Campylobacter spp. infection is one of the leading causes of foodborne diarrhoeal illness in humans worldwide. The purpose of this study was to evaluate the DiaSorin LIAISON®Campylobacter assay for human campylobacteriosis diagnosis.
MethodologyA total of 645 stool samples from 640 patients suspected of having gastrointestinal infection were included. A stool culture was simultaneously performed with the DiaSorin LIAISON®Campylobacter assay to detect the presence of Campylobacter spp.
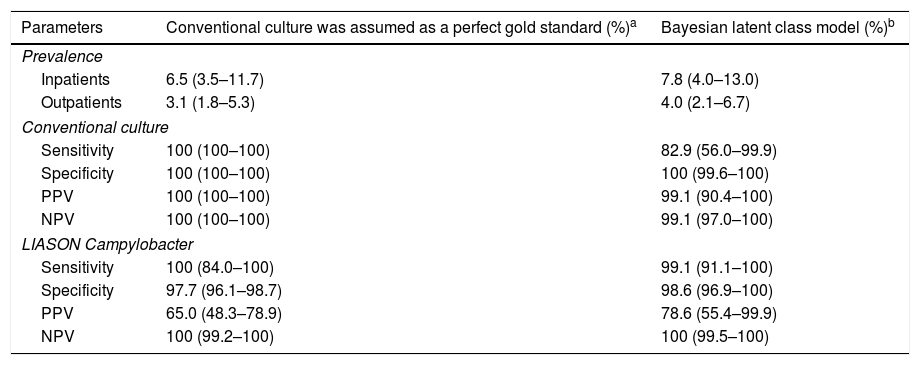
ResultsTaking the conventional culture to be the perfect gold standard, sensitivity and specificity rates of the DiaSorin LIAISON®Campylobacter assay were 100% and 97.7%, respectively; and 99.1% and 98.6%, respectively, when taking the culture to be the imperfect gold standard (Bayesian Model).
ConclusionThis new assay might be a useful tool especially for the screening of negative results.
La infección por Campylobacter spp. es una de las principales causas de enfermedades diarreicas de transmisión alimentaria en el ser humano. Este estudio tuvo como objetivo evaluar la plataforma DiaSorin LIAISON®Campylobacter assay para el diagnóstico de la campilobacteriosis humana.
MetodologíaSe incluyeron un total de 645 muestras de heces de 640 pacientes con sospecha de infección gastrointestinal. Se realizaron simultáneamente coprocultivo y DiaSorin LIAISON®Campylobacter assay para detectar la presencia de Campylobacter spp.
ResultadosAsumiendo el cultivo convencional como el método de referencia perfecto, DiaSorin LIAISON®Campylobacter assay obtuvo una sensibilidad y una especificidad del 100% y 97,7%, respectivamente; y del 99,1% y 98,6%, respectivamente, asumiendo el cultivo como método de referencia imperfecto (modelo bayesiano).
ConclusiónEsta nueva plataforma podría ser una herramienta útil, especialmente para el cribado de resultados negativos.
Campylobacter spp. is the leading cause of foodborne diarrheal illness worldwide. Campylobacter jejuni, with almost 90% of reported cases of campylobacteriosis, is the species that cause most human gastrointestinal infections (GI), characterized by diarrhea, fever, and abdominal cramps.1,2
Campylobacter spp. are bacteria requiring special culture requirements.3 Direct planting onto a selective media, followed by incubation at 42°C under microaerophilic conditions for at least 48h, has long been considered the reference standard for diagnosis. Moreover, this microbiological procedure was designed to recover and identify only the most common pathogenic strains.4
Culture-independent diagnostic tests (CIDTs) for detection of this fastidious organism, have emerged mainly in clinical settings, as an alternative to standard culture methods.2,5,6 CIDTs, including molecular methods or methods based on antigen detection, enable not only the rapid identification of this gastrointestinal pathogen, but also the rapid screening for negative results.7,8 However, culture-based method remains essential for the confirmation of the microorganism viability as well as to perform drug susceptibility tests and epidemiologic studies.2
The aim of this study was to evaluate the antigen-based detection fully-automated random-access platform based on chemiluminescence technology, DiaSorin LIAISON®Campylobacter assay (DiaSorin, Saluggia, Italy), for the diagnosis of human GI caused by Campylobacter species.
MethodologyStudy designA prospective study was conducted at the Microbiology Department of Vall d’Hebron University Hospital of Barcelona (Spain) during two different periods, 10 consecutive days in May 2015 and 5 consecutive days in February 2016. All stool samples from patients suspected of having GI were sent to the laboratory as part of hospital routine diagnosis to perform stool culture. Simultaneously, samples were studied with the DiaSorin LIAISON®Campylobacter assay in order to detect the presence of Campylobacter spp. antigens.
Stool cultureSamples were collected in sterile containers without transport media and delivered to the laboratory under refrigeration (4°C). For Campylobacter spp. culture, a portion of stool was directly plated onto a Charcoal differential agar (Oxoid, Hampshire, United Kingdom) followed by 48h incubation at 42°C under microaerophilic conditions (CampyGen™, Oxoid, United Kingdom). Suspicious colonies were identified by means of oxidase cytochrome tests, fuchsine staining and MALDI-TOF Mass Spectrometry using the VITEK® MS (bioMérieux, Marcy l’Étoile, France).
LIAISON®CampylobacterThe DiaSorin LIAISON®Campylobacter assay was performed according to manufacturer's instructions. Briefly, the stool sample was mixed with 750μL of diluent, using the LIAISON® Stool Extraction Device scoop, in case of solid feces, and 750μL, in case of liquid or semi-solid feces, and screwed the conical blue filter unit onto the mixing tube. The mixture was vortexed vigorously for 20s and centrifuged with the conical tube pointing up at speed of ≥2000×g for 10min. Subsequently, the device was inverted and centrifuged at 200×g for 1min. The mixing tube/blue filter unit was discarded and placed into the DiaSorin Analyzer. The result was considered negative if the index was <0.9, equivocal if ≥0.9, and <1.1, and positive if ≥1.1.
Statistical analysisPerformance parameters as sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of the DiaSorin LIAISON®Campylobacter assay were estimated by using conventional culture as perfect gold standard and as imperfect gold standard using a web-based application (http://mice.tropmedres.ac) Mahidol Oxford Tropical Medicine Research Unit (MORU), Bangkok, Thailand).9
ResultsA total of 645 stool samples from 640 patients suspected of having a GI were included in the study. Of these, 74.4% were outpatients and 15.6% were inpatients. Of the 645 analyzed specimens, 26 (4.0%) were positive (24 identified as C. jejuni and 2 as Campylobacter coli) and 606 (94.0%) were negative by both culture and LIASON®Campylobacter assay. The chemiluminescence-based assay and stool culture methods showed positivity rates of 6% and 4% respectively, and there was no equivocal result of the new platform according to manufacturer instructions. There were 13 (2%) discordant samples all with positive antigen detection and negative culture results. Of these, 9/13 (69.2%) patients presented symptoms compatible with GI while in 4 cases the diagnostic suspicion was not related to GI or the information was not available. Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) parameters are shown in Table 1.
Prevalence, sensitivities, specificities, and positive and negative predictive values (PPV and NPV) estimated by using the conventional culture (assuming that a test is perfect) and imperfect gold standard model (Bayesian latent class model).
| Parameters | Conventional culture was assumed as a perfect gold standard (%)a | Bayesian latent class model (%)b |
|---|---|---|
| Prevalence | ||
| Inpatients | 6.5 (3.5–11.7) | 7.8 (4.0–13.0) |
| Outpatients | 3.1 (1.8–5.3) | 4.0 (2.1–6.7) |
| Conventional culture | ||
| Sensitivity | 100 (100–100) | 82.9 (56.0–99.9) |
| Specificity | 100 (100–100) | 100 (99.6–100) |
| PPV | 100 (100–100) | 99.1 (90.4–100) |
| NPV | 100 (100–100) | 99.1 (97.0–100) |
| LIASON Campylobacter | ||
| Sensitivity | 100 (84.0–100) | 99.1 (91.1–100) |
| Specificity | 97.7 (96.1–98.7) | 98.6 (96.9–100) |
| PPV | 65.0 (48.3–78.9) | 78.6 (55.4–99.9) |
| NPV | 100 (99.2–100) | 100 (99.5–100) |
Additionally, other enteric pathogens isolated by stool culture or detected by antigen detection were: Salmonella spp. (n=9), Shigella flexneri (n=2), enteropathogenic Escherichia coli (EPEC) (n=2), toxin producing Clostridium difficile strains (n=2), non-toxin producing Clostridium difficile strains (n=8), Helicobacter pylori (n=9), rotavirus (n=10), and adenovirus (n=5). None of these pathogens was found in co-infection with Campylobacter spp.
DiscussionCampylobacteriosis is usually a self-limited illness and antimicrobial therapy is not required. However, patients with specific clinical circumstances, such as severe or prolonged illness or immunocompromised state, may benefit of an early diagnosis in order to provide them an appropriate therapy.1,10 A prospective study has been conducted to determine the performance characteristics of the novel stool antigen test, the DiaSorin LIASON®Campylobacter assay. Clinical Microbiology laboratories have assumed increasing responsibility for the rapid and accurate detection of a diverse number of pathogens. DiaSorin LIASON®Campylobacter assay allows reducing the response time from 48hours to less than 2h.
Moreover, DiaSorin LIASON®Campylobacter assay is also a time saving assay taking only about 15min of hands-on-time work. In contrast, culture-based methods require special conditions such as microaerobic environment as well as additional tests in order to confirm the identification of the microorganism.3
Up to now, selective culture techniques are mainly designed for the isolation of C. jejuni/C. coli, the main species associated with human GI.11 However, detection of non-jejuni/coli Campylobacter species, with unclear clinical relevance, by antigen detection CIDTs have been widely reported.12,13 Additionally, recent publications on CIDTs for the detection of Campylobacter spp. claimed that these tests have demonstrated a considerable number of false positives in clinical testing recommending to confirm all positive results by other method.14,15
In the present study DiaSorin LIASON®Campylobacter assay showed a higher positivity rate than conventional culture. This fact could be initially interpreted as an increased sensitivity of this technique against conventional culture, but it should be considered whether discordant results are due to a lack of specificity of the antigen-based assay. On the other hand it is remarkable that 9/13 of the patients with discordant results presented gastrointestinal infection symptoms and no other pathogen was isolated, which would reinforce the results of the new assay. These discordant results could also be explained by the detection of non-jejuni/coli Campylobacter species by DiaSorin LIASON®Campylobacter assay that would not be detected by the conventional culture. Due to the possibility of false positive results of the chemiluminescence assay it would be advisable to confirm these cases by other method. A limitation to our study was the lack of confirmation by a molecular technique of these discordant results to properly examine the nature of these results and to well establish DiaSorin LIASON®Campylobacter assay specificity value and PPV. Finally, regarding the negative results, NPV of both culture and antigen-based methods was 100%. These results point to this antigen-based technic as a good tool for rapid screenings of negative results.
In conclusion, this assay can be considered a useful tool for a rapid campylobacteriosis diagnosis. Additionally, it could be an effective tool for the rapid screening of negative results especially in laboratories assuming an increasingly workload due to its low time-consuming nature. By discarding Campylobacter spp. infection, this test would allow a new diagnostic approach to other organisms causing diarrhea. Unfortunately, up to now there is no available data concerning the cost-effectiveness of the LIASON®Campylobacter assay, both in terms of their impact on laboratory costs and the managing downstream costs of patients with diarrhea. Further studies are needed either to establish the cost-effectiveness of this platform and also to clarify if this test is able to detect other non-jejuni/coli Campylobacter species.
Conflicts of interestThe authors declare that there have no conflicts of interest.