Late diagnosis (LD) of human immunodeficiency virus (HIV) infection (CD4 lymphocytes <350/μl at diagnosis of the disease), deteriorates the condition of those affected and increases the probability of transmission. The objective of the present study was to analyse the prevalence of LD, to identify missed diagnostic opportunities (MDO) and to find out which level of the health care delivery system they took place.
MethodsRetrospective, observational and descriptive study of the population diagnosed with infection of HIV/AIDS in the period 2011–2015 in Aragon. MDO were identified during the 3 years prior to diagnosis of the disease in all levels of the health care delivery system as well as frequentation of consultations. The indicator conditions (IC) that generated more MDO were analysed according to the latest recommendations for early diagnosis of HIV in the health care setting.
Results435 newly diagnosed HIV/AIDS cases were analysed. 45.1% were diagnosed in Primary Healthcare (PH). 49.4% presented criteria of LD and 61.1% were infected through heterosexual contact. The majority of MDO (68.5%) were given in PH. The IC that generated the most MDO were seborrheic dermatitis/exanthema (19.4%) and fever of unknown origin (10.3%). However, the IC that were associated with higher LD were pneumonia acquired in the community and unjustified weight loss.
ConclusionIn Aragon, prevalence of LD is high, the main route of infection is heterosexual and most of MDO go unnoticed in PH. The dissemination of current guidelines for requesting IC guided HIV testing and HIV screening across the preoperative period will result in an effective measure to decrease the LD.
El diagnóstico tardío (DT) de la infección por el virus de la inmunodeficiencia humana (VIH) (linfocitos CD4<350/μl al diagnóstico de la enfermedad) empeora el pronóstico de los afectados y aumenta las probabilidades de transmisión. El objetivo del presente trabajo fue analizar la prevalencia de DT, identificar las oportunidades diagnósticas perdidas (ODP) y averiguar el nivel asistencial donde se produjeron.
MétodosEstudio retrospectivo, observacional, descriptivo de la población diagnosticada de infección por VIH/sida en el periodo 2011-2015 en Aragón. Se identificaron las ODP durante los 3 años previos al diagnóstico de la enfermedad en todos los niveles asistenciales, así como la frecuentación asistencial. Se analizaron las condiciones indicadoras (CI) que generaron más ODP, según las últimas recomendaciones para el diagnóstico precoz del VIH en el medio sanitario.
ResultadosSe analizaron 435 nuevos casos de VIH/sida. El 45,1% fueron diagnosticados en Atención Primaria (AP). El 49,4% presentaron criterios de DT y el 61,1% se contagiaron vía heterosexual. La mayor parte de ODP (68,5%) se dieron en AP. Las CI que generaron más ODP fueron la dermatitis seborreica/exantema (19,4%) y la fiebre sin causa aparente (10,3%). Sin embargo, las CI que se asociaron a mayor DT fueron la neumonía adquirida en la comunidad y la pérdida de peso injustificada.
ConclusiónEn Aragón, la prevalencia de DT es elevada, la principal vía de transmisión es la heterosexual y la mayor parte de las CI pasan desapercibidas en AP. La difusión de las guías actuales para solicitar una prueba de VIH orientada por CI y el screening VIH en todo preoperatorio es una medida eficaz para disminuir el DT.
The late diagnosis (LD) of human immunodeficiency virus (HIV) infection has been defined as the presence of less than 350 CD4/μl at the diagnosis of HIV.1 LD is a significant public health problem both in Spain and in Europe, since it increases the morbidity and mortality of affected patients (up to 8–10 times) and the risk of developing AIDS defining events (ADE), since the patients who initiate antiretroviral therapy (ART) with a lower number of CD4 lymphocytes take longer to regain immunity.2,3 Patients with CD4 lower than 200/μl or an ADE at the time of diagnosis have a risk of death 5.22 times higher than those who do not present with LD.4
Taking into account the efficacy of current ART, missed diagnostic opportunities represent the key point to change the course of the epidemic and reduce its expansion. To make an earlier diagnosis, it is necessary to create guidelines that motivate clinicians to actively search for cases of HIV infection. According to estimates of the Spanish National AIDS Plan, approximately 30% of those infected with HIV in Spain are undiagnosed,5 and the rate of transmission of the infection in patients who do not know their HIV-positive status is 3.5 times higher than that of those who know their infection status, since they do not receive treatment.6,7 The promotion of early diagnosis is one of the priority strategies of prevention and care programmes in HIV infection in developed countries.
Although no consensus has been reached on the criteria regarding the strategies to follow to achieve an early diagnosis of HIV infection and to significantly reduce LD,8 in recent years, two documents have been created with the aim of improving early diagnosis by following a targeted strategy, based on a series of diseases or pathologies associated with a high prevalence of undiagnosed HIV. These two documents are: in Europe, the guideline HIV Indicator Conditions: Guidance for Implementing HIV Testing in Adults in Health Care Settings9 and, in Spain, the Guideline of recommendations for the early diagnosis of HIV in the health field10 (Table 1). In accordance with these recommendations, if any of those pathologies or indicator conditions (IC) occur, it would have been indicated to perform HIV testing.
Indicator conditions in which it would be indicated to order an HIV test.
| AIDS-defining diseases | Diseases associated with undiagnosed HIV prevalence >0.1% | Risk factors |
|---|---|---|
| 1. Cervical cancer | 27. Sexually transmitted infections | |
| 2. Non-Hodgkin's lymphoma | 28. Malignant lymphoma | 57. To all those who request it because risk exposure is suspected |
| 3. Kaposi's sarcoma | 29. Anal cancer/dysplasia | 58. Sexual partners of people infected with HIV |
| 4. Mycobacterium tuberculosis, pulmonary or extrapulmonary | 30. Cervical dysplasia | 59. Current PDUs, or with a history of having been one, and their sexual partners |
| 5. Mycobacterium avium complex (MAC) or Mycobacterium kansasii, disseminated or extrapulmonary | 31. Herpes zoster | 60. MSM and their sexual partners (men and women) |
| 6. Mycobacterium, other species or unidentified species, disseminated or extrapulmonary | 32. Hepatitis B or C (acute or chronic) | 61. People who practise prostitution: women, men and transsexuals, their sexual partners and their clients |
| 7. Recurrent pneumonia (2 or more episodes in 12 months) | 33. Mononucleosis syndrome | 62. Heterosexual people with more than one sexual partner and/or risky practices in the last 12 months |
| 8. Septicaemia due to recurrent salmonella | 34. Leukocytopenia/idiopathic thrombocytopenia lasting >4 weeks | 63. People who want to stop using condoms with their stable partners |
| 9. Cytomegalovirus retinitis | 35. Seborrhoeic dermatitis/rash | 64. People who have suffered sexual assault |
| 10. Cytomegalovirus, other (except liver, spleen, lymph nodes) | 36. Invasive pneumococcal disease | 65. People who have had risk exposure to HIV, occupational or non-occupational (accidental) |
| 11. Herpes simplex bronchitis/pneumonitis, common herpes ulcer(s) >1 month | 37. Fever without apparent cause | |
| 12. Progressive multifocal leukoencephalopathy | 38. Candidemia | |
| 13. Herpes simplex: chronic ulcers (>1 month) or bronchitis, pneumonia or esophagitis | 39. Visceral leishmaniasis | |
| 14. Cerebral toxoplasmosis | Other diseases that are considered likely to have an undiagnosed HIV prevalence >0.1% | |
| 15. Diarrhoeic cryptosporidiosis, >1 month | 40. Primary lung cancer | Other situations |
| 16. Isosporiasis, >1 month | 41. Lymphocytic meningitis | 66. People from countries with high prevalence (>1%) and their sexual partners |
| 17. Atypical disseminated leishmaniasis | 42. Oral hairy leukoplakia | 67. Gestation (implications for the foetus) |
| 18. Reactivation of American trypanosomiasis (meningoencephalitis or myocarditis) | 43. Severe or atypical psoriasis | |
| 19. Fungal infections | 44. Guillain-Barré syndrome | |
| 20. Pneumonia caused by Pneumocystis carinii | 45. Mononeuritis | |
| 21. Thrush, oesophageal | 46. Subcortical dementia | |
| 22. Thrush, bronchial/tracheal/pulmonary | 47. Multiple sclerosis-type disease | |
| 23. Cryptococcosis, extrapulmonary | 48. Peripheral neuropathy | |
| 24. Histoplasmosis, disseminated/extrapulmonary | 49. Unexplained weight loss | |
| 25. Coccidioidomycosis, disseminated/extrapulmonary | 50. Idiopathic lymphadenopathy | |
| 26. Penicilliosis, disseminated | 51. Idiopathic oral thrush | |
| 52. Chronic idiopathic diarrhoea | ||
| 53. Chronic idiopathic renal failure | ||
| 54. Hepatitis A | ||
| 55. Community-acquired pneumonia | ||
| 56. Thrush |
MSM: men who have sex with men; PDU: parenteral drug users; HIV: human immunodeficiency virus.
Source: prepared based on the recommendations collected by the HIV platform in Europe (HIV Indicator Conditions: Guidance for Implementing HIV Testing in Adults in Health Care Settings, 2013) and the Spanish National AIDS Plan (Guide for recommendations for the early diagnosis of HIV in the health field, 2014).
We believe that, up to the time of diagnosis, people living with HIV who have not yet been diagnosed consult for a large number of episodes at different levels of care, generating significant missed diagnostic opportunities (MDO).
The primary endpoint of the study is to determine the prevalence of LD in the new diagnoses of HIV/AIDS infection in Aragón, and to describe the epidemiological characteristics and those related to the diagnosis.
As secondary endpoints, we intend to analyse the contacts with the Health System of Aragón, the MDOs in the three years prior to the diagnosis and their relationship with the LD.
MethodsDesignRetrospective, observational and descriptive study of patients diagnosed with HIV/AIDS infection between 1 January 2011 and 31 December 2015, communicated to the New HIV Infection Diagnoses Information System (SINIVIH) in Aragón.
PopulationThe study population was made up of all the new HIV infection/AIDS diagnoses in the period 2011–2015 collected in the SINIVIH in Aragón. After the review of each case in the electronic medical record (EMR) of the Aragón Health Service, those subjects diagnosed with HIV/AIDS prior to 2011 and erroneously reported to the SINIVIH in the period 2011–2015, whether from Aragón or from any other autonomous community, were excluded.
Data collectionIn Aragón, since 2008, all episodes or reasons why a patient requests health care, regardless of the level of care, are recorded in the EMR. In addition, referrals to Specialist Care (SC) consultations are reflected.
We studied the prevalence of the population with LD (diagnosis of HIV infection with a CD4 lymphocyte level<350/μl) according to established definition criteria,1 the mechanism of transmission of the sample and the area where the diagnosis of HIV infection/AIDS was made. The incidence of the disease per 100,000 inhabitants was calculated, overall, and by sex, age groups, province, nationality, region of origin and years under study.
The existence of ICs justifying HIV testing was analysed, following the recommendations of the WHO, the HIV Platform in Europe and the Spanish National AIDS Plan9,10 (Table 1). Situations or risk factors for the transmission of HIV infection were coded in a unified way in the variable “risk factors” in order to simplify the recording in the data collection. The mortality of the sample up to December 2016 and the survival in the first year from the time of diagnosis of the disease were also recorded.
To identify possible MDOs prior to the diagnosis of HIV/AIDS, the attention given to each new diagnosis of HIV infection/AIDS in the three years prior to diagnosis was reviewed in the EMR, whether in the area of Primary Care (PC), Urgent Care or during possible hospital admissions. In addition, prior visits to the emergency department (ED) were investigated.
Additionally, we analysed the frequency of care in all healthcare areas, the number of MDOs and its association with the main and dependent variable of the study: LD. In this paper, the diagnosis of AIDS only was not included as a variable. The classification of each new HIV infection/AIDS diagnosis in the HIV or AIDS categories is made once the patient is diagnosed. In this study, the factors prior to the diagnosis of the disease are analysed, so we will refer to the sample as new diagnoses of HIV infection/AIDS, without making distinctions between the two categories.
For the analysis of the visits to the different levels of care and of the MDOs associated with the new HIV/AIDS diagnoses in the three years prior to the diagnosis of the disease, those patients whose mechanism of transmission was maternal–foetal were excluded, since the HIV condition was imposed on them from the time of birth.
The study was approved by the Independent Ethics Committee of Aragón (CEICA) on 20/04/2016.
VariablesThe socio-demographic variables (sex, age, date of birth, province where the patient resided at diagnosis and country of origin), those related to the diagnosis (date of diagnosis, mechanism of transmission and centre that requested the first HIV test) and the clinical–analytical variables at the time of diagnosis (CD4/μl lymphocyte numbers and the presence of AIDS-defining diseases) were all investigated.
For the purposes of this study, the event or subsidiary episode of requesting an HIV test has been called an “indicator condition”, taking into account the current recommendations.9,10 On the other hand, a “missed diagnostic opportunity” has been defined as the lack of a request for an HIV test for any person in the study diagnosed with HIV infection/AIDS who, having been treated in the Aragón Public Health System, presented an IC. The number of MDOs for each IC was recorded overall, and by level of care. According to the number of MDOs at each level of care, the following groups were established: “no MDO”, “one MDO” and “more than one MDO”.
The number of consultations in each level of care was counted and if any hospital admission was required in the three years prior to the diagnosis of the disease. According to the number of consultations in each health care area or number of hospital admissions, different categories were established:
- -
In PC: “no consultation”, “one consultation” and “more than one consultation”.
- -
In Urgent Care: “no visits”, “one visit” and “more than one visit”.
- -
Hospital admissions: “requires admission” and “does not require admission”.
- -
In ED: “no consultation”, “one consultation” and “more than one consultation”.
The data was collected in a structured database in Microsoft Excel® and analysed with the software programme IBM SPSS Statistics v. 23.0® for Windows.
For the calculation of the incidence rates per 100,000 population of the socio-demographic variables, the population data of the Statistics Institute of Aragón (IAEST) were used as reference.11
The demographic data provided by the SINIVIH of Europeans diagnosed with HIV infection/AIDS in Aragón are classified, according to their region of origin, as “Western Europe” and “Eastern Europe”. In our study, the calculation in these two groups of the HIV/AIDS incidence rate per 100,000 population had to be carried out jointly, since the classification of Europeans registered in Aragón used by the IAEST follows other criteria.
A descriptive analysis of the data was carried out. The qualitative variables studied (socio-demographic variables, diagnostic characteristics, MDOs for each IC at an overall level and at each healthcare level of the total sample, as well as the prevalence of ICs that generated more MDOs in the population with LD) were presented by the frequency distribution of the percentages of each category. Indicators of central tendency (mean) and dispersion (standard deviation [SD]) were given for the average age of the study population and for the average value of consultations both overall and at each healthcare level.
The effect on the LD of the socio-demographic variables, the variables related to the diagnosis, and on the ICs that generated more MDOs, was investigated by means of the Chi-square test (contrast hypothesis test), with comparison of proportions as they are qualitative variables In the case of multiple proportion comparisons, to find out the effect on the LD of the socio-demographic variables and the variables related to the diagnosis, the Bonferroni correction was applied to the p-value.
To find the risk factors associated with the presence of LD, an analysis was performed using logistic regression models, taking as a dependent variable the presence or absence of LD and as independent variables those variables that were either significant in the bivariate analysis, or were considered important to control its effect.
The odds ratios (OR) were estimated in univariate models (models without adjusting for the other covariates), and the ORs were estimated by adjusting for the rest of the covariates (multivariate regression model). Estimated ORs were given with their 95% confidence interval (CI) and their associated p-value. The goodness of fit of the multivariate model was verified through the Hosmer–Lemeshow test. This test presented a significance higher than 0.05, which indicates that the model is adequate.
The effects were considered significant if p<0.05, and the values presented are two-tailed.
FundingThe development of this study has not required funding sources.
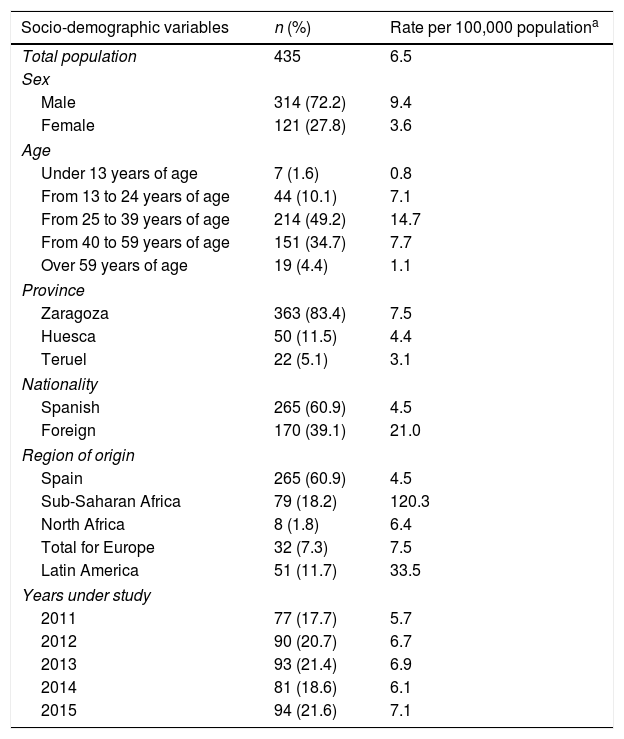
ResultsAccording to data provided by the SINIVIH in Aragón, the number of new HIV infection/AIDS diagnoses in 2011–2015 was 518 cases. A total of 83 cases were ruled out because they were diagnoses prior to 2011. The final study population was 435 new cases of HIV/AIDS, with an incidence of 6.5 new diagnoses per 100,000 population, without adjusting for the delay in notification by the clinicians to SINIVIH (Table 2).
Socio-demographic characteristics (rates per 100,000 population) and related to the diagnosis of new HIV infection/AIDS diagnoses.
| Socio-demographic variables | n (%) | Rate per 100,000 populationa |
|---|---|---|
| Total population | 435 | 6.5 |
| Sex | ||
| Male | 314 (72.2) | 9.4 |
| Female | 121 (27.8) | 3.6 |
| Age | ||
| Under 13 years of age | 7 (1.6) | 0.8 |
| From 13 to 24 years of age | 44 (10.1) | 7.1 |
| From 25 to 39 years of age | 214 (49.2) | 14.7 |
| From 40 to 59 years of age | 151 (34.7) | 7.7 |
| Over 59 years of age | 19 (4.4) | 1.1 |
| Province | ||
| Zaragoza | 363 (83.4) | 7.5 |
| Huesca | 50 (11.5) | 4.4 |
| Teruel | 22 (5.1) | 3.1 |
| Nationality | ||
| Spanish | 265 (60.9) | 4.5 |
| Foreign | 170 (39.1) | 21.0 |
| Region of origin | ||
| Spain | 265 (60.9) | 4.5 |
| Sub-Saharan Africa | 79 (18.2) | 120.3 |
| North Africa | 8 (1.8) | 6.4 |
| Total for Europe | 32 (7.3) | 7.5 |
| Latin America | 51 (11.7) | 33.5 |
| Years under study | ||
| 2011 | 77 (17.7) | 5.7 |
| 2012 | 90 (20.7) | 6.7 |
| 2013 | 93 (21.4) | 6.9 |
| 2014 | 81 (18.6) | 6.1 |
| 2015 | 94 (21.6) | 7.1 |
| Diagnostic characteristics, n (%) | Total patients |
|---|---|
| Diagnostic area | |
| Primary care | 196 (45.1) |
| Specialist care | 239 (54.9) |
| Mechanism of transmission | |
| Heterosexual | 266 (61.1) |
| MSM | 97 (22.3) |
| PDU | 20 (4.6) |
| Maternal–foetal | 7 (1.6) |
| Unknown | 45 (10.3) |
| Late diagnosis | |
| Yes | 215 (49.4) |
| No | 220 (50.6) |
MSM: men who have sex with men; PDU: parenteral drug users.
There were no statistically significant differences in the incidence according to the years under study. The average age of the sample was 37.1 years (SD: 11.9), the diagnosis of the disease was more frequent in the age group between 25 and 39 years, and most of the new cases resided in the province of Zaragoza. More than half of the new diagnoses were Spanish; however, the incidence of HIV/AIDS was five times higher in the foreign population. Of these, those from sub-Saharan Africa were the majority group, representing half of the total, followed by Latin Americans. The incidence in individuals from sub-Saharan Africa is notable, almost 30 times higher than that of the native population (Table 2).
The HIV/AIDS diagnosis of the sample was performed almost equally in both PC and in ED. The main mechanism of transmission of the disease were heterosexual relationships, accounting for more than half of the total cases, followed by transmission between men who have sex with men (MSM). More than 80% of those affected were transmitted sexually. Half of the new HIV infection/AIDS diagnoses presented criteria compatible with LD (Table 2).
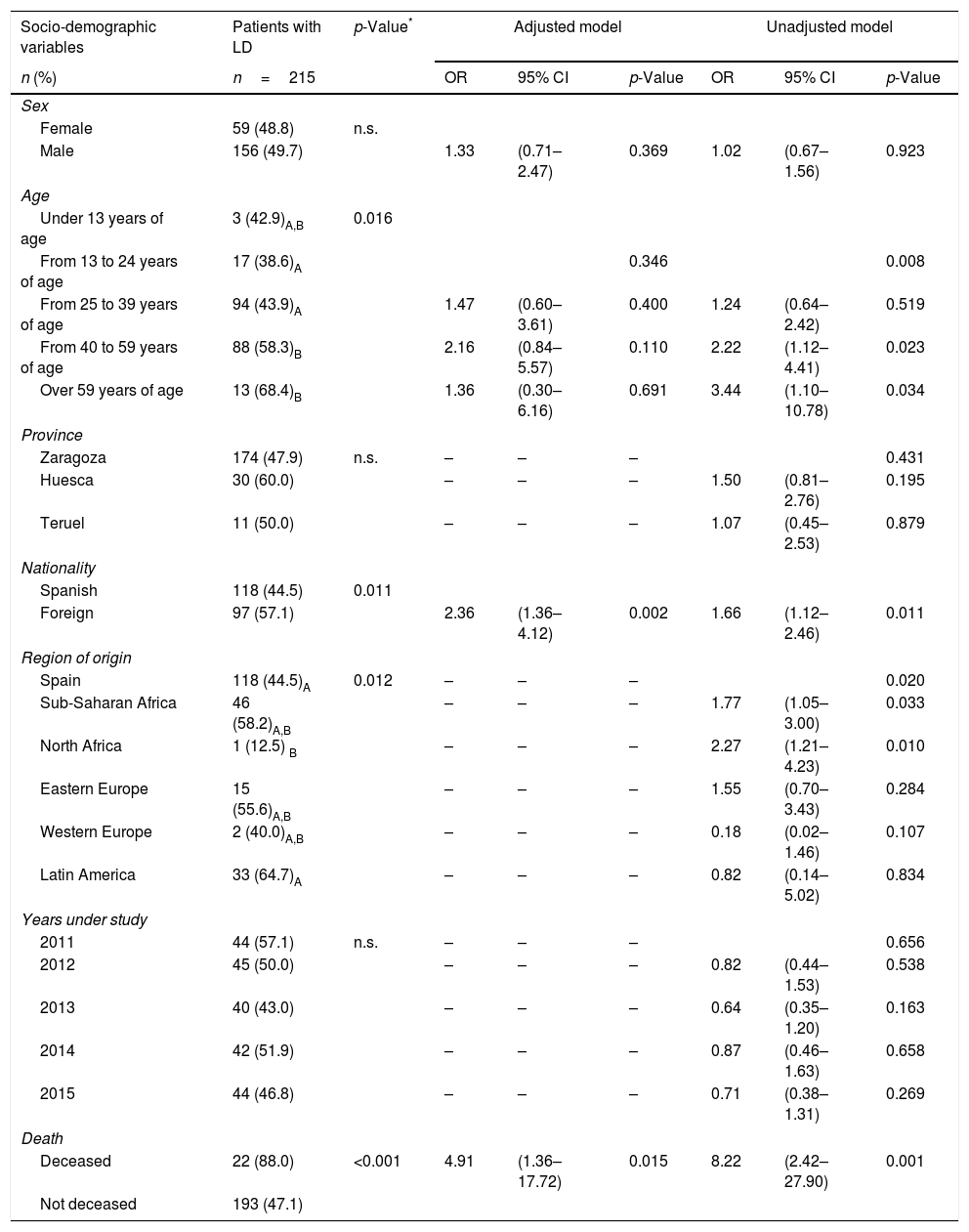
LD of HIV/AIDS was more common as age increased, especially after the age of 40 (Table 3). The prevalence of LD was higher in the foreign population than in the native population (p=0.011) (Table 3). Of the eight cases originating in North Africa, only one had LD. The mortality of our population up to December 2016 was 5.7%, and the survival in the first year was 95.4%. 88% of the deceased up to December 2016 had LD criteria, compared to 47.1% of the non-deceased, with p<0.001.
Socio-demographic characteristics, related to the diagnosis, frequency of health care visits and number of missed diagnostic opportunities of the population with late diagnosis. Logistic regression of the factors associated with late diagnosis.
| Socio-demographic variables | Patients with LD | p-Value* | Adjusted model | Unadjusted model | ||||
|---|---|---|---|---|---|---|---|---|
| n (%) | n=215 | OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| Sex | ||||||||
| Female | 59 (48.8) | n.s. | ||||||
| Male | 156 (49.7) | 1.33 | (0.71–2.47) | 0.369 | 1.02 | (0.67–1.56) | 0.923 | |
| Age | ||||||||
| Under 13 years of age | 3 (42.9)A,B | 0.016 | ||||||
| From 13 to 24 years of age | 17 (38.6)A | 0.346 | 0.008 | |||||
| From 25 to 39 years of age | 94 (43.9)A | 1.47 | (0.60–3.61) | 0.400 | 1.24 | (0.64–2.42) | 0.519 | |
| From 40 to 59 years of age | 88 (58.3)B | 2.16 | (0.84–5.57) | 0.110 | 2.22 | (1.12–4.41) | 0.023 | |
| Over 59 years of age | 13 (68.4)B | 1.36 | (0.30–6.16) | 0.691 | 3.44 | (1.10–10.78) | 0.034 | |
| Province | ||||||||
| Zaragoza | 174 (47.9) | n.s. | – | – | – | 0.431 | ||
| Huesca | 30 (60.0) | – | – | – | 1.50 | (0.81–2.76) | 0.195 | |
| Teruel | 11 (50.0) | – | – | – | 1.07 | (0.45–2.53) | 0.879 | |
| Nationality | ||||||||
| Spanish | 118 (44.5) | 0.011 | ||||||
| Foreign | 97 (57.1) | 2.36 | (1.36–4.12) | 0.002 | 1.66 | (1.12–2.46) | 0.011 | |
| Region of origin | ||||||||
| Spain | 118 (44.5)A | 0.012 | – | – | – | 0.020 | ||
| Sub-Saharan Africa | 46 (58.2)A,B | – | – | – | 1.77 | (1.05–3.00) | 0.033 | |
| North Africa | 1 (12.5) B | – | – | – | 2.27 | (1.21–4.23) | 0.010 | |
| Eastern Europe | 15 (55.6)A,B | – | – | – | 1.55 | (0.70–3.43) | 0.284 | |
| Western Europe | 2 (40.0)A,B | – | – | – | 0.18 | (0.02–1.46) | 0.107 | |
| Latin America | 33 (64.7)A | – | – | – | 0.82 | (0.14–5.02) | 0.834 | |
| Years under study | ||||||||
| 2011 | 44 (57.1) | n.s. | – | – | – | 0.656 | ||
| 2012 | 45 (50.0) | – | – | – | 0.82 | (0.44–1.53) | 0.538 | |
| 2013 | 40 (43.0) | – | – | – | 0.64 | (0.35–1.20) | 0.163 | |
| 2014 | 42 (51.9) | – | – | – | 0.87 | (0.46–1.63) | 0.658 | |
| 2015 | 44 (46.8) | – | – | – | 0.71 | (0.38–1.31) | 0.269 | |
| Death | ||||||||
| Deceased | 22 (88.0) | <0.001 | 4.91 | (1.36–17.72) | 0.015 | 8.22 | (2.42–27.90) | 0.001 |
| Not deceased | 193 (47.1) | |||||||
| Diagnostic characteristics | Patients with LD | p-Value* | Adjusted model | Unadjusted model | ||||
|---|---|---|---|---|---|---|---|---|
| n (%) | n=215 | OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| Diagnostic area | ||||||||
| Primary Care | 79 (40.3) | 0.001 | ||||||
| Specialist Care | 136 (56.9) | 2.80 | (1.62–4.86) | <0.001 | 1.99 | (1.35–2.93) | <0.001 | |
| Mechanism of transmission | ||||||||
| Heterosexual | 130 (48.9) | n.s. | 0.688 | 0.801 | ||||
| MSM | 47 (48.5) | 1.25 | (0.71–2.18) | 0.572 | 0.98 | (0.62–1.57) | 0.944 | |
| PDU | 12 (60.0) | 1.06 | (0.36–3.13) | 0.588 | 1.57 | (0.62–3.96) | 0.340 | |
| Maternal-foetal | 3 (42.9) | |||||||
| Unknown | 23 (51.1) | 1.21 | (0.59–2.48) | 0.625 | 1.09 | (0.58–2.06) | 0.781 | |
| Level of care | Patients with LD | p-Value* | Adjusted model | Unadjusted model | ||||
|---|---|---|---|---|---|---|---|---|
| n (%) | n=212 | OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| Primary care | ||||||||
| No consultation | 12 (54.5) | n.s. | 0.518 | 0.775 | ||||
| One consultation | 11 (55.0) | 1.17 | (0.29–4.69) | 0.819 | 1.02 | (0.30–3.44) | 0.976 | |
| More than one consultation | 189 (49.0) | 0.71 | (0.25–1.98) | 0.508 | 0.80 | (0.34–1.89) | 0.611 | |
| Urgent care | ||||||||
| No visits | 47 (35.6)A | 0.001 | 0.059 | <0.001 | ||||
| One visit | 57 (51.8)B | 2.07 | (0.98–4.37) | 0.056 | 1.95 | (1.16–3.26) | 0.012 | |
| More than one visit | 108 (58.1)B | 2.38 | (1.15–4.93) | 0.019 | 2.20 | (1.58–3.97) | <0.001 | |
| Hospital admission | ||||||||
| No admission | 167 (47.3) | 0.046 | ||||||
| One or more admissions | 45 (60.0) | 1.19 | (0.66–2.12) | 0.563 | 1.67 | (1.01–2.77) | 0.047 | |
| Number of MDOs | ||||||||
| No MDO | 19 (32.2)A | <0.001 | 0.021 | 0.001 | ||||
| One MDO | 24 (38.1)A | 1.67 | (0.28–3.07) | 0.902 | 1.30 | (0.61–2.73) | 0.469 | |
| More than one MDO | 169 (55.2)B | 2.65 | (1.01–6.93) | 0.047 | 2.60 | (1.44–4.69) | 0.002 | |
The first category is taken in the form of a reference in the event that there are more than two categories. In dichotomous variables, the value of the variable that is contrasted in relation to the complementary value is indicated.
CI: confidence interval; LD: late diagnosis; MDO: missed diagnostic opportunities; MSM: men who have sex with men; n.s.: not significant; OR: odds ratio; PDU: parenteral drug users.
The population diagnosed in the ED had a higher prevalence of LD. There was no statistical significance in the prevalence of LD by sex, provinces, years under study or among the different mechanisms of transmission of the disease, despite the fact that 60% of the new HIV/AIDS diagnoses that were transmitted through the use of injected drugs had LD criteria (Table 3).
The 428 new HIV infection/AIDS diagnoses in 2011–2015 generated a total of 7475 consultations between PC, Urgent Care services, hospital admissions and ED consultations in the three years prior to diagnosis. 65% of these consultations were made in PC, 12.2% in Urgent Care, 1.2% during hospital admissions and 21.6% in ED consultations.
The average number of consultations in PC, Urgent Care and ED of each new case of HIV infection/AIDS was 17.2 (SD: 14.7): 11.4 consultations in PC (SD: 9.9), 2.1 assessments in Urgent Care (SD: 3.0) and 3.7 consultations in ED (SD: 5.7).
The most frequently consulted specialists were: first the group of purely surgical specialities, second Dermatology and third Digestive, representing 34.6% of the total number of consultations in this health area.
One in every five new HIV/AIDS cases was admitted in the three years prior to diagnosis (mean: 0.2; SD: 0.5). The three departments in which our population were most frequently admitted were, in order: grouped surgical services, Internal and Digestive Medicine, representing more than half of the total admissions.
The prevalence of LD increased the higher the frequency of care in emergency services and the greater the number of hospital admissions required by our population, prior to the diagnosis of the disease (Table 3).
13.8% (59 cases) of the new HIV infection/AIDS diagnoses did not present any MDO in the three years prior to the diagnosis of the disease at any level of care, 14.7% (63 cases) a single MDO and 71.5% (306 cases) more than one MDO.
The LD increased the greater the number of MDOs: 32.2% of those affected without any MDO had LD criteria, compared to 55.2% with more than one MDO in the three years prior to HIV infection/AIDS diagnosis, with statistically significant differences being seen between both groups (Table 3).
Table 3 shows the variables that were shown to be risk factors associated with the presence of LD in HIV infection/AIDS:
- 1.
The risk of presenting LD is seen to be twice as high if the patient is in the age group of 40–59 years compared to the group of 13–24 years.
- 2.
Being a foreigner results in the risk of having LD being twice as high compared to a patient born in Spain (Table 3).
- 3.
The risk of having LD increases two-fold if the disease was diagnosed in the ED.
- 4.
The risk of starting with LD criteria is twice as high if a visit to Urgent Care is made compared to not going at any time.
- 5.
Although requiring a hospital admission increases the risk of having LD almost two-fold, when adjusting the model for the rest of the variables it is not significant.
- 6.
Having more than one MDO increases the risk of having LD almost three times compared to those who do not have any MDO in the three years prior to the diagnosis of the disease.
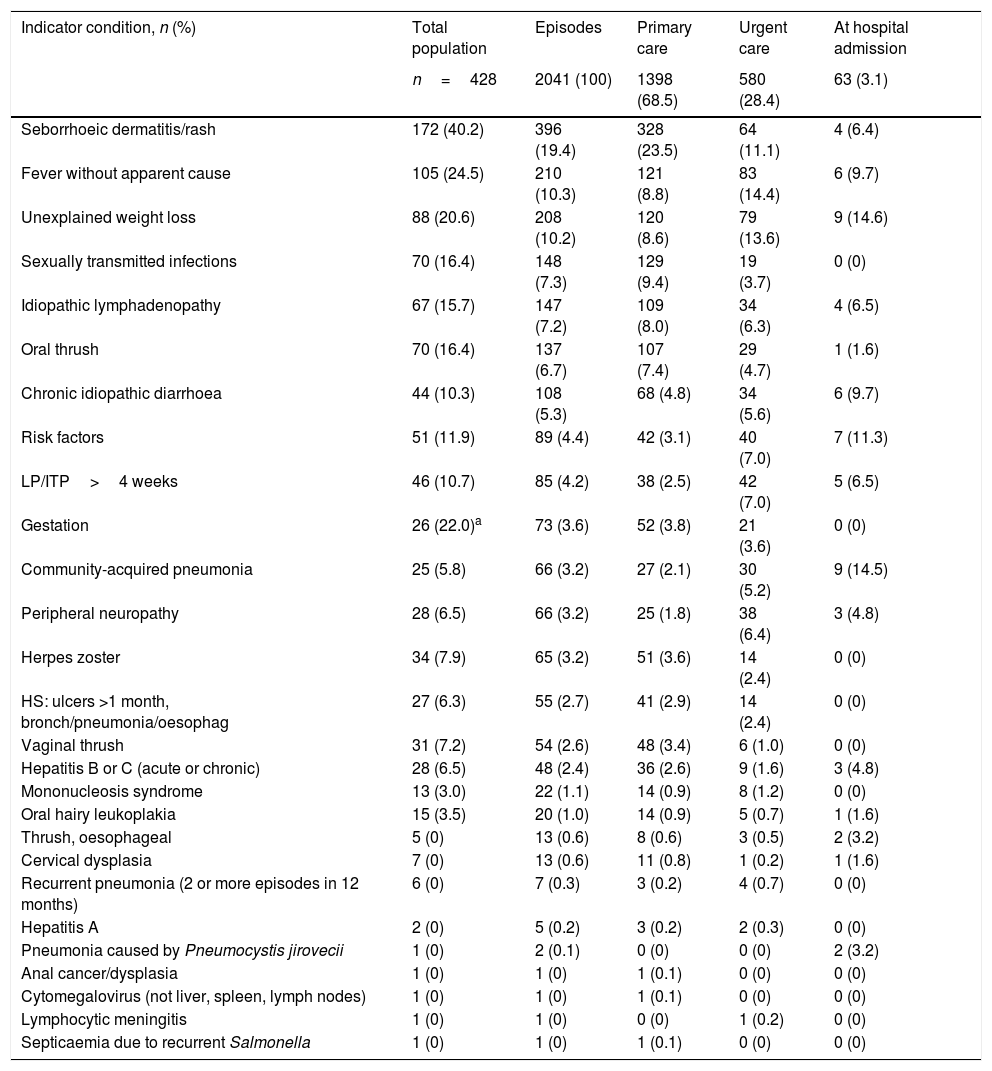
The five most frequent ICs that generated more MDOs in the study population were: seborrhoeic dermatitis or rash, fever without apparent cause, unexplained weight loss, sexually transmitted infections and oral thrush (Table 4).
Prevalence and number of missed diagnostic opportunities for each indicator condition of the new HIV infection/AIDS diagnoses at the different levels of care.
| Indicator condition, n (%) | Total population | Episodes | Primary care | Urgent care | At hospital admission |
|---|---|---|---|---|---|
| n=428 | 2041 (100) | 1398 (68.5) | 580 (28.4) | 63 (3.1) | |
| Seborrhoeic dermatitis/rash | 172 (40.2) | 396 (19.4) | 328 (23.5) | 64 (11.1) | 4 (6.4) |
| Fever without apparent cause | 105 (24.5) | 210 (10.3) | 121 (8.8) | 83 (14.4) | 6 (9.7) |
| Unexplained weight loss | 88 (20.6) | 208 (10.2) | 120 (8.6) | 79 (13.6) | 9 (14.6) |
| Sexually transmitted infections | 70 (16.4) | 148 (7.3) | 129 (9.4) | 19 (3.7) | 0 (0) |
| Idiopathic lymphadenopathy | 67 (15.7) | 147 (7.2) | 109 (8.0) | 34 (6.3) | 4 (6.5) |
| Oral thrush | 70 (16.4) | 137 (6.7) | 107 (7.4) | 29 (4.7) | 1 (1.6) |
| Chronic idiopathic diarrhoea | 44 (10.3) | 108 (5.3) | 68 (4.8) | 34 (5.6) | 6 (9.7) |
| Risk factors | 51 (11.9) | 89 (4.4) | 42 (3.1) | 40 (7.0) | 7 (11.3) |
| LP/ITP>4 weeks | 46 (10.7) | 85 (4.2) | 38 (2.5) | 42 (7.0) | 5 (6.5) |
| Gestation | 26 (22.0)a | 73 (3.6) | 52 (3.8) | 21 (3.6) | 0 (0) |
| Community-acquired pneumonia | 25 (5.8) | 66 (3.2) | 27 (2.1) | 30 (5.2) | 9 (14.5) |
| Peripheral neuropathy | 28 (6.5) | 66 (3.2) | 25 (1.8) | 38 (6.4) | 3 (4.8) |
| Herpes zoster | 34 (7.9) | 65 (3.2) | 51 (3.6) | 14 (2.4) | 0 (0) |
| HS: ulcers >1 month, bronch/pneumonia/oesophag | 27 (6.3) | 55 (2.7) | 41 (2.9) | 14 (2.4) | 0 (0) |
| Vaginal thrush | 31 (7.2) | 54 (2.6) | 48 (3.4) | 6 (1.0) | 0 (0) |
| Hepatitis B or C (acute or chronic) | 28 (6.5) | 48 (2.4) | 36 (2.6) | 9 (1.6) | 3 (4.8) |
| Mononucleosis syndrome | 13 (3.0) | 22 (1.1) | 14 (0.9) | 8 (1.2) | 0 (0) |
| Oral hairy leukoplakia | 15 (3.5) | 20 (1.0) | 14 (0.9) | 5 (0.7) | 1 (1.6) |
| Thrush, oesophageal | 5 (0) | 13 (0.6) | 8 (0.6) | 3 (0.5) | 2 (3.2) |
| Cervical dysplasia | 7 (0) | 13 (0.6) | 11 (0.8) | 1 (0.2) | 1 (1.6) |
| Recurrent pneumonia (2 or more episodes in 12 months) | 6 (0) | 7 (0.3) | 3 (0.2) | 4 (0.7) | 0 (0) |
| Hepatitis A | 2 (0) | 5 (0.2) | 3 (0.2) | 2 (0.3) | 0 (0) |
| Pneumonia caused by Pneumocystis jirovecii | 1 (0) | 2 (0.1) | 0 (0) | 0 (0) | 2 (3.2) |
| Anal cancer/dysplasia | 1 (0) | 1 (0) | 1 (0.1) | 0 (0) | 0 (0) |
| Cytomegalovirus (not liver, spleen, lymph nodes) | 1 (0) | 1 (0) | 1 (0.1) | 0 (0) | 0 (0) |
| Lymphocytic meningitis | 1 (0) | 1 (0) | 0 (0) | 1 (0.2) | 0 (0) |
| Septicaemia due to recurrent Salmonella | 1 (0) | 1 (0) | 1 (0.1) | 0 (0) | 0 (0) |
HS: ulcers >1 month, bronch/pneumonia/oesophag: herpes simplex as the causative agent of chronic ulcers lasting more than one month, bronchitis, pneumonia or oesophagitis; LP/ITP >4 weeks: leukopenia/idiopathic thrombocytopenia lasting more than 4 weeks.
More than two thirds (68.5%) of all MDOs were in PC (Table 4). In other words, 1398 ICs that justified HIV testing went unnoticed by PC physicians. Almost one third (28.4%) of all MDOs occurred in emergency services and 3.1% during hospital admissions, with the disease being diagnosed later.
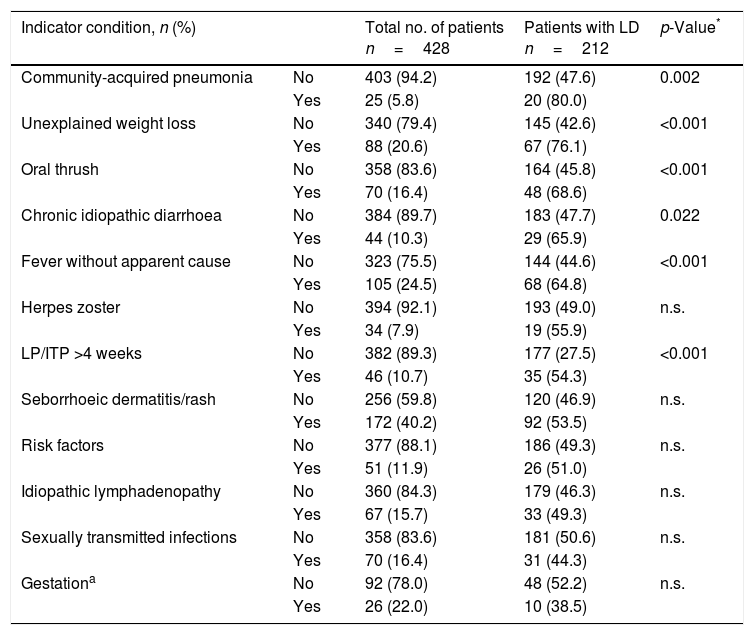
Table 5 shows the number and proportion of patients with LD for each of the most prevalent ICs in the study population. There are statistically significant differences in the prevalence of LD in the population that had, prior to the diagnosis of HIV/AIDS: community-acquired pneumonia, unexplained weight loss, oral thrush, chronic idiopathic diarrhoea, fever without apparent cause and leukopenia or idiopathic thrombocytopenia lasting for more than 4 weeks, compared to the population with LD that did not have these ICs.
Prevalence of the indicator conditions that generated more missed diagnostic opportunities in the population with late diagnosis.
| Indicator condition, n (%) | Total no. of patients n=428 | Patients with LD n=212 | p-Value* | |
|---|---|---|---|---|
| Community-acquired pneumonia | No | 403 (94.2) | 192 (47.6) | 0.002 |
| Yes | 25 (5.8) | 20 (80.0) | ||
| Unexplained weight loss | No | 340 (79.4) | 145 (42.6) | <0.001 |
| Yes | 88 (20.6) | 67 (76.1) | ||
| Oral thrush | No | 358 (83.6) | 164 (45.8) | <0.001 |
| Yes | 70 (16.4) | 48 (68.6) | ||
| Chronic idiopathic diarrhoea | No | 384 (89.7) | 183 (47.7) | 0.022 |
| Yes | 44 (10.3) | 29 (65.9) | ||
| Fever without apparent cause | No | 323 (75.5) | 144 (44.6) | <0.001 |
| Yes | 105 (24.5) | 68 (64.8) | ||
| Herpes zoster | No | 394 (92.1) | 193 (49.0) | n.s. |
| Yes | 34 (7.9) | 19 (55.9) | ||
| LP/ITP >4 weeks | No | 382 (89.3) | 177 (27.5) | <0.001 |
| Yes | 46 (10.7) | 35 (54.3) | ||
| Seborrhoeic dermatitis/rash | No | 256 (59.8) | 120 (46.9) | n.s. |
| Yes | 172 (40.2) | 92 (53.5) | ||
| Risk factors | No | 377 (88.1) | 186 (49.3) | n.s. |
| Yes | 51 (11.9) | 26 (51.0) | ||
| Idiopathic lymphadenopathy | No | 360 (84.3) | 179 (46.3) | n.s. |
| Yes | 67 (15.7) | 33 (49.3) | ||
| Sexually transmitted infections | No | 358 (83.6) | 181 (50.6) | n.s. |
| Yes | 70 (16.4) | 31 (44.3) | ||
| Gestationa | No | 92 (78.0) | 48 (52.2) | n.s. |
| Yes | 26 (22.0) | 10 (38.5) | ||
LD: late diagnosis; LP/ITP >4 weeks: leukopenia/idiopathic thrombocytopenia lasting more than 4 weeks; n.s.: not significant.
The incidence of new HIV infection/AIDS diagnoses in Aragón in 2011–2015 was 6.5 cases per 100,000 population, lower than that calculated in Spain and worldwide,12,13 and similar to that estimated in Europe14 in the same year. The incidence of HIV infection/AIDS was five times higher in the foreign population than in the native population; however, it is necessary to mention that, for the calculation of this datum, the demographic data of the IAEST were taken into account11 (based on the Municipal Register), and this organisation does not include foreigners in an irregular situation. There are no real censuses of the foreign population in Spain in all situations regarding legality, which is why we believe that there is a possible overestimation of the incidence of HIV/AIDS in the foreign population in our study.
Considering that the delay in notification is similar in recent years, the overall incidence in our study, once all new HIV/AIDS diagnoses in the study period were reported to the SINIVIH in Aragón, would be 7.7 cases per 100,000 population.
The LD was slightly higher than the whole of Europe and Spain.12,14,15 These differences may be due to the greater percentage of population from endemic regions, which in our study showed greater immunosuppression at diagnosis of the disease.
The proportion of women diagnosed with HIV infection/AIDS was higher in our study than in Spain and Europe,12,14 and lower than what is estimated worldwide.13 These differences with Spain and Europe are due to the higher percentage of affected women from other countries (65.3%).
The most common age group was 25–39 years old. Among foreigners, those from sub-Saharan Africa and Latin America were the most numerous groups, and this situation is similar to that of Spain and Europe.12,14,15 The percentage of foreign population was slightly higher than that of Spain and Europe.12,14,15 These differences may also be due to the influence exerted in our study by the greater proportion of women from other countries. The main mechanism of transmission of our population was heterosexual followed by MSM transmission, a situation inverse to that observed in Spain and Europe.12,14,15 The influence of the sub-Saharan and North African population, as well as the greater proportion of women in our study, could justify these differences.
The proportion of patients diagnosed with HIV infection/AIDS in PC was higher than that found by Mahendran et al.16 in the United Kingdom, a country that has a similar public health system. In this sense, PC is the health resource most frequently used, so we can expect a higher proportion of new cases of the disease, diagnosed at this level of care, than in many regions of the world with more privatised health systems. The health care characteristics in Spain allow the foreign population in an irregular situation to access only with an emerging health problem in urgent care services.
LD affected both genders equally and increased the older the age at the time of diagnosis of the disease. LD was also higher in the foreign population (especially in those from sub-Saharan Africa and Latin America), in those who died during the first year after the diagnosis of the disease and in parenteral drug users (PDUs). These same situations were repeated in Spain and Europe.12,14,15
The one-year survival of our sample was slightly lower than that of Spain15 and higher than that of the reviewed investigations, whose populations presented higher LD.17–19 In addition, therapeutic advances in ART have also contributed to an increase in survival.20,21
Missed diagnostic opportunities86.2% of our study population had at least one MDO in the three years prior to the HIV infection/AIDS diagnosis. When comparing the number of MDOs of our population with the rest of the investigations, the differences are also variable: Levy et al.,22 with a population that had a lower prevalence of LD (33.2%), found in all cases at least one MDO in the five years prior to the diagnosis of the disease. However, Wohlgemut et al. (36.8%),23 Rivero et al. (49.1%),24 Gullón et al. (46.6%)25 and Joore et al. (58.8%26and 60.7%27) found a proportion of cases with at least one MDO lower than that of our study. These differences are due to the characteristics of the sample and the time of MDO search prior to the diagnosis of the disease. In most of the referenced studies, the sample had lower LD and the search for MDOs was less than three years, so we expect a smaller number of MDOs.
The distribution of MDOs by level of care of our population is similar to the visits if the classification is simplified in PC and ED. The proportion of MDOs (Table 4) in PC in our study was 68.5%, lower than that found by Gullón et al.25 (79.3%) and greater than that found by Rivero et al.24 (47.2%) in two studies conducted in Spain. The proportion of MDOs in PC was lower in other studies22,23,28,29 conducted outside of Spain. This situation seems reasonable considering the characteristics of our health system and the greater number of visits to PC.
The prevalence of MDOs associated with the five most frequent ICs of our study was higher than that found by most authors22–25,29, except for Ellis et al., whose study population showed greater immunosuppression at the diagnosis of the disease (LD: 71.1%). There is a strong association between LD and a greater frequency of IC onset prior to the HIV/AIDS diagnosis. In contrast, we have not found studies that relate the prevalence of LD for each of the ICs.
Taking into account the excellent results of HIV screening in pregnancy, which is a cost-effective test,13,30 and that surgical services was the set of specialities where most of our population were admitted and consulted prior to the HIV/AIDS diagnosis, we believe that an effective measure to improve early diagnosis is HIV screening in all pre-operative procedures.
Although seborrhoeic dermatitis/rash and fever without apparent cause were the most frequent ICs, community-acquired pneumonia and unexplained weight loss were the ICs that were associated with higher LD, thus we hope that the dissemination of the current guidelines for the realisation of HIV in IC,9,10 mainly in PC, favour the progressive increase in the offer of HIV testing under IC. It should be mentioned that the guidelines used to define IC were published in 2013 and 2014, and the study includes cases diagnosed in 2011–2015, so these recommendations could not be taken into account by health professionals before their publication. In other words, some of the ICs included were not considered as such before 2013.
The study has limitations. As it is a retrospective observational study, in which the electronic medical records of each patient were reviewed, it is possible that information not reflected in the EMR was missed. Another limitation is the delay in the notification to SINIVIH. This problem has occurred in all the autonomous communities for years in Spain, so new cases of HIV infection/AIDS reported after 2015 possibly correspond to our study period. When evaluating MDOs, those that could have occurred in ED consultations were not taken into account, since in this last level of care the information is still collected in the physical record on paper. In the same way, and continuing with the analysis of the MDOs, it can be expected that, as it is a study that analyses the ICs that went unnoticed in the public health system of Aragón, it has not been possible to register all the possible MDOs of patients treated in parallel in the private sphere.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Our most sincere thanks to Mr Javier Toledo Pallarés, for his collaboration in the writing of this study.
Please cite this article as: Gargallo-Bernad C, Sangrós-González FJ, Arazo-Garcés P, Martínez-Álvarez R, Malo-Aznar C, Gargallo-Bernad A, et al. Oportunidades perdidas en el diagnóstico de la infección por el virus de inmunodeficiencia humana en la Comunidad de Aragón. Importancia del diagnóstico tardío. Enferm Infecc Microbiol Clin. 2019;37:100–108.