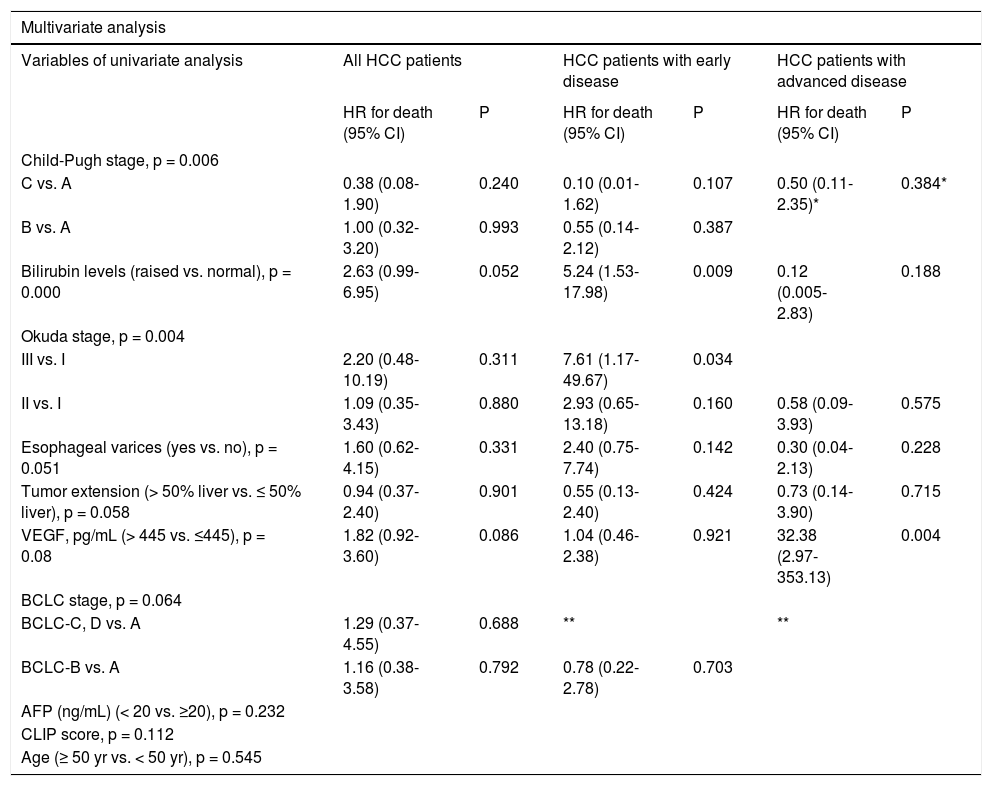
Variables that demonstrated a p value of < 0.1 in our univariate survival model were subsequently evaluated in a multivariate Cox regression model for all HCC patients, patients with early disease, and with advanced disease separately. The hazard ratio (HR) indicated a highly significant effect for our VEGF cutoff 445 pg/mL on survival for advanced HCC disease (p = 0.004; HR, 32.38; 95% CI, 2.97353.13), but not for early HCC disease (p = 0.921; HR, 1.04; 95% CI, 0.46–2.38). Bilirubin level was the independent prognostic factor identified for patients with early HCC disease (p = 0.009; HR, 5.24; 95% CI, 1.53–17.98) (Table 3).
Survival predictors in patients with early and advanced HCC by Cox regression analysis.
| Multivariate analysis | ||||||
|---|---|---|---|---|---|---|
| Variables of univariate analysis | All HCC patients | HCC patients with early disease | HCC patients with advanced disease | |||
| HR for death (95% CI) | P | HR for death (95% CI) | P | HR for death (95% CI) | P | |
| Child-Pugh stage, p = 0.006 | ||||||
| C vs. A | 0.38 (0.08-1.90) | 0.240 | 0.10 (0.01-1.62) | 0.107 | 0.50 (0.11-2.35)* | 0.384* |
| B vs. A | 1.00 (0.32-3.20) | 0.993 | 0.55 (0.14-2.12) | 0.387 | ||
| Bilirubin levels (raised vs. normal), p = 0.000 | 2.63 (0.99-6.95) | 0.052 | 5.24 (1.53-17.98) | 0.009 | 0.12 (0.005-2.83) | 0.188 |
| Okuda stage, p = 0.004 | ||||||
| III vs. I | 2.20 (0.48-10.19) | 0.311 | 7.61 (1.17-49.67) | 0.034 | ||
| II vs. I | 1.09 (0.35-3.43) | 0.880 | 2.93 (0.65-13.18) | 0.160 | 0.58 (0.09-3.93) | 0.575 |
| Esophageal varices (yes vs. no), p = 0.051 | 1.60 (0.62-4.15) | 0.331 | 2.40 (0.75-7.74) | 0.142 | 0.30 (0.04-2.13) | 0.228 |
| Tumor extension (> 50% liver vs. ≤ 50% liver), p = 0.058 | 0.94 (0.37-2.40) | 0.901 | 0.55 (0.13-2.40) | 0.424 | 0.73 (0.14-3.90) | 0.715 |
| VEGF, pg/mL (> 445 vs. ≤445), p = 0.08 | 1.82 (0.92-3.60) | 0.086 | 1.04 (0.46-2.38) | 0.921 | 32.38 (2.97-353.13) | 0.004 |
| BCLC stage, p = 0.064 | ||||||
| BCLC-C, D vs. A | 1.29 (0.37-4.55) | 0.688 | ** | ** | ||
| BCLC-B vs. A | 1.16 (0.38-3.58) | 0.792 | 0.78 (0.22-2.78) | 0.703 | ||
| AFP (ng/mL) (< 20 vs. ≥20), p = 0.232 | ||||||
| CLIP score, p = 0.112 | ||||||
| Age (≥ 50 yr vs. < 50 yr), p = 0.545 | ||||||
CI: confidence interval. HCC: hepatocellular carcinoma. HR: hazard ratio. VEGF: vascular endothelial growth factor. "Child-Pugh stage C vs. B comparison. “Not applicable since early HCC patients consists of only BCLC-A or B patients, advanced HCC patients consists of only BCLC-C,D patients.
We made further analysis by stratifying patients within each stage of four prognostic systems, and early-advanced HCC disease according to our VEGF cutoff to assess whether patients with higher VEGF had shorter median survival.
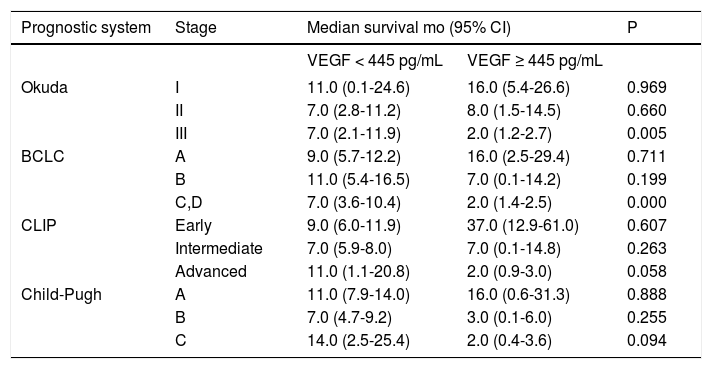
Using the log-rank test, we found that at advanced stages of Okuda (stage III) and BCLC (BCLC stage C, D) systems, the median survival of VEGF-high patients were shorter than the median survival of VEGF-low patients (p = 0.005 and p = 0.000 respectively), and for advanced stages of CLIP (CLIP advanced stage) and Child-Pugh (Child-Pugh stage C) systems, the VEGF-high vs. VEGF-low comparison for survival tended to be significant (p = 0.058 and p = 0.094 respectively). Table 4 shows the median survivals of stages of four prognostic systems when stratified by VEGF cutoff 445 pg/mL.
Comparison of median survivals of stages of four prognostic systems when stratified by VEGF cutoff 445 pg/mL.
| Prognostic system | Stage | Median survival mo (95% CI) | P | |
|---|---|---|---|---|
| VEGF < 445 pg/mL | VEGF ≥ 445 pg/mL | |||
| Okuda | I | 11.0 (0.1-24.6) | 16.0 (5.4-26.6) | 0.969 |
| II | 7.0 (2.8-11.2) | 8.0 (1.5-14.5) | 0.660 | |
| III | 7.0 (2.1-11.9) | 2.0 (1.2-2.7) | 0.005 | |
| BCLC | A | 9.0 (5.7-12.2) | 16.0 (2.5-29.4) | 0.711 |
| B | 11.0 (5.4-16.5) | 7.0 (0.1-14.2) | 0.199 | |
| C,D | 7.0 (3.6-10.4) | 2.0 (1.4-2.5) | 0.000 | |
| CLIP | Early | 9.0 (6.0-11.9) | 37.0 (12.9-61.0) | 0.607 |
| Intermediate | 7.0 (5.9-8.0) | 7.0 (0.1-14.8) | 0.263 | |
| Advanced | 11.0 (1.1-20.8) | 2.0 (0.9-3.0) | 0.058 | |
| Child-Pugh | A | 11.0 (7.9-14.0) | 16.0 (0.6-31.3) | 0.888 |
| B | 7.0 (4.7-9.2) | 3.0 (0.1-6.0) | 0.255 | |
| C | 14.0 (2.5-25.4) | 2.0 (0.4-3.6) | 0.094 | |
BCLC: Barcelona Clinic Liver Cancer. CLIP: Cancer of the Liver Italian Program. HCC: hepatocellular carcinoma. VEGF: vascular endothelial growth factor. CI: confidence interval.
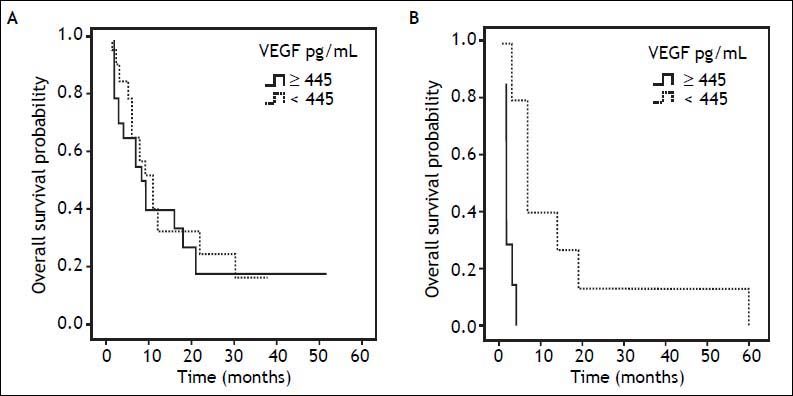
When patients with advanced HCC disease were considered, VEGF levels higher than 445 pg/mL predicted median survival of 2.0 months when compared with the lower VEGF levels which predicted 7.0 months of survival (p = 0.000) (log-rank test shown in table 5). Kaplan-Meier curves in figure 2 shows the prognostic impact of serum VEGF levels on overall survival by using the cutoff 445 pg/mL.
Comparison of median survivals of early and advanced HCC disease when stratified by VEGF cutoff 445 pg/mL.
| Median survival mo (95% CI) | P | ||
|---|---|---|---|
| VEGF < 445 pg/mL | VEGF > 445 pg/mL | ||
| HCC patients with early disease | 11.0 (7.4–14.6) | 8.0(3.7–12.3) | 0.448 |
| HCC patients with advanced disease | 7.0 (3.6–10.4) | 2.0 (1.4–2.6) | 0.000 |
HCC: hepatocellular carcinoma. VEGF: vascular endothelial growth factor. CI: confidence interval.
Prognostic influence of serum VEGF level on overall survival. The survival of patients with serum VEGF levels of < 445 and ≥ 445 pg/mL for (A) early HCC disease were 11.0 and 8.0 months, respectively (p = 0.448), (B) advanced HCC disease were 7.0 and 2.0 months, respectively (p = 0.000).
No significant correlation was observed between serum VEGF levels and sex, age, serum AFP level, metastatic disease, presence of varices, etiology of underlying cirrhosis, and extension of tumor.
Serum levels of VEGF in patients with HCC (median 347.09 pg/mL) were significantly higher than those in healthy controls (median 143.35 pg/mL) (p = 0.000), and higher than those in cirrhotic patients without HCC (median 175.55 pg/mL) (p = 0.011). There was also a significant association between high serum VEGF levels and presence of abdominal pain (p = 0.038).
DiscussionIn our study, we compared the discriminatory abilities of four prognostic staging systems in terms of predicting survival using c-index analysis. As concordance probabilities did not reveal any meaningful differences among each other, none of the systems were proved to be more accurate at discriminating survival. The reason for lack of difference may be related to the characteristics of our study population, as we included a heterogeneous group of HCC patients with different underlying etiologies of liver cirrhosis (hepatitis B and C, cryptogenic, alcohol), receiving different forms of treatments (radiofre-quency ablation, chemoembolization, alcohol ablation, supportive, surgery). Several other studies comparing HCC staging systems in surgical and nonsurgical series reported varying performances regarding their prognostic stratification and prediction abilities.30.–35 The characteristics of a study population might be significantly different from the population which the staging systems were designed originally in terms of tumor extension, treatment strategy, liver function status, genetics, sex, age, geographic area, ethnic group and other demographics. Thus, the relevance of certain parameters and the predictive ability and applicability of staging systems will vary according to the characteristics of the population studied; therefore, it may be challenging to compare them. Certain parameters such as tumor size may only have predictive value in early HCC patients undergoing curative therapies,22,36 whereas some parameters can only be predictive in the setting of advanced HCC patients who benefit from palliative treatments. One other factor complicating the staging process is the incomplete understanding of the highly complex biologic characteristics of these tumors, thus lack of integration of the intrinsic tumor characteristics in staging systems.37
We performed a detailed analysis to evaluate the prognostic influence of various variables including VEGF as an intrinsic tumor feature and a series of clinical, radiologic and laboratory parameters, considering early and advanced HCC disease separately. For early HCC disease, we identified high serum bilirubin, a parameter representing liver function as an independent unfavorable prognostic factor for overall survival. BCLC system which was constructed based on the independent prognostic factors derived from the analysis of various studies, has been validated as the best staging system to select early-stage HCC patients who could benefit from curative thera-pies.34,38 In BCLC system portal hypertension and bilirubin levels > 1.5 mg/dL were identified as factors negatively correlating with survival for early HCC patients. For advanced HCC patients, we found serum VEGF level higher than its optimal cutoff of 445 pg/mL as a significant independent predictor of poor survival, while any independent prognostic influence of tumor extension, esophageal varices or bilirubin levels on overall survival were not observed. There was also no significant correlation between serum VEGF levels and tumor extension in our study. Tumor extension tend to influence survival in our univariate model, but this influence disappeared in our multivariate model. This suggests that the correlation between survival and tumor extension may be mainly indirect, and circulating VEGF levels reflect the tumor angiogenic activity rather than the tumor burden. Furthermore, as reported by other studies, there was no correlation between serum VEGF and AFP levels in our study.18,39,40 Therefore, in advanced HCC disease setting, VEGF is an independent and the most important prognostic factor and survival seems to be more related to the intrinsic characteristics of tumor itself rather than liver function parameters for this setting.
Our results indicating that high circulating VEGF levels had a negative prognostic impact on survival are consistent with previous studies that included a substantial proportion of advanced HCC patients, undergoing different types of therapies like resection, transarterial chemoembolization or che-motherapy.16,18.–20,41.–43 A meta-analysis evaluating 16 studies reported that high liver tissue and high serum VEGF levels predicted poor overall survival (HR = 2.15, 95% CI, 1.26–3.68 and HR= 2.35, 95% CI, 1.80–3.07 respectively).13 They also found no difference in the VEGF levels between surgically and non-surgically treated groups, and suggested that choice of therapy was not potentially associated with serum VEGF levels.13 According to this, heterogeneity of the therapies received in our patient population would not complicate the generalisability of our results.
In this study, our biomarker cutoff point 445 pg/ mL determined by recursive partitioning method was in agreement with two previous studies based on the same method identifying their cutoff as 450 pg/mL.41,42 Different VEGF cutoffs were identified with different methods in some other studies using the median level or ROC curve method as a cutoff value.16,18,19,44.–46 In our study, the serum median level of VEGF was 347.09 pg/mL in HCC patients and was significantly higher than the value in healthy controls and in cirrhotic patients without HCC, but our cutoff value 445 pg/mL best correlated with the survival of our patient population.
When we compared the stages of each scoring system by pairwise log-rank test, significant survival differences were mostly revealed between early and intermediate-advanced stages. Importantly, we could not show a progressive decrease in survival from the intermediate to the advanced stages in each of all four systems (log-rank Child-Pugh B vs. C p = 0.571; Okuda II vs. III p = 0.103; CLIP intermediate vs. advanced p = 0.537; BCLC B vs. C-D p = 0.174).
These proved that the prognostic parameters for intermediate or advanced stages were not well represented in these four conventional prognostic systems and thus, they were inadequate for accurate prognostic stratification. On the other hand, we could stratify advanced stages of four prognostic systems by our VEGF cutoff into risk subgroups with different median survivals; patients with a VEGF level higher than the cutoff value had a worse median survival than VEGF level lower patients (Okuda stage III 2.0 vs. 7.0 mo; BCLC stage C-D 2.0 vs. 7.0 mo; CLIP advanced stage 2.0 vs. 11.0 mo; Child-Pugh stage C 2.0 vs. 14.0 mo). These findings revealed a substantial variation in prognosis among patients within the advanced stages of all four conventional prognostic systems, and showed that VEGF could define more homogeneous populations of patients with different outcomes for this group of patients.
Accurate prognostic stratification of advanced HCC population is particularly important, because this population represents the classic patient population of therapeutic clinical trials. One of the major challenges in designing HCC clinical trials is the heterogeneity of advanced HCC disease which makes the results difficult to compare, analyze, and interpret. Stratification systems should have an optimal capacity to define homogeneous populations with different outcomes. Thus, tumor prognostic parameters should be well-represented in HCC staging systems, and it seems that this should involve more than the number and size of the tumor or liver function status, and should reflect the intrinsic biologic characteristics of the tumor like angiogenic biomarkers.
Non-invasive means of measuring tumor parameters like circulating angiogenic biomarkers is advantageous over evaluation in tumor samples; because it is technically simple, easily accessible and will not induce any bleeding risk in HCC patients with coagulopathy following needle biopsies. Serum VEGF levels have been found to be correlated with VEGF expression in HCC, and can serve as a valid surrogate marker of tumor tissue levels; it seems reasonable to measure serum VEGF levels as a reflection of tumor angiogenic activity in HCC.47
Our study has some limitations. First of all, this is a single-center study. Even though different centers may have their own experience and practice, our patients were followed and treated uniformly based on consensus algorithms widely used by many centers. As a tertiary referral center, although we were able to analyze a broad spectrum of patients with early, intermediate, and advanced tumors, some variables had relatively large confidence intervals, which were related to the lack of representative sampling for some subgroups of staging systems. Validation across different geographic populations is especially needed before generalisability of our results due to the global variations related to the complex etiology of HCC, leading to distinct outcomes as revealed between eastern and western patient populations in therapeutic HCC trials.29,48 Due to large variations in serum VEGF levels which made the reliability of cuttoff estimate an issue, additional studies are warranted to optimize the value of VEGF to be used in advanced HCC setting.
In conclusion, this research adequately addressed that staging systems with conventional parameters did not provide good prognostic stratification in terms of survival for advanced HCC population, and that serum VEGF defined more homogeneity in terms of survival within the advanced stages of all four staging systems. Assessment of tumor angiogenic activity by serum VEGF and using this noninvasive biomarker stratification approach by incorporation into the commonly used HCC staging systems, may prove beneficial for more refined prognostic stratification after prospective validation, and ultimately by affecting therapeutic decisions may capture benefits in advanced HCC outcome. Active research is needed to identify other biomarkers which may allow the stratification of patients not only according to the conventional prognostic measures like liver function or tumor burden, but also more accurately by characterizing intrinsic tumor features.
Abbreviations- •
AFP: α-fetoprotein.
- •
BCLC: Barcelona Clinic Liver Cancer.
- •
CI: confidence interval.
- •
CLIP: Cancer of the Liver Italian Program.
- •
HCC: hepatocellular carcinoma.
- •
VEGF: vascular endothelial growth factor.
This study was supported by Research Foundation of Marmara University Faculty of Medicine.