Embryo development is a very complex process and depends not only on the culture system but also on the entire environment of the in vitro fertilization laboratory (IVF). That is why the correlation between the contaminants present in the IVF laboratory and the specific harmful effects on human gametes and embryos is necessary but scientific evidence, especially on early stages of development, is insufficient.
Materials & methodsTherefore, information and data on how these contaminants could affect the health of oocytes and embryos has been compiled, making an extensive literature search. Types of contaminants, sources, environmental control and the harmful effects they can cause are described.
ResultsDifferent types of particles (PM) and volatile organic compounds (VOCs) could affect cellular structures interrupting cellular communication, modifying viability and changing their molecular profile, making them more vulnerable to hereditary mutations. Some adverse effects on embryonic maturation, morphology, segmentation, blastocyst development, hatching and implantation are described, which are related to negative clinical results.
DiscussionDespite the existing scientific evidence on how pollutants are detrimental to reproduction and development, the literature is scarce and dispersed. This literature provides an idea about the extent of the damage that pollutants can produce and the chemical or molecular mechanisms that could be involved. More studies with similar designs are needed to investigate further, especially about VOCs.
El desarrollo embrionario es un proceso muy complejo y no solo depende del sistema de cultivo sino también de todo el entorno del laboratorio de fecundación in vitro (FIV). Por esto, la correlación entre los contaminantes presentes en el laboratorio de FIV y los efectos nocivos específicos sobre gametos y embriones humanos es necesaria pero la evidencia científica, especialmente sobre etapas de desarrollo tempranas, es insuficiente.
Materiales y métodosPor lo tanto, se ha recopilado información y datos sobre cómo estos contaminantes podrían afectar la salud de los ovocitos y los embriones, realizando una extensa búsqueda bibliográfica. Los tipos de contaminantes, las fuentes, el control ambiental y los efectos nocivos que pueden causar, son descritos.
ResultadosDiferentes tipos de partículas (PM) y compuestos orgánicos volátiles (COV), podrían afectar estructuras celulares interrumpiendo la comunicación celular, modificando la viabilidad y cambiando su perfil molecular haciéndolos más vulnerables a las mutaciones hereditarias. Se describen algunos efectos adversos sobre maduración embrionaria, morfología, segmentación, desarrollo de blastocitos, eclosión y la implantación, los cuales están relacionados a resultados clínicos negativos.
DiscusiónA pesar de la evidencia científica existente sobre cómo los contaminantes son perjudiciales para la reproducción y el desarrollo, la literatura es escasa y dispersa. Esta literatura proporciona una idea sobre la magnitud del daño que los contaminantes pueden producir, y los mecanismos químicos o moleculares que podrían estar involucrados. Se necesitan más estudios con diseños similares para investigar más a fondo, especialmente sobre los COV.
The majority of assisted reproductive techniques (ART) facilities are located in urban areas and they are exposed to the pollutants produced in the external environment but, due to the daily routine activities and equipment within IVF laboratories, the air quality also diminish from the outside of a building throughout the laboratory (De Los Santos, 2001; Esteves and Bento, 2016). In addition, although the incubators have controlled conditions, the environment provided is strongly influenced by unexpected sources of pollutants (Khoudja et al., 2013) as it has been found that the highest values of toxic compounds were inside the IVF incubators because every time an incubator is opened, gas concentrations and temperature conditions can be significantly disturbed due to the large air-exchange volume with the laboratory's ambient air (94–95%) or because the chemicals are released from the gas bottles (5%), especially if they have not received a routinely maintenance (Cohen et al., 1997; Fujiwara et al., 2007; Meseguer et al., 2012). Similarly, a 5–6-fold increase of VOCs has been found inside the incubators and even higher in the IVF's adjacent areas (Hall et al., 1998).
These observations can be relevant as the fertilization, cleavage and blastocyst formation rates increase after improving the air quality of the laboratory. Interestingly, certain compounds are capable of diffusing into the culture media and adversely affect gametes and embryos, at a sensitive stage, with devastating outcomes (Fabro, 1978; Khoudja et al., 2013; Munch et al., 2015). So guidelines have been developed to prevent these but not much is yet known about what types of pollutants and their concentrations represent danger to embryos and future offspring.
Here, we review the information currently available describing known pollutants and sources, how they might be controlled inside IVF laboratories, as well as possible impacts on early embryonic development and IVF outcomes.
Materials and methodsThis is a comprehensive review that presents information and some data on how environment-related pollutants could impact the reproductive outcomes, especially in female gametes and embryos that are exposed to the IVF laboratory's ambient air. Semen quality studies were not included. An electronic literature and the PubMed database search was performed using Spanish and English keywords such as IVF, ambient air pollution, particulate matter, volatile organic compounds and human embryo, or specific compounds that are known to be in the ambient air of IVF laboratories. Publications older than 5 years old are included because key information on this subject is still too dispersed in time. No language limits were established.
Specific studies in animal models were included due to the void of experimentation in humans possibly due to the legal and/or ethical restrictions that may exist. 135 references have been selected for this comprehensive review. 46 references are studies related to pollution over human health (prospective: 16, retrospective: 30). However, only 16 studies are specific on the deleterious effects of pollution over the IVF environment.
Types, sources, environmental control and some pollutants effects have been compiled for a better understanding of the evolution of the IVF's environment, and the path that remains ahead for waging against known and unknown pollution.
ResultsTypes of pollutantsAtmospheric air pollutants can be categorized as primary pollutants (directly emitted from their sources into the atmosphere) or secondary pollutants (formed from photochemical reactions from primary pollutants), or classified according to chemical composition (organic or inorganic), sources (natural or anthropogenic), degradation properties (degradable or non-degradable), place of generation (indoor or outdoor), or based on the state of matter (Daly and Zannetti, 2007). Each pollutant has its very own reaction properties, emission, persistence in the environment, ability to be transported in long and short distances and their specific impact on human and/or animal health. Additionally, the pollutants that are known or suspected to cause irreversible illnesses because of their toxicity, such as cancer or reproductive effects, are classified as Hazardous Air Pollutants (HAP) (Kampa and Castanas, 2008; U.S. Environmental Protection Agency, 2017a). Inside the IVF laboratories, there are two general types of pollutants that have our attention: the particulate matter and the VOCs.
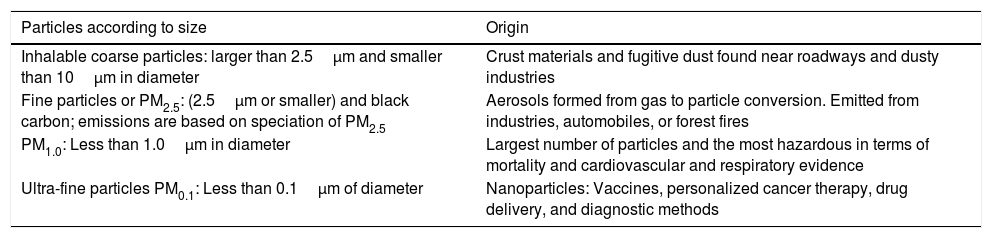
Particulate matterPM is a complex mixture of extremely small solid and liquid particles (droplets) that can contain a wide range of inorganic and organic components. These are the most common atmospheric pollutants and their mass and composition are strongly influenced by climatic and meteorological conditions. PM can be categorized by aerodynamic diameter as size is a critical determinant of the likelihood and site of deposition within the respiratory tract shown below (Table 1).
Classification of PM according to size and origin. (Samet et al., 2005; U.S. Environmental Protection Agency, 2016; Centre Interprofessionnel Technique d’Etudes de la Pollution Atmosphérique, 2016).
| Particles according to size | Origin |
|---|---|
| Inhalable coarse particles: larger than 2.5μm and smaller than 10μm in diameter | Crust materials and fugitive dust found near roadways and dusty industries |
| Fine particles or PM2.5: (2.5μm or smaller) and black carbon; emissions are based on speciation of PM2.5 | Aerosols formed from gas to particle conversion. Emitted from industries, automobiles, or forest fires |
| PM1.0: Less than 1.0μm in diameter | Largest number of particles and the most hazardous in terms of mortality and cardiovascular and respiratory evidence |
| Ultra-fine particles PM0.1: Less than 0.1μm of diameter | Nanoparticles: Vaccines, personalized cancer therapy, drug delivery, and diagnostic methods |
Most PM, especially the smallest fractions, are known to cause serious health problems due to their ability to penetrate deep into the blood stream and through different mechanisms or interactions that will depend on the type of exposition (acute and chronic, or outdoor or indoor exposure) (Shah et al., 2013, 2015; U.S. Environmental Protection Agency, 2016). Some of the most common PM associated with human health problems comprises the heavy metals (Suvarapu and Baek, 2016; World Health Organization, 2007), the Polycyclic Aromatic Hydrocarbons (Januario et al., 2009; Kim et al., 2013; Mesquita et al., 2014) or other organic components originated by the oxidation of VOCs, nanoparticles (Ray et al., 2009) and the endotoxins (not discussed in this review) etc. (Centre Interprofessionnel Technique d́Etudes de la Pollution Atmosphérique, 2016).
The heavy metals (HM) particles are responsible of toxic effects when they are involved in the biochemical reactions of living organisms. Lead (Pb), cadmium (Cd), and mercury (Hg) are non-essential HM are some of the most common toxic HM to which humans are exposed due to their persistent, accumulative, and toxic nature (Suvarapu and Baek, 2016). These non-essential HM are emitted through industrial activities and they can be found in almost every region. Cd, for example, is a compound that can be found in almost everything and it can travel long distances from its source via natural and anthropogenic atmospheric transports. Cd can be found attached to 0.1–1-μm size particles, which have an atmospheric lifetime of a few days, depending on the particle density and meteorological parameters. Other HM may be present for weeks or even months (World Health Organization, 2007).
The polycyclic aromatic hydrocarbons (PAHs) are organic toxicants formed from incomplete combustion processes, which can be absorbed by PM with carbonaceous cores contributing to the strong toxic potential of submicron-sized particles. Moreover, atmospheric organic compounds (both in gas and particulate phase) generate oxidized derivatives by photochemical processes (Kim et al., 2013; Mesquita et al., 2015). A few PAHs can be found in both, particulate and volatile phase because they can be vaporized when exposed to above room temperatures; for this reason they can be called semi VOCs as well. High molecular weight PAHs are preferentially attached or absorbed by particles because of their low pressure in the air; but because of the impact of the indoor characteristics and activities, the low molecular weight PAHs can be or become, volatile: naphthalene (most volatile: 98%), fluorene, acenaphtene, acenaphtylene, phenanthrene, anthracene, fluoroanthene, pyrene (less volatile: 55%). Some of the diesel exhaust particles (DEP) are composed from both particle and semi volatile PAHs (Januário et al., 2009; World Health Organization, 2000; Kim et al., 2013; Mesquita et al., 2014).
Ultrafine particles or nanoparticles (NPs) are synthesized from materials such as cadmium selenide (CdSe), gold (Au), silver (Ag), perylene (C20H12), carbon, polystyrene, iron oxide (Fe2O3), silica (SiO2), titanium dioxide (TiO2), and organics such as latex, polylactic acid, polyglycolic acid, and polyalkylcyanoacrylate. As a result, a large number of products exist in any clinical setting that can release NPs into the environment. They have been widely applied in biomedicine for different purposes in human and veterinary medicine, during preclinical or clinical phases (in the development of vaccines, personalized cancer therapy, drug delivery, and diagnostic methods, among others) (Bosman et al., 2005). But it is uncertain which NPs are related to an IVF setting.
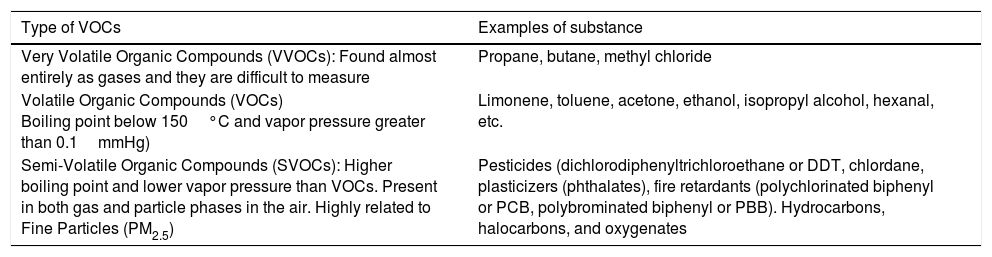
Volatile organic compoundsVOCs are gaseous emissions of organic compounds that participate in forming ozone (O3) and have health implications such as cancer and reproductive toxicity (Webb et al., 2014). VOCs are chemicals that contain carbon (C) along with other elements (hydrogen, oxygen, fluorine, chlorine, bromine, sulfur, or nitrogen). However, carbon monoxide, carbon dioxide, carbonic acid, metallic carbides, or carbonates and ammonium carbonate are not VOCs. Their composition makes it possible for them to evaporate under normal indoor atmospheric conditions of temperature and pressure (U.S. Environmental Protection Agency, 2017b). VOCs are formed as intermediate compounds during the combustion, decomposition, or breakdown of longer-chain carbon compounds, as well as during the photosynthesis process in vegetation (Pinto et al., 2010; Eller et al., 2016; Dutta et al., 2016).
VOCs have an initial boiling point less than or equal to 250°C, measured at a standard atmospheric pressure of 101.3kPa. The higher the volatility (lower boiling point) the more likely the compounds will be emitted into the air due to weakened intermolecular forces. The United States Environmental Protection Agency (EPA) has technically categorized these compounds depending on the ease with which they are emitted (Table 2) (U.S. Environmental Protection Agency, 2017b).
Classification of indoor VOCs according to volatile properties by the WHO. (U.S. Environmental Protection Agency, 2017b; Wei and Li, 2010; Butterwick et al., 1997).
| Type of VOCs | Examples of substance |
|---|---|
| Very Volatile Organic Compounds (VVOCs): Found almost entirely as gases and they are difficult to measure | Propane, butane, methyl chloride |
| Volatile Organic Compounds (VOCs) Boiling point below 150°C and vapor pressure greater than 0.1mmHg) | Limonene, toluene, acetone, ethanol, isopropyl alcohol, hexanal, etc. |
| Semi-Volatile Organic Compounds (SVOCs): Higher boiling point and lower vapor pressure than VOCs. Present in both gas and particle phases in the air. Highly related to Fine Particles (PM2.5) | Pesticides (dichlorodiphenyltrichloroethane or DDT, chlordane, plasticizers (phthalates), fire retardants (polychlorinated biphenyl or PCB, polybrominated biphenyl or PBB). Hydrocarbons, halocarbons, and oxygenates |
Outdoor pollution contributes to indoor air quality; the type of ventilation (natural or forced), the ventilation rate (air changes per hour), and the nature of the contaminants are variables that affect the environment (Daly and Zannetti, 2007; Wei and Li, 2010; Butterwick et al., 1997; Jones, 1999; Perin et al., 2010a). Moreover, PM and VOCs are present inside IVF laboratories through various vectors such as Heating, Ventilation, and Air Conditioning (HVAC) systems, diffusion of volatiles from adjacent rooms and hallways, off-gassing materials, equipment, people (perfumes and personal odors), medical and anesthetic gases, etc. Additional sources include potable water, dust, glass fragments, alcohol burners, disposable plastic ware and their shavings, markers, disinfectants, microscopes, television monitors, furniture, etc. VOCs are the most worrisome airborne pollutants because the embryo toxicity risk in the clinical setting could in fact, be two to five times higher. VOCs are constantly released as different types of unsaturated volatiles and accumulate through the oxidation of air and light over routinely used materials, even when laboratories use appropriate materials to accomplish clean room standards (Hall et al., 1998; Jones, 1999; Lawrence et al., 2007; Khoudja et al., 2013; Wale and Gardner, 2016; ESHRE Guideline Group on Good Practice in IVF Labs et al., 2016). Within 18 to 300 volatile compounds have been reported inside the laboratories, linked to overhauls such as constructions, refurbishments (installation of filters), punctual events such as fumigations (Cohen et al., 1997), or to inner activities (Hall et al., 1998) that increase certain pollutants concentrations found on specific laboratory's spots: inside incubators (Cohen et al., 1998; Hall et al., 1998) or minihoods used for oocyte retrieval (Boone et al., 1999), etc., but it has not been done an official compilation on this matter on peer review and the majority of related investigations were carried out years ago (Khoudja et al., 2013; Cohen et al., 1997; Gilligan et al., 1997; Nijs et al., 2009). The biggest concern is that VOCs, such as benzene, can be produced inside the incubators (CO2 gas cylinders) and may contaminate gametes and embryos (De Los Santos, 2001; Cohen et al., 1998; Mehta, 2013). Additionally, VOCs are difficult to remove from IVF's ambient air and from the incubators, and they can interact with PM as well (Khoudja et al., 2013; Wale and Gardner, 2016).
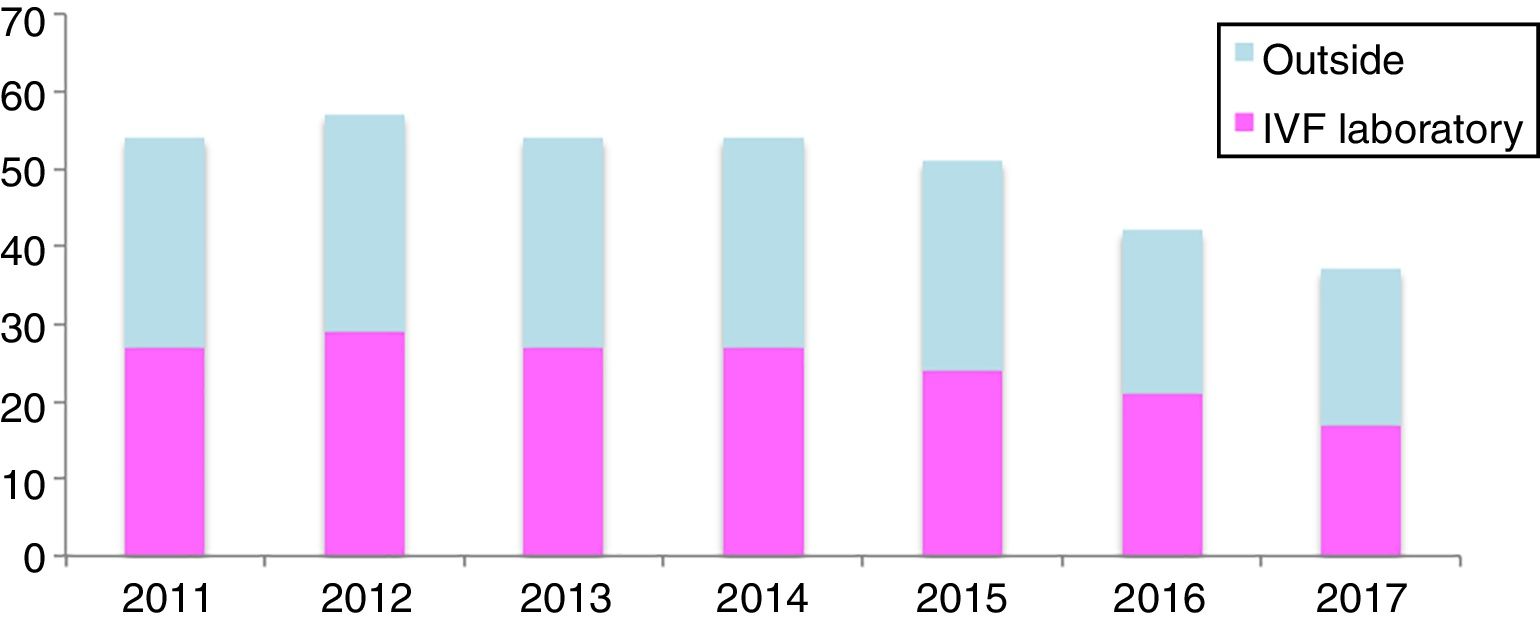
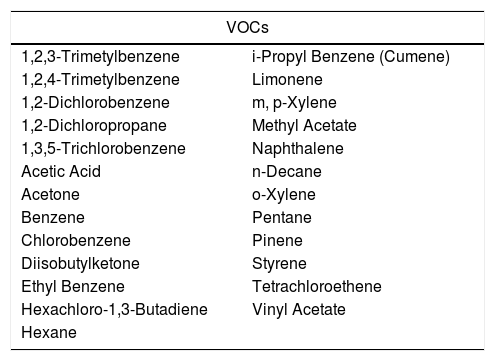
For example, in our experience (unpublished), when analyzing the VOCs concentrations, detected in all our clinics in Spain (13 clinics) over a seven-year period (2011–2017), we were able to recognize that there are certain VOCs present constantly inside the IVF laboratories. The concentrations analyzed were measured outside, inside the clinics and inside the IVF laboratories, taken once per year by an external audit service. 54 compounds were screened and each one of them was detected in at least 1 of the clinics per year. 25 VOCs are detected every year (46.3%) (Fig. 1), and 5 more are very common despite not being present every year: 1,3,5-trimetylbenzene, 1,4-dichlorobenzene, dichloromethane, 1,2,4-trichlorobenzene and toluene (Table 3).
25 VOCs present every year inside all IVI clinics in Spain.
| VOCs | |
|---|---|
| 1,2,3-Trimetylbenzene | i-Propyl Benzene (Cumene) |
| 1,2,4-Trimetylbenzene | Limonene |
| 1,2-Dichlorobenzene | m, p-Xylene |
| 1,2-Dichloropropane | Methyl Acetate |
| 1,3,5-Trichlorobenzene | Naphthalene |
| Acetic Acid | n-Decane |
| Acetone | o-Xylene |
| Benzene | Pentane |
| Chlorobenzene | Pinene |
| Diisobutylketone | Styrene |
| Ethyl Benzene | Tetrachloroethene |
| Hexachloro-1,3-Butadiene | Vinyl Acetate |
| Hexane | |
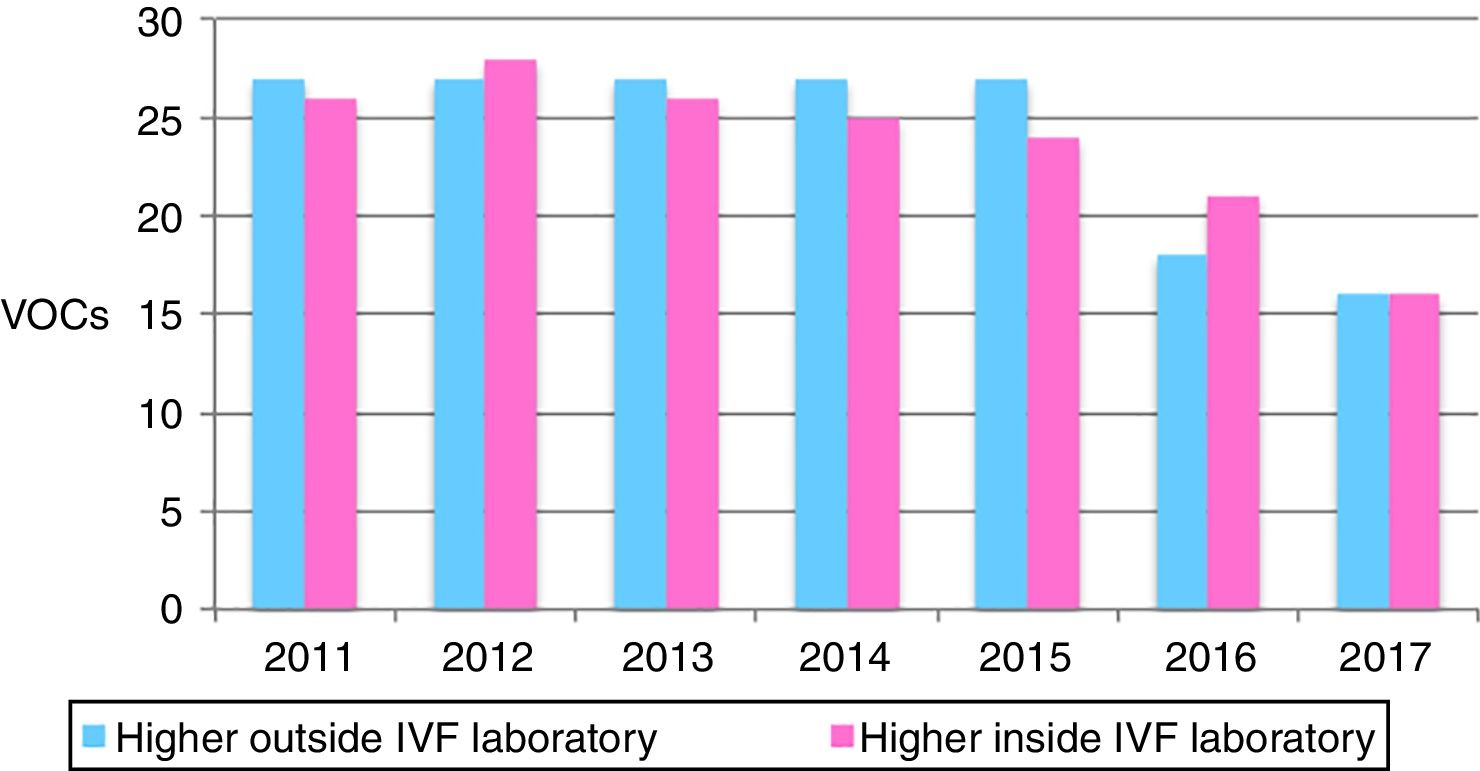
Within sixteen to twenty five VOCs were in higher concentrations inside the IVF compared to the outside, per year. The years 2012 and 2013 registered the highest levels of VOCs inside the IVF (Fig. 2), which when looking retrospectively we found there were some renovations done.
Additionally, 6 different VOCs exceeded the 1% occupational limit values (OLV) empirically established by the IVF laboratory directors, inside the laboratory: acetic acid, acetone, hexachloro-1,3-butadiene, methyl acetate, tetrachloroethene and vinyl acetate, and low values of volatile PAHs (semi VOCs) were also detected inside the IVF laboratories: naphthalene, although this compound was not present every year.
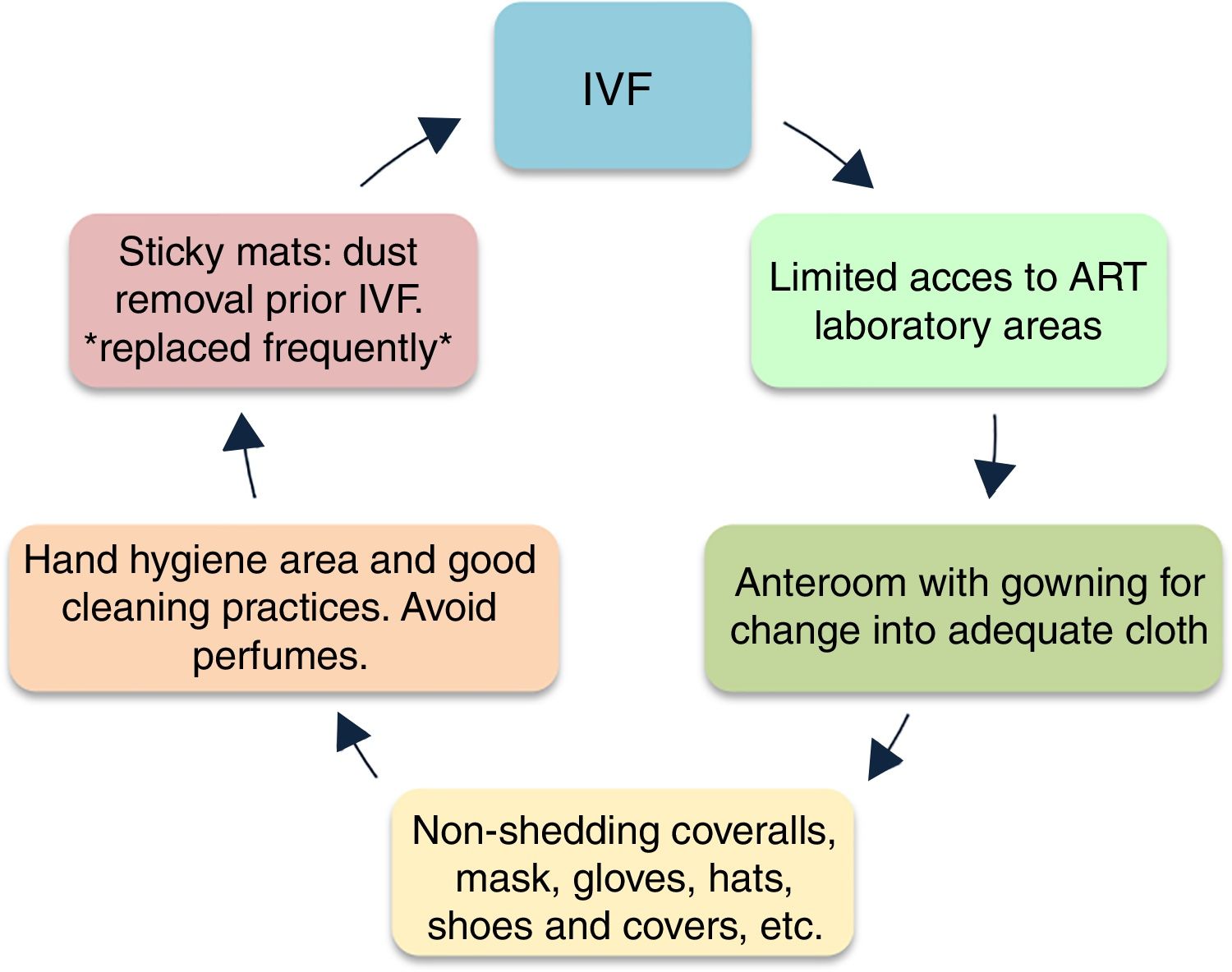
Control of pollutantsThe understanding on the infiltration and production of pollutants inside the laboratories and incubators has been essential to improve the design and preventive strategies to minimize contamination (Wale and Gardner, 2016; Hyslop et al., 2012). The first improvement concepts aimed at transforming other clean room designs, minimizing pollutants like vapors and particles accomplishing specific permitted values (Lawrence et al., 2007; Thomas, 2012). Nowadays, the quality control involves the improvement of the culture media formulations, contact supplies and gases used in IVF procedures (Cohen et al., 2009; ESHRE Guideline Group on Good Practice in IVF Labs et al., 2016) (Fig. 3).
Cleanroom preventive strategies against contamination (Boone et al., 1999; Esteves and Agarwal, 2013; De Los Santos, 2001; ESHRE Guideline Group on Good Practice in IVF Labs et al., 2016; Esteves and Bento, 2016).
The isolation of the IVF lab, retrieval room, transfer room (Boone et al., 1999; Dickey et al., 2010; Esteves and Agarwal, 2013) and the design and adaptability of the laboratory to future improvements is essential (Khoudja et al., 2013; Hyslop et al., 2012; Forman et al., 2014; Agarwal et al., 2017). Basic preventing strategies include: clean access for personnel and materials, double doors with windows for the anterooms between the operating room and the IVF lab to minimize the air mixing, a separated laboratory with a safety fume hood to use fixatives and toxic reagents and a separate area for cleaning and sterilization of materials. On the inside, the air management can be achieved through laminar flow cabinets, positive pressure and air filtration systems considering that dust particles of <0.5um in diameter often carry bacteria and/or fungi as well (De Los Santos, 2001; ESHRE Guideline Group on Good Practice in IVF Labs et al., 2016; Boone et al., 1999; Esteves and Agarwal, 2013; Dickey et al., 2010). There are different filtration systems such as High Efficiency Particulate Air filtration systems (HEPA) which removes particles larger than approximately 0.3μm (Cohen et al., 1997, Higdon et al., 2003, Esteves et al., 2004). The Ultra Low Penetration Air (ULPA) (Boone et al., 1999, Dickey et al., 2010), activated carbon filters, potassium-permanganate filters (Esteves et al., 2004, Sene et al., 2009, Munch et al., 2015), photo-catalytic units (Lawrence, 2007), UV radiation (Gea-Izquierdo et al., 2009), and filtration units within the incubators, chambers and filters in the incoming gas lines (Racowsky et al., 1999, Merton et al., 2007; Khoudja et al., 2013). Activated carbon absorbs higher molecular weight hydrocarbons (PAHs) through pores of varying size and a field of molecular attraction that captures large flat electron-rich molecules. Low molecular weight organics, alcohols, ketones and aldehydes can be oxidized and degraded by potassium permanganate. Photo catalytic oxidation technologies are also used to filter VOCs (Munch et al., 2015; Worrilow, 2017a). New proposals in IVF isolation, engineered molecular media and genomically modeled biological inactivation are also in development and have shown significant increase in blastocyst conversion rates (Hyslop et al., 2012; Forman et al., 2014).
As to control the IVF pollutants, various parameters could be measured, such as: compound concentration and composition, solubility and vapor pressure (specially for VOCs), particle size, shape, surface modification and degree of agglomeration, as well as the ambient temperature and the surface area from which they could be released (Thomas, 2012; Worrilow, 2017b; Celá et al., 2014). Specifically, the degree of solubility of compounds should be taken into account because they might penetrate the mineral oil layer and pass into the culture medium. The molecules penetration can be estimated by partition coefficients from air to oil (with vegetable oil) and oil to water (with octanol) to determine if it is soluble in both oil and water. The latter technique has been used to evaluate the risk of absorption of several compounds into the culture media; negative values of the octanol–water partition coefficients indicate that a compound is most likely to be absorbed by the media (e.g.: acrolein, −0.01 and methanol, −0.74 from a range of −0.01 to 6.25) (Finizio et al., 1997; Cohen et al., 1998; Thomas, 2012).
Mineral oil, used for embryo culture and embryo research applications, can act as a sink for toxins as well as a source because is not inert (Morbeck et al., 2010), so each lot can vary widely in quality. It is derived from crude oil, which is the same starting material used for benzene which is an IVF-related chemical used for polystyrene to make petri dishes. It is a mixture of straight chain, cyclic and aromatic hydrocarbons, mostly saturated but also low levels of unsaturated hydrocarbons, which include the more reactive PAHs, and compounds like peroxides, aldehydes, alkenals can be present as well (Morbeck et al., 2010; Morbeck 2012; Khan et al., 2013; Martinez et al., 2017). It can also be affected by oxygen exposition (peroxidation and free radicals formation) and storage conditions (varying temperature) (Ainsworth 2017). Actually, despite passing the manufacturers bioassays, mineral oils have affected embryo development, meaning that there is a lack of sensitivity of the tests. Nonetheless, the use of a good quality mineral oil can be achieved by washing it (Otsuki et al., 2009; Morbeck et al., 2010) and likewise, by improving the toxins screening through the improvement of the bioassays (Human Sperm Motility Assays (HSMA) + 1-cell Mouse Embryo Assays (MEA) or MEA with time-lapse) (Morbeck et al., 2010; Hughes et al., 2010; Khan et al., 2013; Wolff et al., 2013). Recently, it was described a modification that could be more suitable for many laboratories: the extended MEA (eMEA) is more simple and sensitive when assessing the cells number and blastocyst formation rate was at 144h (instead of the 96h assessment as the regular technique) of individually cultured embryos, because group culture can stabilize the embryo environment and mask toxicity (Ainsworth 2017).
The specific requirements for IVF laboratories are different due to variations in regulations among regions (Morbeck, 2015). Environmental and work health institutions (WHO), Occupational Safety and Health Administration (OSHA) and the “Instituto Nacional de Seguridad e Higiene en el Trabajo” (INSHT) in Spain, have chemical standards for evaluating industrial hygiene and health. These were only designed to cover workers that could be exposed every day without adverse effects, but they are not designed for cultured and largely unprotected cells such as the embryos and gametes as they lack physical barriers (epithelial surfaces), immunological defense or detoxifying mechanisms (Cohen et al., 1997; Thomas, 2012; Instituto Nacional de Seguridad e Higiene en el Trabajo, 2016; Worrilow, 2017a; European Commission, 2010). For general contamination, threshold limit values can be obtained and registered in concentrations of milligrams (mg/m3), parts per million (ppm), or micromoles (me). To measure and evaluate the negative effects in cultured cells, limit values need to be in much lower concentrations (μg/m3, ppm, or ppb). Unfortunately, specific quality standards and specific threshold levels at which contaminants cause harm to embryos have not been determined (Thomas, 2012). Further, the measured composition of air pollutants, such as VOCs, can vary significantly depending on the methods and recognized terminology, lending to confusion. Still, at the “Cairo consensus on the IVF laboratory environment and air quality” performed, experts suggest different “aspirational benchmarks” for existing ART laboratories:
- Less than 352,000 particles larger than 0.5μm to 10μm per metre3 (equivalent to <10,000 such particles per cubic foot).
- Total VOCs less than 500μg/m3 (∼400–800ppb total VOC, depending on molecular species); less than 5μg/m3 aldehydes.
- Fifteen total air changes per hour, including three fresh air changes per hour, i.e. 20% outside air.
Type of VOC filtration and filters’ manufacturer instructions concerning ACH should also be considered when setting the fresh to recirculated air ratio.
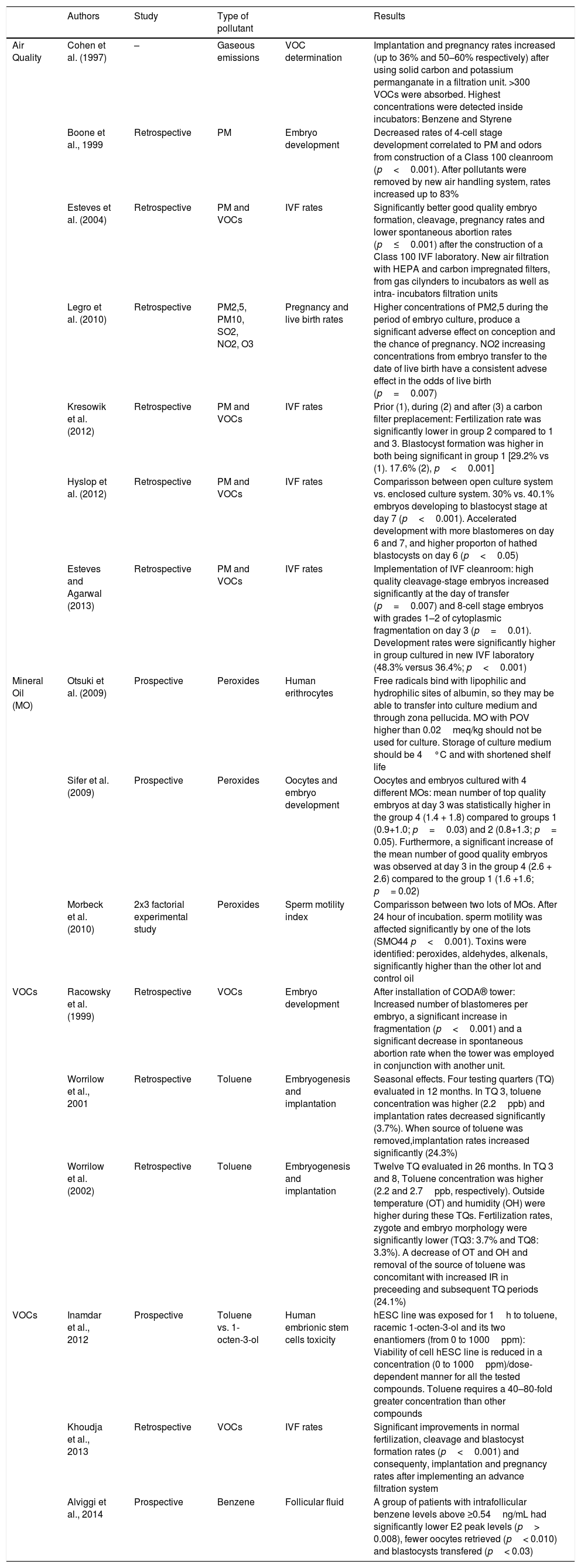
Effects of pollutants on IVF outcomes.The reported pollutants effects have been primarily related to acute and chronic cardiopulmonary affections through the activation of local and systemic inflammatory pathways, which promote systemic oxidative stress and inflammatory responses, thrombosis and coagulation, vascular dysfunction, epigenetic changes and genotoxicity (suppression of DNA repair and more DNA errors) (Kannan et al., 2006; Chin, 2015; Nemmar et al., 2013). However, the adverse effects over human reproduction can vary widely and are not entirely understood (Table 4). hypotheses about how pollutants affect the embryonic development are still weak because they are based on limited data (Cohen et al., 1997; Hall et al., 1998; Boone et al., 1999). Studies and reviews had focused mainly in the relationship between pollutants and birth defects (Tanner et al., 2015), or Low Birth Weight (LBW) and preterm births (almost 60% of LBW) which have been related mostly to pregnant women exposed during their 1st trimester (Medeiros and Gouveia, 2005; Bell et al., 2007; Santos et al., 2016; Diaz et al., 2016), lack of fetal immune development (Herr et al., 2010) or menstrual disorders and their possible relationship with higher incidence rates of spontaneous abortion (Huang, 1991) etc., that can also lead to intrauterine and infant mortality (Pereira et al., 1998; Racowsky et al., 1999; Sram et al., 2005). But less is known about sub-fertile patients undergoing reproductive treatments, whose gametes and embryos are more susceptible to environmental influences because they lack of the physiological maturity of a differentiated mammal to protect themselves (Hall et al., 1998; Legro et al., 2010; Cohen et al., 1997). Lower implantation and pregnancy rates and early pregnancy loss have been related to specific pollution events inside ART facilities (Cohen et al., 1998; Perin et al., 2010b; Heitmann et al., 2015). Actually, the pioneer pre-implantation toxicology research known demonstrated that the gaseous emissions produced after a construction of a laboratory arrested 90% of the two-cell stage mouse embryos exposed; blastocyst development rates (BDR) were higher before and after the construction period (Cohen et al., 1997). Decreased fertilization, cleavage and BDRs have been reported due to the peroxidation of the mineral oil; however, even when these rates can improve after washing it, there are different commercial oils which vary in quality; it has been found that there are significant differences between the embryo development and quality on D3 among different brands (Otsuki et al., 2007; Sifer et al., 2009; Morbeck et al., 2010; Martinez et al., 2017).
Most relevant IVF- human related studies.
| Authors | Study | Type of pollutant | Results | ||
|---|---|---|---|---|---|
| Air Quality | Cohen et al. (1997) | – | Gaseous emissions | VOC determination | Implantation and pregnancy rates increased (up to 36% and 50–60% respectively) after using solid carbon and potassium permanganate in a filtration unit. >300 VOCs were absorbed. Highest concentrations were detected inside incubators: Benzene and Styrene |
| Boone et al., 1999 | Retrospective | PM | Embryo development | Decreased rates of 4-cell stage development correlated to PM and odors from construction of a Class 100 cleanroom (p<0.001). After pollutants were removed by new air handling system, rates increased up to 83% | |
| Esteves et al. (2004) | Retrospective | PM and VOCs | IVF rates | Significantly better good quality embryo formation, cleavage, pregnancy rates and lower spontaneous abortion rates (p≤0.001) after the construction of a Class 100 IVF laboratory. New air filtration with HEPA and carbon impregnated filters, from gas cilynders to incubators as well as intra- incubators filtration units | |
| Legro et al. (2010) | Retrospective | PM2,5, PM10, SO2, NO2, O3 | Pregnancy and live birth rates | Higher concentrations of PM2,5 during the period of embryo culture, produce a significant adverse effect on conception and the chance of pregnancy. NO2 increasing concentrations from embryo transfer to the date of live birth have a consistent advese effect in the odds of live birth (p=0.007) | |
| Kresowik et al. (2012) | Retrospective | PM and VOCs | IVF rates | Prior (1), during (2) and after (3) a carbon filter preplacement: Fertilization rate was significantly lower in group 2 compared to 1 and 3. Blastocyst formation was higher in both being significant in group 1 [29.2% vs (1). 17.6% (2), p<0.001] | |
| Hyslop et al. (2012) | Retrospective | PM and VOCs | IVF rates | Comparisson between open culture system vs. enclosed culture system. 30% vs. 40.1% embryos developing to blastocyst stage at day 7 (p<0.001). Accelerated development with more blastomeres on day 6 and 7, and higher proporton of hathed blastocysts on day 6 (p<0.05) | |
| Esteves and Agarwal (2013) | Retrospective | PM and VOCs | IVF rates | Implementation of IVF cleanroom: high quality cleavage-stage embryos increased significantly at the day of transfer (p=0.007) and 8-cell stage embryos with grades 1–2 of cytoplasmic fragmentation on day 3 (p=0.01). Development rates were significantly higher in group cultured in new IVF laboratory (48.3% versus 36.4%; p<0.001) | |
| Mineral Oil (MO) | Otsuki et al. (2009) | Prospective | Peroxides | Human erithrocytes | Free radicals bind with lipophilic and hydrophilic sites of albumin, so they may be able to transfer into culture medium and through zona pellucida. MO with POV higher than 0.02meq/kg should not be used for culture. Storage of culture medium should be 4°C and with shortened shelf life |
| Sifer et al. (2009) | Prospective | Peroxides | Oocytes and embryo development | Oocytes and embryos cultured with 4 different MOs: mean number of top quality embryos at day 3 was statistically higher in the group 4 (1.4 + 1.8) compared to groups 1 (0.9+1.0; p=0.03) and 2 (0.8+1.3; p= 0.05). Furthermore, a significant increase of the mean number of good quality embryos was observed at day 3 in the group 4 (2.6 + 2.6) compared to the group 1 (1.6 +1.6; p= 0.02) | |
| Morbeck et al. (2010) | 2x3 factorial experimental study | Peroxides | Sperm motility index | Comparisson between two lots of MOs. After 24 hour of incubation. sperm motility was affected significantly by one of the lots (SMO44 p<0.001). Toxins were identified: peroxides, aldehydes, alkenals, significantly higher than the other lot and control oil | |
| VOCs | Racowsky et al. (1999) | Retrospective | VOCs | Embryo development | After installation of CODA® tower: Increased number of blastomeres per embryo, a significant increase in fragmentation (p<0.001) and a significant decrease in spontaneous abortion rate when the tower was employed in conjunction with another unit. |
| Worrilow et al., 2001 | Retrospective | Toluene | Embryogenesis and implantation | Seasonal effects. Four testing quarters (TQ) evaluated in 12 months. In TQ 3, toluene concentration was higher (2.2ppb) and implantation rates decreased significantly (3.7%). When source of toluene was removed,implantation rates increased significantly (24.3%) | |
| Worrilow et al. (2002) | Retrospective | Toluene | Embryogenesis and implantation | Twelve TQ evaluated in 26 months. In TQ 3 and 8, Toluene concentration was higher (2.2 and 2.7ppb, respectively). Outside temperature (OT) and humidity (OH) were higher during these TQs. Fertilization rates, zygote and embryo morphology were significantly lower (TQ3: 3.7% and TQ8: 3.3%). A decrease of OT and OH and removal of the source of toluene was concomitant with increased IR in preceeding and subsequent TQ periods (24.1%) | |
| VOCs | Inamdar et al., 2012 | Prospective | Toluene vs. 1-octen-3-ol | Human embrionic stem cells toxicity | hESC line was exposed for 1h to toluene, racemic 1-octen-3-ol and its two enantiomers (from 0 to 1000ppm): Viability of cell hESC line is reduced in a concentration (0 to 1000ppm)/dose-dependent manner for all the tested compounds. Toluene requires a 40–80-fold greater concentration than other compounds |
| Khoudja et al., 2013 | Retrospective | VOCs | IVF rates | Significant improvements in normal fertilization, cleavage and blastocyst formation rates (p<0.001) and consequenty, implantation and pregnancy rates after implementing an advance filtration system | |
| Alviggi et al., 2014 | Prospective | Benzene | Follicular fluid | A group of patients with intrafollicular benzene levels above ≥0.54ng/mL had significantly lower E2 peak levels (p> 0.008), fewer oocytes retrieved (p< 0.010) and blastocysts transfered (p< 0.03) |
The adaptation of new filtration systems have improved the air conditions inside the IVF laboratories and incubators significantly (p≤0.001), increasing fertilization, embryo development, implantation and pregnancy rates (Boone et al., 1999; Esteves et al., 2004; Dickey et al., 2010; Kresowik et al., 2012; Munch et al., 2015). For example, it has been reported an accelerated progression of development from early embryos up to blastocysts stage, a higher proportion of expanded and hatched blastocysts and a significantly higher number of blastomeres when cultured inside enclosed systems that protect oocytes and embryos throughout the IVF process (Hyslop et al., 2012). However, some pollutants are still difficult to erradicate; so detailed information about the relationship between pollution and developmental parameters (morphology, cleavage rate, symmetry, fragmentation, multi-nucleation, embryo development rate inside incubators, hatching process and defense mechanisms) require more attention (Esteves et al., 2004; Maluf et al., 2009; Legro et al., 2010; Thomas, 2012). One of the main mechanisms related to exposure to air pollution (mainly studied for cardiopulmonary diseases) is the oxidative stress and few studies have related this in early human development with clinical outcomes in pregnant women (Mohorovic, 2004). Oxidative damage produced by pollutants has showed time-dependent cumulative effects and it can affect the membranes potential of mitochondria or produces apoptosis. Embryonic stem cells have shown different responses compared to somatic cells when exposed to pollutants or antioxidant treatments (Ramos-Ibeas et al., 2017). Oxidative stress-related genes and pancreatic and eye-lens gene markers appear de-regulated in embryos exposed to urban pollution, whereas exposure to rural extracts affected genes implicated in basic cellular functions (Nemmar et al., 2013; Mesquita et al., 2015).
For this reason, it is necessary to understand the toxic mechanisms, the pattern of substance distribution and their action, which varies with each compound due to their molecular weight, solubility, and degree of ionization at a physiological pH over the early development because (Fabro, 1978).
Effects of particulate matter on IVFSpecific effects of airborne PM have been described mainly in animal models. It has been observed a significant impairment in fertilization, zygotes, embryo development, lineage of specification in blastocysts (decreased inner cell mass (ICM), trophectoderm (TE) and cell count and ratio), hatching, survival and post implantation potential after being exposed to PM2.5. ICM and TE could have a different susceptibility to embryotoxic agents as well (Maluf et al., 2009). Mixtures of PM2,5, PM10 and nitrogen dioxide (NO2), after acute or chronic exposition may decline the follicular growth, the number of top embryos, the implantation rates (IR) and decrease live births (Legro et al., 2010; Perin et al., 2010a, 2010b; Carre et al., 2016). PM may have influence over specific mechanisms such as the Zonula Occludens (ZO-1), a protein that regulates tight junction formation between cells. This protein has been seen expressed first during the compaction of eight-cell mouse embryos and it has been suggested as a necessary mechanism for blastocyst formation, helping in the differentiation of the TE and ICM. When its function is altered, the number of formed blastocysts can decrease significantly and produce degeneration as it affects the number of embryonic cells, the bi-functional barrier that limits the diffusion of solutes and the epithelial cell polarity (Wang et al., 2008). Although this study was not correlated with a specific substance, a follow up study found negative effects associated with the interaction between different types of PM and pulmonary cells; the protein's degradation was evident as proteins were relocated from the cell periphery, disrupting the epithelial barrier (Wang et al., 2012).
Some HM have physiological functions, however pathologies may develop from deficiencies and/or excesses of essential metals, and more so with non-essential metals (those with no physiological functions), because of noxious effects caused by binding to macromolecules or by activating or inactivating cellular processes (Vinken et al., 2010). Toxic responses may include growth inhibition, suppression of oxygen consumption, and impairment of reproduction and tissue repair (Suvarapu and Baek, 2016). Despite of this, the HM are not considered critical for humans but it has been reported that there are negative effects on the viability, maturation, morphological abnormalities, morula/blastocyst yield and hatching when exposing animal models to Hb an Cd for example. It is known to inhibit gap junction intercellular communication (GJIC) and connexin phosphorylation, both of which are essential processes in the progression from the four-cell stage in humans (eight-cell stage in mice) through compaction (Hardy et al., 1996). The toxic mechanisms of Cd include ionic and molecular mimicry, interference with cell adhesion and signaling, oxidative stress, apoptosis, genotoxicity, and cell cycle disturbance due to either synergism or just one mechanism predominating in a cell specific manner (Thompson and Bannigan, 2008). With increasing concentrations there are gradual abnormalities in oocytes (from absence of the 1st polar body in the perivitelline space, degenerated ooplasm, and abnormal perivitelline space to presence of multiple abnormalities with higher doses), and deleterious effects over embryos: from a reduction in the morula/blastocyst yield and blastocyst hatching, to the arrest of four- to eight-cell embryos and increased degeneration and/or asynchronous division, or healthy embryos exposed during the two- to four- cell stage may present declined cell count and ICM values (Nandi et al., 2010). Actually, toxicity may increase with development; if exposition happens previous or during morula stages, these will be seen with less cells and blastocysts can be found degenerated with evidence of apoptosis (shrunken cells, pyknotic nuclei and debris accumulation) (Abraham et al., 1986; De et al., 1993).
The NPs can be a source of different developmental malformations with fatal impacts on exposed animals and their offspring. Reduced fetal growth and genetic abnormalities in infants are known to be associated with NP exposure past the threshold dose of toxicity during vulnerable times of embryonic and fetal development. However, there is little information regarding the specific effects of NPs during the early stages of human development, while many deleterious effects have been found in different animals (Celá et al., 2014). It has been found a numerical trend toward to fewer hatched mouse embryos previously exposed to mixed sized polystyrene (C8H8)n NPs at the two-cell stage, with no significant differences in developmental capacity. Additionally, smaller NPs can be internalized by the trophoblast cells (by endocytosis or pinocytosis) and a few NPs can be internalized in the ICM as well but these can expel the NPs during the compaction phase, while the trophoblasts have a larger dimension to absorb them. Despite of this, no negative effects on cellular processes or expression of factors needed for development were seen (Bosman et al., 2005). But different particles derived from nanomaterials, or in higher doses, might behave differently. After tagging D2 embryos through intracytoplasmic injection or by co-incubation with Ps-NPs and polyacrylonitrile (C3H3N) NPs, it was noticed that D2 development was lower in both types of NPs and on D6, Pa-NP embryos did not develop and the hatching percentage was lower with both types of NPs. The Pa-NPs could have had effects on the eight-cell embryo which is a cell stage characterized by compaction, blastomere membrane fusion, and GJIC (Fynewever et al., 2007).
Celá et al. reviewed some of the most relevant results in different animal models, highlighting the fact that the negative effects of NPs in other mammalian models can show possible deleterious effects in human oocytes and embryos. Some of the effects described in mouse oocytes were after a 24-h exposure to CdSe-NPs that resulted in decreased cell numbers, induced apoptosis, and inhibited post-implantation development, possibly due to a teratogenic effect. Blastocysts exposed to CdSe-NPs had induced apoptosis, inhibited cell proliferation, retarded post-implantation blastocyst development, and increased early-stage blastocyst death in vitro and in vivo; cytotoxicity was significantly reduced by the addition of a zinc sulfide (ZnS) coating. Ag-NPs exposition caused TE and ICM apoptosis and significantly inhibited cell proliferation. Exposing mouse morulas to chitosan (CS-NPs) induced defects in blastocysts such as small or no blastocoel cavity, lower expression of TE associated genes and pluripotent marker genes and a reduction in total cell numbers with enhanced apoptosis at this stage. The IR was reduced and embryos that implanted were subsequently reabsorbed (Celá et al., 2014). A recent study reported that CS-NPs can be internalized into the zona pellucida, periviteline space and cytoplasm of mouse blastocysts, due to their high aqueous solubility, initiating an intercellular oxidative stress reaction in which they reported swollen mitochondrias, mitophagosomes, lipophagy, lysosomes, degenerated organelles and early signs of apoptosis. The expression levels of the endoplasmic reticulum stress-related genes were also increased and epigenetic reprogramming was affected (Choi et al., 2016).
PAHS reproductive negative health effects have been documented mainly in animals or humans that were exposed to smoke (Dechanet et al., 2011; Cinar et al., 2014). Some of these compounds can be found inside the IVF laboratories but no reports have been published. PAHs can alter embryo and fetal development at the molecular level, reducing the allocation of embryonic and placental cell lineages and inducing apoptosis. Some of the most potent PAHs (7,12-dimethylbenz(a)anthracene, benzo[a]anthracene, benzo[a]pyrene, and dibenz[ah]anthracene) have shown clear evidence of mutagenicity/genotoxicity in somatic cells (Detmar et al., 2006; Kim et al., 2013).
When exposing mouse embryos to 7,12-dimethylbenz(a)anthracene it was found that some were smaller, with a significant lower cell number and in the grade of apoptosis between the non-exposed and the exposed embryos: higher cellular death in the ICM and TE cells with characteristics such as nuclear condensation and fragmentation. Despite this, the progression of the embryo from the eight-cell stage to the blastocyst stage was not interrupted. There was also a significant increase in Bax levels (a Bcl-2 group family member) and therefore the activation of caspase 3, which is known to induce cellular death and consequently could affect the developmental potential of the embryos, increasing their resorption potential (Detmar et al., 2006). Later, the authors described that high levels of PAHs have immunosuppressive effects and suggesting that pregnancy resorption could be an immune-mediated mechanism on the part of the mother after being chronically exposed to low doses (Detmar and Jurisicova, 2010). Another group exposed mouse embryos to different doses of DEP finding that all doses led to the disruption of the normal segregation pattern (cell lineage specification); without affecting the total cell count, the ICM/TE ratio was significantly affected because there were fewer ICM cells. Lower doses had low rates of apoptosis but when increasing the dose, the ICM morphological integrity was significantly impaired and the blastocysts developmental potential and hatching status were significantly affected. On a molecular level, a reduction in Oct-4 expression was observed in the embryos, which can be associated with impaired formation of a proper ICM with fewer cells. There was also an over-expression of Cdx-2, which it could trigger the differentiation toward TE as a compensatory mechanism to preserve blastocyst cells number (Januário et al., 2009). Brevik et al. demonstrated that the parental origin of mutations happen even after an acute exposition to PAHs, and this may lead to affect early embryonic transcription, the activation of the embryonic genome and may lead to transgenerational effects in humans (Brevik et al., 2012).
Effects of volatile organic compounds on IVFIn routine ART laboratory audits, it is recommended to evaluate the concentrations of VOCs in the air among other pollutants to prevent occupational hazards; however, while these substances do not often surpass the OLV due to current environment management strategies, there is still no certainty that they will not interact with gametes or embryos during culture. Past studies have demonstrated lethal effects on early mouse embryos by gaseous emissions produced after the construction of a new laboratory: 90% of the two-cell stage embryos were arrested despite of the use of freestanding ionization units and HEPA filtration. BDR were higher before and after the construction period. Other studies have been able to corroborate the same pattern of an increase and decrease in VOC measurement before and after the redesign of clinics and laboratories or the construction of new ones (Cohen et al., 1997; Hall et al., 1998). Khoudja et al. stated that more than 1ppm of VOCs can be considered high and directly toxic to animal embryos and that VOC levels around 0.5ppm allow for acceptable blastocyst development and reasonable pregnancy rates, although there could be high percentage of miscarriages and that VOC levels should be below 0.2ppm to have an ideal IVF environment (Khoudja et al., 2013).
The seasonal influence of the IVF laboratory‘s ambient air over the embryo development has been found to be related to the outside temperature (OT) and humidity (OH). Two studies by Worrilow et al. observed negative effects on IR by evaluating different testing quarters. In both studies they found that when toluene was in higher levels IR decreased, and in one study the fertilization rates, zygote quality, and embryo morphology scoring decreased significantly as well. The toluene presence was also related to high OT and OH (Worrilow et al., 2001; Worrilow et al., 2002). Other groups have correlated the presence of the VOCs filtration systems with higher rates of blastocyst development and higher number of blastomeres and one of these groups also suggested that higher embryo fragmentation could be a mechanism that may provide means through which embryos improve developmental competency and for that there was a reduction in spontaneous abortion (Racowsky et al., 1999; Higdon et al., 2003; Merton et al., 2007). Koudhja et al. established that morphological and intrinsic embryo parameters: fertilization, cleavage, D5 blastocyst formation, implantation, and pregnancy rates significantly improved when reducing the presence of VOCs after the installation of an air filtration system. The concentrations of formaldehyde, ethylene, acetylene, ethane, propylene, SO2, NOx, isobutene, cis-butene, cyclopentane, benzene, CFC-11, chloroform, carbon tetrachloride, halon-1211, and alcohol, were reduced with the new air filtration system (Khoudja et al., 2013).
Benzene (C6H6) is one of the most common VOC air pollutants found inside laboratories. It is a colorless, clear liquid that is fairly stable and highly volatile and has a general ambient concentration between 1 and 50ppb. The EPA estimates that exposure over 0.4ppb in air over a lifetime pose a significant risk of cancer. OSHA allows 1ppm as the maximum allowable amount of benzene in workroom air during an eight-hour workday over the course of a 40-hour workweek. The National Institute for Occupational Safety and Health (NIOSH) recommends that all workers wear special breathing equipment when they are likely to be exposed to benzene at levels exceeding the recommended (eight hour) exposure limit of 0.1ppm (ATSDR 2007). There are not specific threshold levels for this substance inside laboratories and there is little evidence linking benzene to IVF reproductive outcomes. Through a pilot study Alviggi et al. evaluated if the levels of benzene in the follicular fluid could influence the response of controlled ovarian stimulation and the outcome of IVF/ICSI cycles. Two groups of exposure were established: low intra-follicular concentration (A) and high intra-follicular concentration (B) (≥0.54ng/mL). Benzene levels were significantly related to a positively trend in baseline FSH levels and negatively trend of E2 peak levels, average number of oocytes retrived and average number of embryos transfered in the B-group. The A-group had a higher number of embryos transferred. The intra-ovarian levels of benzene are associated with hypo-sensitivity of follicles to endogenous and exogenous gonadotropin, leading to an unknown mechanism of resistance (Alviggi et al., 2014).
Studies on different cell types have evaluated the intrinsic damage that benzene produces inside laboratories. Meiotic delay of MI oocytes and frequencies of aneuploidies in MII mouse oocytes were observed after a dose-dependent inhalation of benzene, especially with higher doses in a “multiple inhalations” group (Zeng et al., 2001). Tsutsui et al. demonstrated a marked dose-dependent genotoxicity on Syrian hamster embryo cells when exposed to benzene and its metabolites. Some of the effects seen were disturbed cell growth, cells transformation, increased frequency of chromosomal aberrations (gaps and breaks), alterations in chromosome numbers, and genetic mutations. Catechol is the most harmful metabolite for cells at lower concentrations, but hydroquinone and phenol also have negative effects (Tsutsui et al., 1997). Benzene or its metabolites can produce diverse effects in individuals of different ages and genders as well. Corti et al. exposed 16-day-old male and female, adult males, females, and pregnant females mouse fetuses colony forming units, erythroid or CFU-e (precursor cells), finding that hydroquinone and benzoquinone were the most cytotoxic, producing a dose-dependent decrease in all cell types growth. The adult male cells showed more susceptibility to these metabolites while fetal cells were more resistant to catechol but showed more deleterious effects when exposed to binary mixtures containing catechol. Phenol was found to affect fetal male cells at 40μM (Corti and Snyder, 1998). The possible confounding factors (individual genetic susceptibility or previous exposure of the parents) can cause genotoxicity, as well as individual cell and tissue responses.
Because benzene is a carcinogenic substance, in utero exposure studies have suggested that predisposition of the embryo and/or fetal tissues to carcinogenesis as well, caused by alterations in the redox signaling pathways, excess of production of reactive oxygen species (ROS) and therefore oxidative stress, affecting the regulation of gene expression, cell growth, and cell death. But there are a lot of intrinsic differences in the susceptibility of the target cells (type, age, gender) as well. In utero benzene can cause alterations in critical cell signaling pathways necessary for normal hematopoiesis; male fetuses have been found to be more susceptible to benzene-induced ROS production after two hours of exposure. Benzene was observed to be very deleterious for unprotected embryos given their rapid growth and developmental changes and reliance on the cellular signaling that occurs during embryonic development (Badham et al., 2010).
Another well-known VOC is Toluene, which is an organic solvent (an aromatic hydrocarbon) that can be easily found in commercial products such as paints, varnishes, glue, and gasoline. Toluene and other organic solvents have a high affinity for lipid-rich tissues and can readily cross the placenta allowing direct exposure of the fetus. Significant degenerative changes were found in preimplantation embryos exposed to toluene in vitro. Higher doses decreased the fertilization rate of exposed eggs and enhanced embryo degeneration resulting in increased embryonic lethality (Yelian and Dukelow, 1992). Other studies in animal models have demonstrated that morphological anomalies and congenital defects, such as reduced placental weight, fetal weight and growth, reduced skeletal ossification, negative neurobehavioral effects as well as fetal resorptions and death, are produced specially in early female fetuses (gestational D8 to D20) or during other stages of pregnancy after exposing mothers to inhaled toluene (Bowen et al., 2009; Callan et al., 2015, 2016). It has been described also that toluene has cytotoxicity effects on human embryonic stem cells, however it is needed a higher concentration compared to the toxic effects produced by lower concentrations of 1-octen-3-ol and its enantiomers (a major fungal VOCs associated to indoor mold and odors) (Inamdar et al., 2012). When exposing murine bone marrow stem cells to [(E)-2-octenal and oct-1-en-3-ol], a shift to unsaturated fatty acids and lower cholesterol levels in the cells membrane was produced, which means increased the membrane fluidity, and this could be related to malfunction of the immune system (Hokeness et al., 2014).
Acrolein is an airborne VOC with widespread environmental prevalence produced during lipid peroxidation and by burning tobacco or liquid fuels. Preliminary studies have already demonstrated negative effects on cleavage, cell number, and blastocyst development when exposed to acrolein (Little and Mirkes, 1990; Hall et al., 1998). However, a recent study of short-term exposure demonstrated that the negative effects depend on the protein concentration in the medium and the quality of the oil. Mouse embryos incubated with 500ppb of acrolein inside the embryoscope were found to develop to the blastocyst stage at a normal rate (80%) when also exposed to 5mg/mL of human serum albumin (HAS) in the culture medium. Embryos incubated with low-protein medium were arrested at the four-cell stage and the remainder arrested at the morula stage. The embryos of the protein-free group were also exposed to mineral oil that contained peroxides, and were arrested and lysed at the one-cell stage within 24h of culture. The protein concentration in the groups that contained HSA did not affect the timing of the cell cycles (Karaouga et al., 2014).
Trichloroethylene (TCE), is a highly volatile inhalation anesthetic used mainly in short surgical procedures where light anesthesia with good analgesia is required or as an industrial solvent. Significant anomalies in skeletal and soft tissues, indicative of delay in the development, have been observed in groups exposed to TCE during pregnancy in rats (Dorfmueller et al., 1979). TCE effects over the cardiac tissue, function and development (National Center for Biotechnology Information, 2016) have been studied in animal models describing embryonic genotoxicity as well, causing cardiac valvular and septal malformations or it can disrupts calcium (Ca**) flux regulation in embryonic myocites (Johnson et al., 1998; Boyer et al., 2000; Caldwell et al., 2010). Caldwell et al. determined that TCE produces gene expression errors of the calcium homeostasis and that, could be the mechanism by which cardiac malformations can occur. The research was conducted in mice embryonic hearts obtained from pregnant females that had received folate supplementation in 3 different doses: high folate levels in combination with TCE induced over-expression of many genes of the ion channel pathway. The majority encodes for potassium (K), Ca**, and other cation transportation channels indicating that TCE may alter the permeability of the cell membrane causing an electrolyte imbalance. TCE caused great changes in gene expression during critical phases of the heart development, but folate may have a dual effect on cardiogenesis too, depending on the nutritional variations and the presence of environmental toxins (Caldwell et al., 2010).
Limonene it is another well-known VOC that can be present inside of the IVF laboratory, because it is used as an additive in cleaning and cosmetic products. Despite of this, few studies have been performed evaluating possible early life deleterious effects, perhaps because it is known as a low toxicity VOC and it is considered that it does not have strong mutagenic, carcinogenic or nephrotoxic effects. However, a couple of studies have reported that even though it is not highly toxic or carcinogenic it does induce a few cell transformations in Syrian hamster embryo cells (Rivedal et al., 2000), or that influences mechanisms of increase intracellular Ca++ pathways and Ca++ activated potassium (BKCa) channels, which could be correlated to increasing myometrium contractions (Hajagos-Tóth et al., 2015).
DiscussionWhile the development of human embryos happens in a very controlled environment, several aspects of this process (morphology, developmental kinetics, physiology and metabolism) can be affected by the exposure to a variety of conditions in which the laboratory environment participates actively. Additionally, we must keep in mind that if there is a significant exposure to a suboptimal condition of the environment, this may predispose embryos to be more vulnerable to a second stressor having cumulative negative effects and compromising their implantation potential (Gardner and Kelley, 2017) and eventually increasing the risk of disease in the offspring.
Nowadays, these culture systems not only focus on the culture media and how the supplements or the incubation conditions (oxygen, the number of embryos cultured at the same time, “one-step” cultures, etc.) influence the development but also about how the ambient air of the laboratory and inside the incubators can influence the embryo's ability to develop into a blastocyst and reproductive outcomes. Industry and mostly laboratories are aware of this and that is how most of them are trying to improve the official operational requirements, the ambient air conditions through improving air filtration management, the quality of products of daily use (such as free- VOCs disinfectants) and the monitoring of common environmental threads through the development of IVF specific equipment with high sensitivity that can be used during laboratory activities and even inside the working stations and incubators (e.g. Oosafe® VOC-log).
In a recent meeting, at the “Cairo consensus on the IVF laboratory environment and air quality”, experts agreed that evidence on how IVF specific pollutants may affect outcomes is lacking and that controlling the laboratories contamination positively impacts these outcomes. However, the environmental monitoring of laboratories and surrounding spaces will continue to be insufficient until the harmful pollutant values can be standardized into official embryo-toxicity thresholds. The data gathered has progressively allowed to research about specific parameters warning about possible embryonic hazards, but so far, the repro- toxicologic studies with specific effects reported on different stages of human embryo development in vitro are very limited, in some specific pollutants non- existent. Most have been performed in other animals and these results are not completely translatable to humans due to the differences in species or type of culture (medium, oil overlay and protein supplementation, etc.) or just by the differences of pollutants, doses and studies designs. The absorption mechanisms of each substance should also be considered due to the variability of their physical and chemical characteristics, most specifically solubility parameters, which may determine the brevity in which the exposure will result in developmental failure and long-term negative effects on fetuses. Specific morphologic parameters of the gametes, zygotes, and embryos should be assessed systematically when evaluating air quality due to the evidence that pollutants can affect development at the earliest stages (the four cell stage and compaction up to the differentiation of the ICM and TE), which have fundamental roles in embryo survival, implantation, and fetal viability (Boone et al., 1999; Hardy et al., 1996; Esteves and Agarwal, 2013; Karaouga et al., 2014). However, since embryos often develop even in the presence of contaminants, other molecular parameters should be considered and an epigenetic bio monitoring is necessary as well as an international methodological accordance (Pacchierotti and Spano, 2015). It would be remarkable to ascertain the role of embryonic self-defense and repair mechanisms against pollutants, such as the mechanisms of embryonic fragmentation, as well as long-term events such as abortion or deleterious effects on children conceived through IVF techniques.
Therefore, it must be considered the importance of performing and sharing studies that demonstrate the potential deleterious effects produced by IVF-recognized compounds (for e.g. NPs, Naphtalene PAHs, PM, VOCs) over human gametes and embryos, which will only lead to improve the risk management. Future studies should have similar designs so that results can be easily interrelated and associated with previous findings so as to establish specific developmental rates after exposure and perhaps develop an official database with IVF known pollutants and their effects. Additionally, further investigations are needed to develop sensitive and relevant quality control assays for culture system as well (Khan et al., 2013).
ConclusionsChanges in IVF laboratory air quality have been crucial in influencing conception rates, embryonic development, implantation, live birth rates in human reproduction and long- term studies in newborns. The in vitro environment monitoring must be a normal procedure of any IVF laboratory, bearing in mind that it is not only about the culture media formulations (Karaouga et al., 2014; Mortimer et al., 2018). Due to the existing risk of exposure to different pollutant compounds present inside the IVF laboratories and inside incubators and during routine handling inside in the laboratory of the gametes and embryos, laboratories have to invest in installations, air filtration systems and incubators with VOCs filters included, which will allow them to improve outcomes. It is equally important the routine environmental audits and the development of preventive strategies despite the high degree of uncertainty about how all pollutants interact with human embryos.
Because all the studies reviewed originate from different sources, study designs and findings, scientists all agree that IVF environment pollution affects the human embryo development and the future reproductive outcomes. However, morphological and genomic information needs to be described on key points of the early development, so the prediction can be accurate.
FundingThis manuscript has not been funded by any organization.
Conflict of interestBoth authors certify that we do not have affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
The authors would like to express their gratitude to Dr. Fernanda Insúa (Senior Embryologist) for her interest in this work and her selflessly collaboration by reviewing the draft of this manuscript.